Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
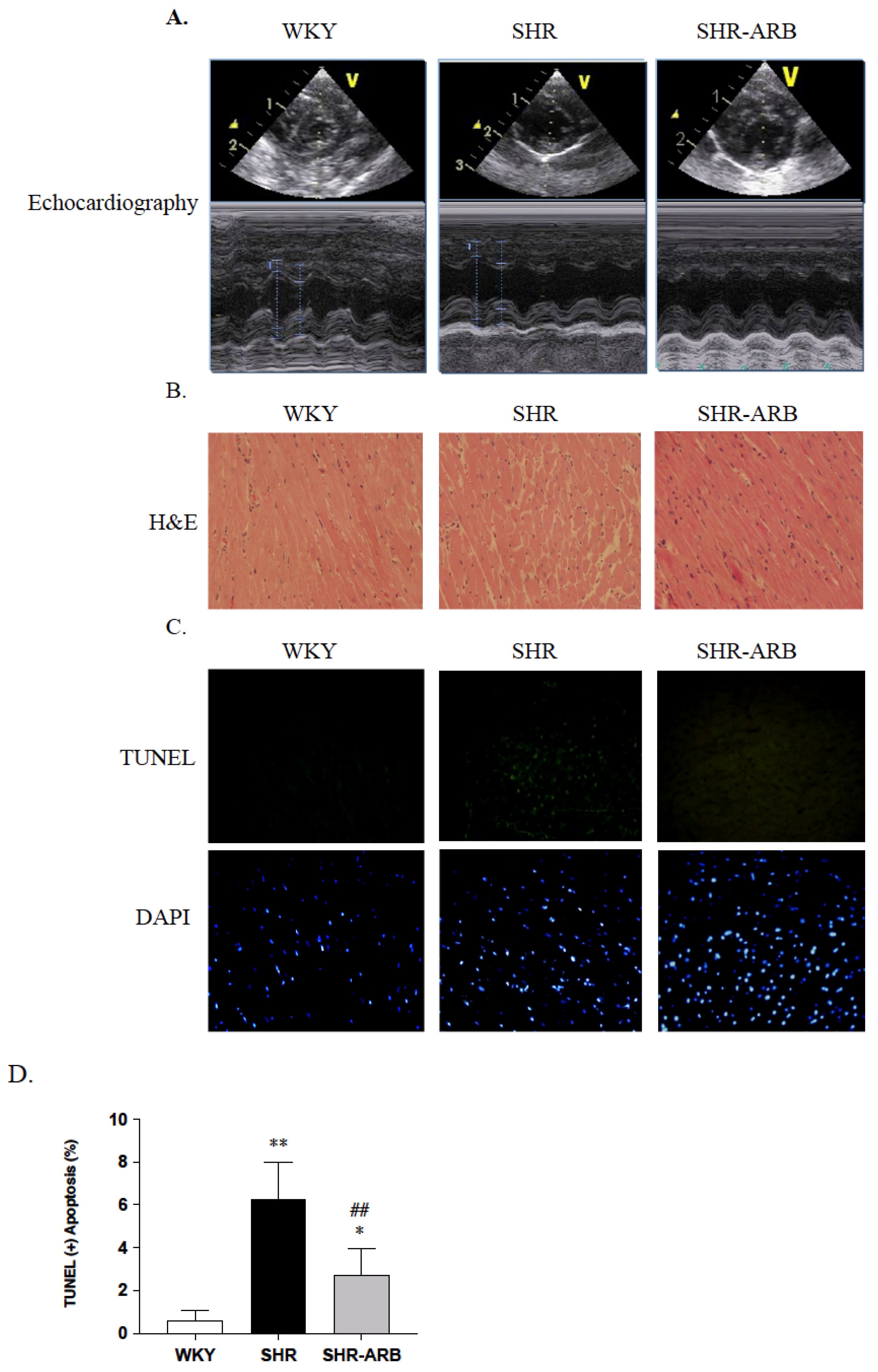
2.2. Tail Cuff Resting Blood Pressure and Echocardiography
2.3. Histological Analysis
2.4. TUNEL Assay in Heart Tissue
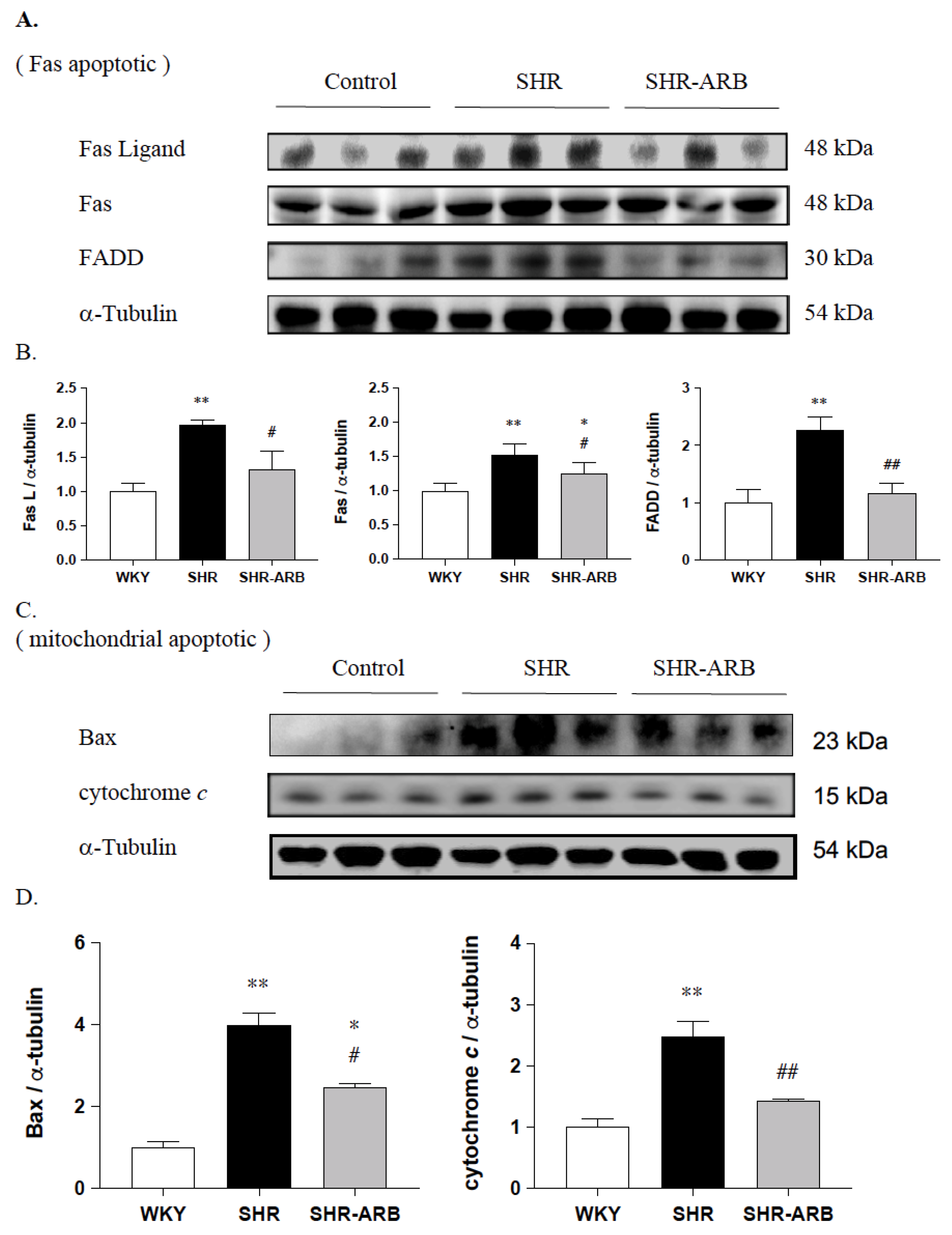
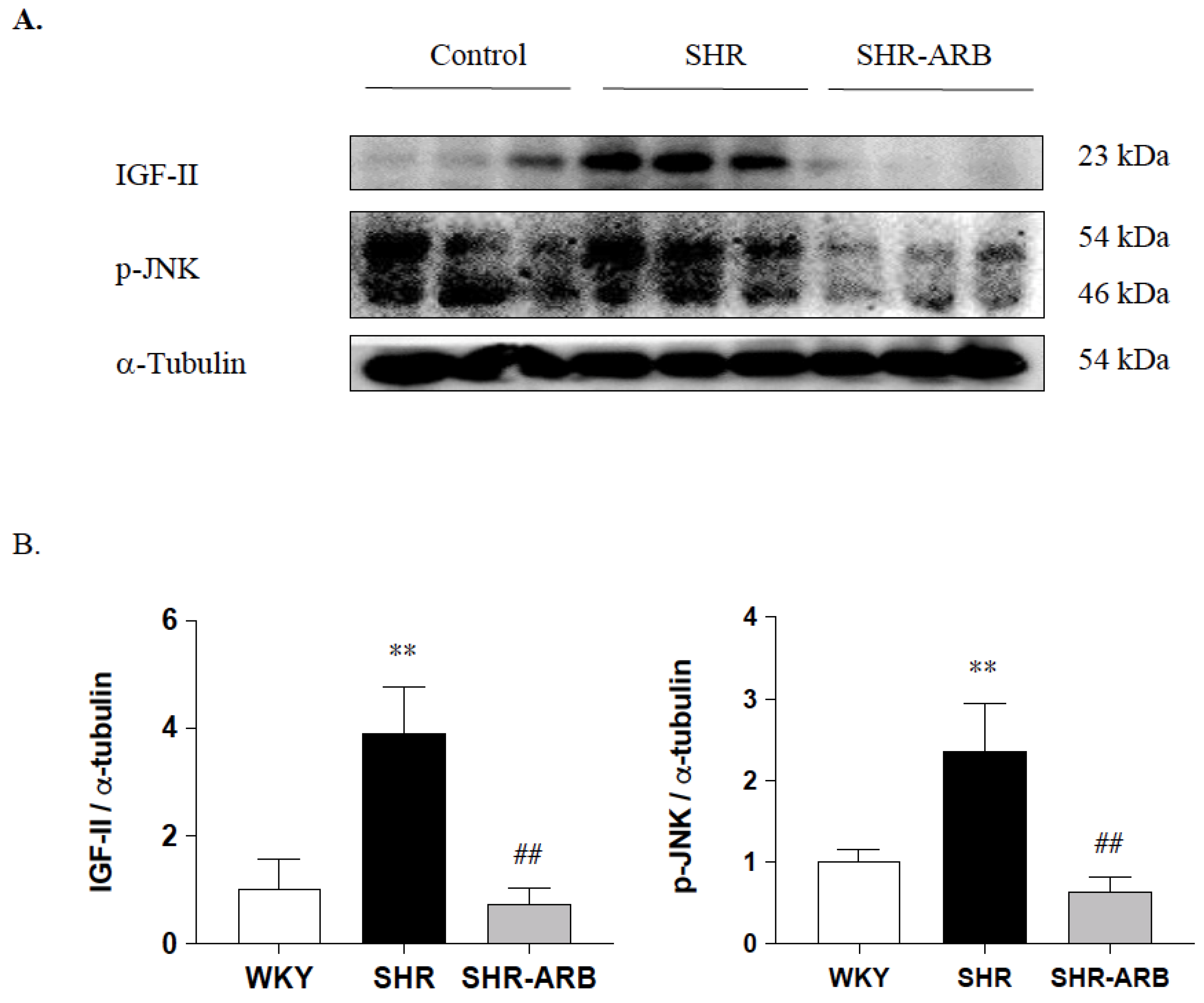
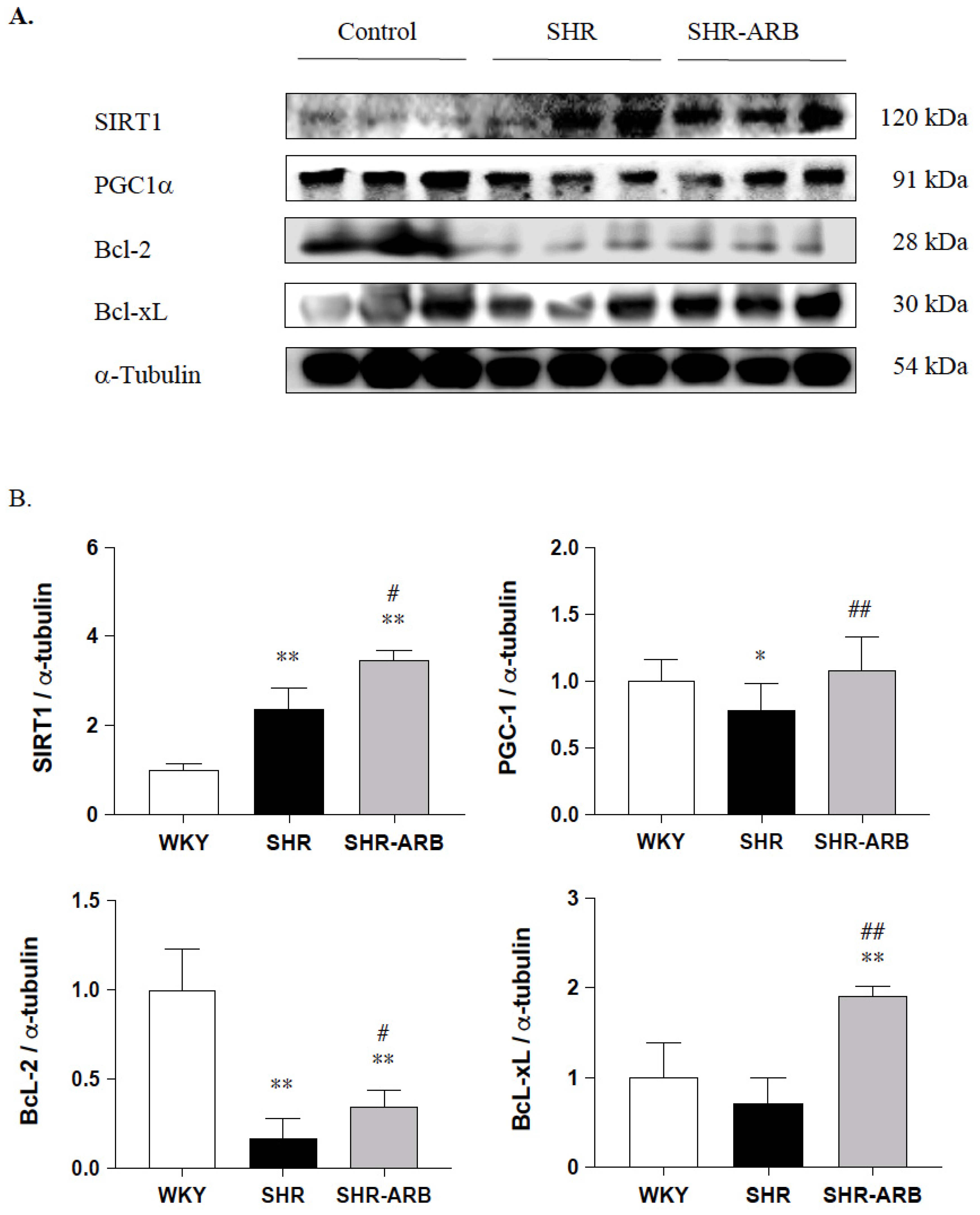
2.5. Immunoblot Analysis
2.6. Statistical Analysis
3. Results
3.1. Body Weight and Cardiac Characteristics
3.2. DAPI Staining and TUNEL-Positive Apoptotic Cells of Left Ventricle
3.3. Upstream of Fas/FasL- to Mitochondria-Mediated Apoptotic Pathways
3.4. Downstream of Fas/FasL- to Mitochondria-Mediated Apoptotic Pathways
3.4.1. Effect of ARB on IGF-II and p-JNK Protein Expression
3.4.2. Cardiac SIRT1/PGC-1α Pro-Survival Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muller-Brunotte, R.; Kahan, T.; Lopez, B.; Edner, M.; Gonzalez, A.; Diez, J.; Malmqvist, K. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: Results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J. Hypertens. 2007, 25, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Fortuno, M.A.; Querejeta, R.; Ravassa, S.; Lopez, B.; Lopez, N.; Diez, J. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc. Res. 2003, 59, 549–562. [Google Scholar] [CrossRef]
- Hunyady, L.; Catt, K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006, 20, 953–970. [Google Scholar] [CrossRef]
- Lee, S.D.; Chu, C.H.; Huang, E.J.; Lu, M.C.; Liu, J.Y.; Liu, C.J.; Hsu, H.H.; Lin, J.A.; Kuo, W.W.; Huang, C.Y. Roles of insulin-like growth factor II in cardiomyoblast apoptosis and in hypertensive rat heart with abdominal aorta ligation. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E306–E314. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kuo, W.W.; Yeh, Y.L.; Ho, T.J.; Lin, J.Y.; Lin, D.Y.; Chu, C.H.; Tsai, F.J.; Tsai, C.H.; Huang, C.Y. ANG II promotes IGF-IIR expression and cardiomyocyte apoptosis by inhibiting HSF1 via JNK activation and SIRT1 degradation. Cell Death Differ. 2014, 21, 1262–1274. [Google Scholar] [CrossRef]
- Bishopric, N.H.; Andreka, P.; Slepak, T.; Webster, K.A. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr. Opin. Pharmacol. 2001, 1, 141–150. [Google Scholar] [CrossRef]
- Crow, M.T.; Mani, K.; Nam, Y.J.; Kitsis, R.N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Yang, A.L.; Lin, Y.M.; Wu, F.N.; Lin, J.A.; Chan, Y.S.; Tsai, F.J.; Tsai, C.H.; Kuo, C.H.; Lee, S.D. Anti-apoptotic and pro-survival effects of exercise training on hypertensive hearts. J. Appl. Physiol. 2012, 112, 883–891. [Google Scholar] [CrossRef]
- Narula, J.; Haider, N.; Arbustini, E.; Chandrashekhar, Y. Mechanisms of disease: Apoptosis in heart failure—Seeing hope in death. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R. Apoptosis in the cardiovascular system. Heart 2002, 87, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Li, Y.; Yu, S.; Zhao, Y. SIRT1/PGC-1alpha Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front. Mol. Neurosci. 2017, 10, 443. [Google Scholar] [CrossRef]
- Forni, V.; Wuerzner, G.; Pruijm, M.; Burnier, M. Long-term use and tolerability of irbesartan for control of hypertension. Integr. Blood Press Control 2011, 4, 17–26. [Google Scholar] [CrossRef]
- Riveiro, A.; Mosquera, A.; Alonso, M.; Calvo, C. Angiotensin II type 1 receptor blocker irbesartan ameliorates vascular function in spontaneously hypertensive rats regardless of oestrogen status. J. Hypertens. 2002, 20, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Shang, Q.H.; Jin, H.Y.; Song, B.; Oudit, G.Y.; Lu, L.; Zhou, T.; Xu, Y.L.; Gao, P.J.; Zhu, D.L.; et al. Cardiac protective effects of irbesartan via the PPAR-gamma signaling pathway in angiotensin-converting enzyme 2-deficient mice. J. Transl. Med. 2013, 11, 229. [Google Scholar] [CrossRef]
- Bramlage, P.; Pittrow, D.; Kirch, W. The effect of irbesartan in reducing cardiovascular risk in hypertensive type 2 diabetic patients: An observational study in 16,600 patients in primary care. Curr. Med. Res. Opin. 2004, 20, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Bramlage, P.; Paar, W.D.; Thoenes, M.; Unger, T. Irbesartan for the treatment of hypertension in patients with the metabolic syndrome: A sub analysis of the Treat to Target post authorization survey. Prospective observational, two armed study in 14,200 patients. Cardiovasc. Diabetol. 2007, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Suzuki, J.; Wakayama, K.; Kumagai, H.; Ikeda, Y.; Akazawa, H.; Komuro, I.; Isobe, M. Angiotensin II receptor blocker irbesartan attenuates cardiac dysfunction induced by myocardial infarction in the presence of renal failure. Hypertens. Res. 2016, 39, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.Y.; Lin, Y.Y.; Yu, S.H.; Lin, C.Y.; Liou, Y.F.; Wu, X.B.; Wong, J.K.S.; Huang, C.Y.; Lee, S.D. Angiotensin II receptor blocker irbesartan attenuates sleep apnea-induced cardiac apoptosis and enhances cardiac survival and Sirtuin 1 upregulation. Sleep Breath 2021. [Google Scholar] [CrossRef]
- Brunner, H.R. The new angiotensin II receptor antagonist, irbesartan: Pharmacokinetic and pharmacodynamic considerations. Am. J. Hypertens. 1997, 10, 311S–317S. [Google Scholar] [CrossRef]
- Fujino, M.; Miura, S.; Kiya, Y.; Tominaga, Y.; Matsuo, Y.; Karnik, S.S.; Saku, K. A small difference in the molecular structure of angiotensin II receptor blockers induces AT(1) receptor-dependent and -independent beneficial effects. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2010, 33, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Watanabe, A.; Zhao, S.; Kobayashi, T.; Fukao, K.; Tanaka, Y.; Nakano, T.; Yoshida, T.; Takemoto, H.; Tamaki, N.; et al. Suppressive effects of irbesartan on inflammation and apoptosis in atherosclerotic plaques of apoE-/- mice: Molecular imaging with 14C-FDG and 99mTc-annexin A5. PLoS ONE 2014, 9, e89338. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Nithiyanantham, S.; Huang, C.Y.; Pai, P.Y.; Chang, T.T.; Hu, L.C.; Chen, R.J.; VijayaPadma, V.; Kuo, W.W.; Huang, C.Y. Synergistic cardiac pathological hypertrophy induced by high-salt diet in IGF-IIRalpha cardiac-specific transgenic rats. PLoS ONE 2019, 14, e0216285. [Google Scholar] [CrossRef]
- Finck, B.N.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 2007, 115, 2540–2548. [Google Scholar] [CrossRef]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 2000, 106, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Karamanlidis, G.; Garcia-Menendez, L.; Kolwicz, S.C., Jr.; Lee, C.F.; Tian, R. Promoting PGC-1alpha-driven mitochondrial biogenesis is detrimental in pressure-overloaded mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1307–H1316. [Google Scholar] [CrossRef] [PubMed]
- Sihag, S.; Cresci, S.; Li, A.Y.; Sucharov, C.C.; Lehman, J.J. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J. Mol. Cell Cardiol. 2009, 46, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Hong, Y.; Yu, S.H.; Wu, X.B.; Shyu, W.C.; Chen, J.S.; Ting, H.; Yang, A.L.; Lee, S.D. Antiapoptotic and mitochondrial biogenetic effects of exercise training on ovariectomized hypertensive rat hearts. J. Appl. Physiol. 2019, 126, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, H.; Taniyama, Y.; Rakugi, H.; Morishita, R. Cardiac and renal protective effects of irbesartan via peroxisome proliferator-activated receptorgamma-hepatocyte growth factor pathway independent of angiotensin II Type 1a receptor blockade in mouse model of salt-sensitive hypertension. J. Am. Heart Assoc. 2013, 2, e000103. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1375–H1389. [Google Scholar] [CrossRef] [PubMed]
- Alcendor, R.R.; Gao, S.; Zhai, P.; Zablocki, D.; Holle, E.; Yu, X.; Tian, B.; Wagner, T.; Vatner, S.F.; Sadoshima, J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 2007, 100, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Ren, Y.; Sun, C.; Sun, Y.; Tan, H.; Wu, Y.; Cui, B.; Wu, Z. PPAR gamma protects cardiomyocytes against oxidative stress and apoptosis via Bcl-2 upregulation. Vascul. Pharmacol. 2009, 51, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.M.; Gustafsson, A.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022. [Google Scholar] [CrossRef]


| Parameters/Groups | WKY | SHR | SHR-ARB |
|---|---|---|---|
| Number of animals | 8 | 8 | 8 |
| BW (g) | 345 ± 35.46 | 400.6 ± 32.08 * | 406.1 ± 19.86 ** |
| WHW (g) | 1.54 ± 0.37 | 1.78 ± 0.19 | 1.45 ± 0.06 ## |
| LVW (g) | 0.996 ± 0.26 | 1.42 ± 0.17 * | 1.09 ± 0.05 ## |
| WHW/BW (×104) | 45.84 ± 8.76 | 45.94 ± 2.27 | 35.69 ± 7.34 * ## |
| LVW/BW (×104) | 29.49 ± 5.53 | 36.58 ± 2.24 * | 26.93 ± 9.30 ## |
| LVW/WHW | 0.645 ± 0.038 | 0.796 ± 0.012 ** | 0.754 ± 0.014 ** ## |
| WHW/TL (g/mm) | 0.041 ± 0.009 | 0.046 ± 0.005 | 0.037 ± 0.001 ## |
| LVW/TL (g/mm) | 0.027 ± 0.007 | 0.037 ± 0.004 * | 0.028 ± 0.001 ## |
| IVSd (mm) | 1.78 ± 0.15 | 2.26 ± 0.28 ** | 1.84 ± 0.24 ## |
| LVPWd (mm) | 1.80 ± 0.33 | 1.92 ± 0.25 | 1.88 ± 0.23 |
| LVIDd (mm) | 7.74 ± 0.59 | 8.63 ± 0.80 | 8.04 ± 0.44 |
| LVIDs (mm) | 4.75 ± 0.73 | 5.73 ± 0.80 * | 4.76 ± 0.65 ## |
| Fractional Shortening (FS), % | 38.6 ± 8.2 | 33.8 ± 4.2 | 41.04 ± 5.66 ## |
| Heart rate | 424 ± 14 | 423 ± 13 | 404 ± 11 |
| Systolic blood pressure (mmHg) | 137 ± 9.36 | 225 ± 11.30 ** | 160 ± 12.90 ## |
| Diastolic blood pressure (mmHg) | 81 ± 14.04 | 155 ± 21.30 ** | 97 ± 15.90 * ## |
| Mean blood pressure (mmHg) | 100 ± 10.40 | 178 ± 15.70 ** | 118 ± 12 ** ## |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, P.-Y.; Wong, J.K.S.; Cui, Z.-Y.; Lin, Y.-Y.; Lee, S.-D. Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis. J. Cardiovasc. Dev. Dis. 2022, 9, 266. https://doi.org/10.3390/jcdd9080266
Pai P-Y, Wong JKS, Cui Z-Y, Lin Y-Y, Lee S-D. Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis. Journal of Cardiovascular Development and Disease. 2022; 9(8):266. https://doi.org/10.3390/jcdd9080266
Chicago/Turabian StylePai, Pei-Ying, James K. S. Wong, Zhen-Yang Cui, Yi-Yuan Lin, and Shin-Da Lee. 2022. "Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis" Journal of Cardiovascular Development and Disease 9, no. 8: 266. https://doi.org/10.3390/jcdd9080266
APA StylePai, P.-Y., Wong, J. K. S., Cui, Z.-Y., Lin, Y.-Y., & Lee, S.-D. (2022). Angiotensin II Receptor Blocker Irbesartan Enhanced SIRT1 longevity Signaling Replaces the Mitochondrial Biogenetic Survival Pathway to Attenuate Hypertension-Induced Heart Apoptosis. Journal of Cardiovascular Development and Disease, 9(8), 266. https://doi.org/10.3390/jcdd9080266







