Pulmonary Embolism in Women: A Systematic Review of the Current Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Extraction
3. Results
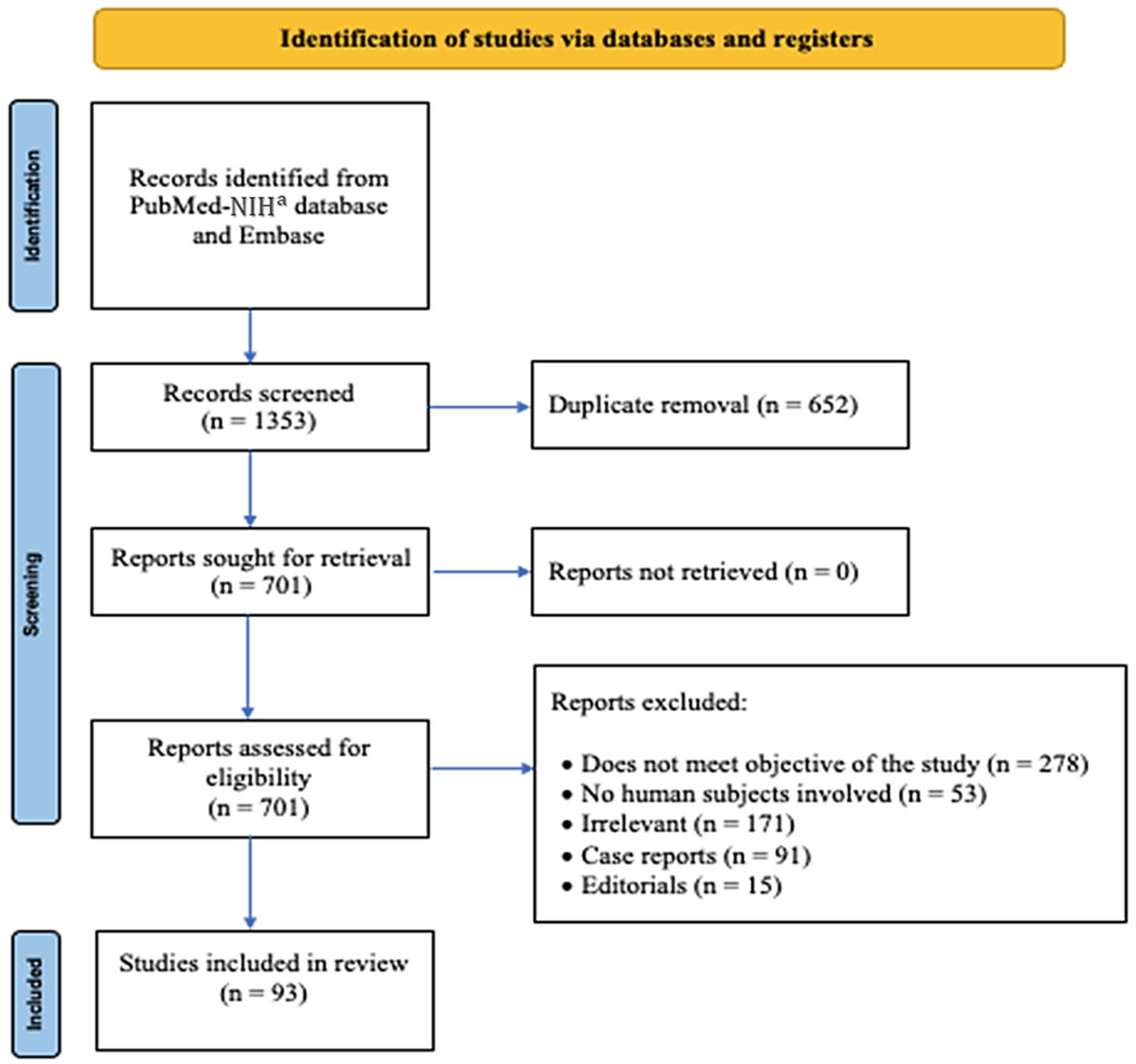
3.1. Search Results
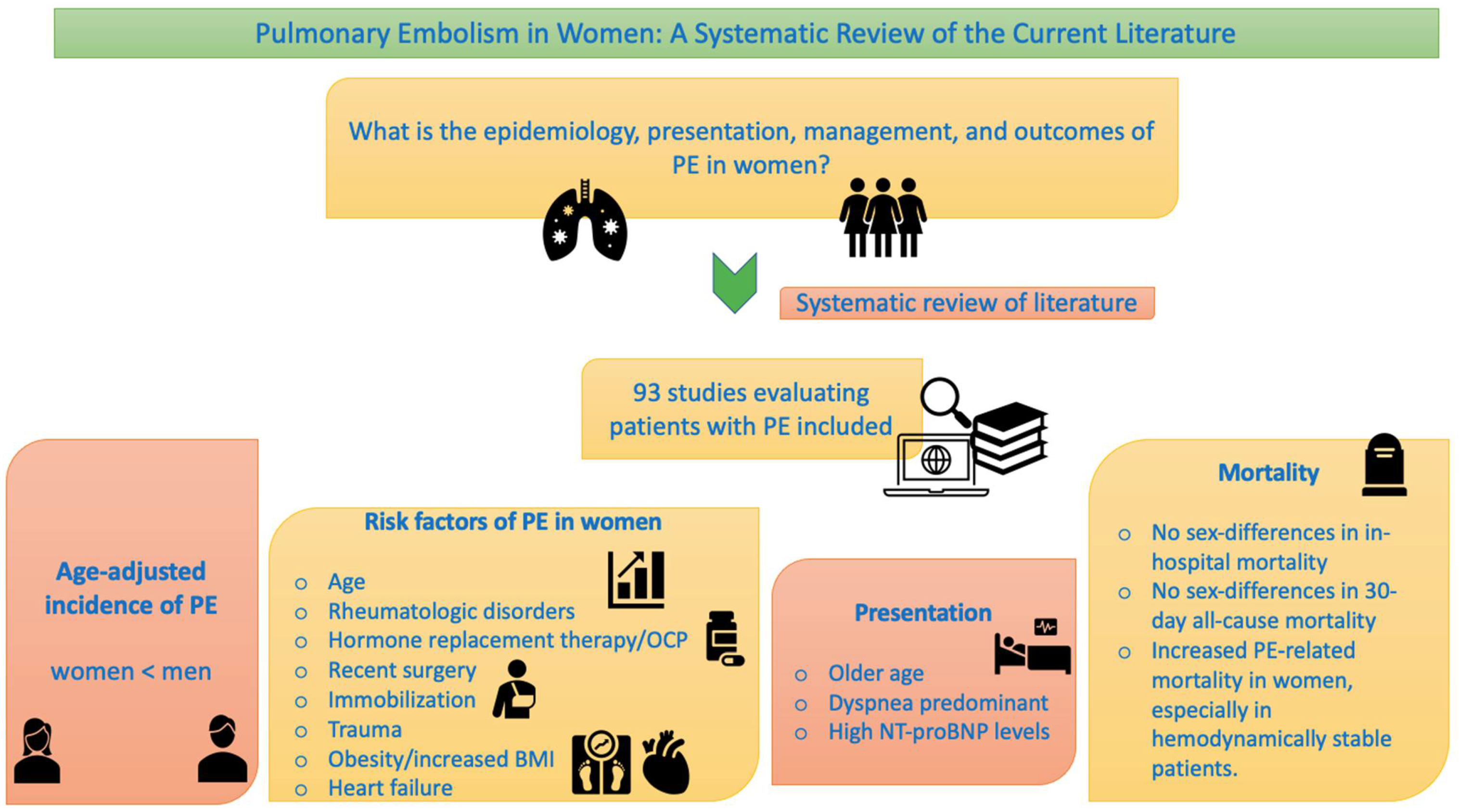
3.2. Epidemiology
3.2.1. Incidence
3.2.2. Risk Factors for PE
3.3. Clinical Features
3.4. Diagnosis
3.5. Management
3.6. Prognostication
3.7. Short-Term Outcomes
3.7.1. Complications
3.7.2. Short-Term Mortality
3.8. Long-Term Outcomes
3.8.1. CTEPH
3.8.2. Long-Term Mortality
3.9. Special Focus
3.9.1. PE in Pregnancy
3.9.2. PE in COVID-19
4. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldhaber, S.Z.; Bounameaux, H. Pulmonary embolism and deep vein thrombosis. Lancet 2012, 379, 1835–1846. [Google Scholar] [CrossRef]
- Martin, K.A.; Molsberry, R.; Cuttica, M.J.; Desai, K.R.; Schimmel, D.R.; Khan, S.S. Time Trends in Pulmonary Embolism Mortality Rates in the United States, 1999 to 2018. J. Am. Heart Assoc. 2020, 9, e016784. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A.; Perrier, A.; Konstantinides, S.; Agnelli, G.; Galie, N.; Pruszczyk, P.; Bengel, F.; Brady, A.J.; Ferreira, D.; Janssens, U.; et al. Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur. Heart J. 2008, 29, 2276–2315. [Google Scholar] [CrossRef] [PubMed]
- Coventry, L.L.; Finn, J.; Bremner, A.P. Sex differences in symptom presentation in acute myocardial infarction: A systematic review and meta-analysis. Heart Lung 2011, 40, 477–491. [Google Scholar] [CrossRef]
- Anderson, R.D.; Pepine, C.J. Gender differences in the treatment for acute myocardial infarction: Bias or biology? Circulation 2007, 115, 823–826. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Gialeraki, A.; Valsami, S.; Pittaras, T.; Panayiotakopoulos, G.; Politou, M. Oral Contraceptives and HRT Risk of Thrombosis. Clin. Appl. Thromb. Hemost. 2018, 24, 217–225. [Google Scholar] [CrossRef]
- Miro, O.; Jimenez, S.; Mebazaa, A.; Freund, Y.; Burillo-Putze, G.; Martin, A.; Martin-Sanchez, F.J.; Garcia-Lamberechts, E.J.; Alquezar-Arbe, A.; Jacob, J.; et al. Pulmonary embolism in patients with COVID-19: Incidence, risk factors, clinical characteristics, and outcome. Eur. Heart J. 2021, 42, 3127–3142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Heit, J.A. The epidemiology of venous thromboembolism in the community. Arter. Thromb. Vasc. Biol. 2008, 28, 370–372. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Tsai, A.W.; White, R.H.; Heckbert, S.R.; Rosamond, W.D.; Enright, P.; Folsom, A.R. Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am. J. Med. 2004, 117, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Naess, I.A.; Christiansen, S.C.; Romundstad, P.; Cannegieter, S.C.; Rosendaal, F.R.; Hammerstrøm, J. Incidence and mortality of venous thrombosis: A population-based study. J. Thromb. Haemost. 2007, 5, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Jo, J.Y.; Kwon, Y.S.; Kim, J.B.; Lee, M.Y. Incidence of pulmonary embolism among hospitalized patients. Thromb. Res. 2012, 129, 523–525. [Google Scholar] [CrossRef]
- Barrios, D.; Morillo, R.; Guerassimova, I.; Barbero, E.; Escobar-Morreale, H.; Cohen, A.T.; Becattini, C.; Tapson, V.; Yusen, R.; Jimenez, D. Sex differences in the characteristics and short-term prognosis of patients presenting with acute symptomatic pulmonary embolism. PLoS ONE 2017, 12, e0187648. [Google Scholar] [CrossRef] [PubMed]
- Bakebe, A.; Kashongwe, I.; Mulenga, C.; Tshiasuma, M.; Kabengele, B.; Bisuta, S.F.; Makulo, J.R.; Kashongwe, Z.; Kayembe, J.M. Pulmonary embolism: Epidemiological data and diagnosis in Kinshasa hospitals. Int. J. Tuberc. Lung Dis. 2017, 21, 875–879. [Google Scholar] [CrossRef]
- Pai, N.; Ghosh, K.; Shetty, S. Cause of deep venous thrombosis and pulmonary embolism in young patients from India as compared with other ethnic groups. Blood Coagul. Fibrinolysis 2012, 23, 257–261. [Google Scholar] [CrossRef]
- Jarman, A.F.; Mumma, B.E.; Singh, K.S.; Nowadly, C.D.; Maughan, B.C. Crucial considerations: Sex differences in the epidemiology, diagnosis, treatment, and outcomes of acute pulmonary embolism in non-pregnant adult patients. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12378. [Google Scholar] [CrossRef]
- Roach, R.E.; Cannegieter, S.C.; Lijfering, W.M. Differential risks in men and women for first and recurrent venous thrombosis: The role of genes and environment. J. Thromb. Haemost. 2014, 12, 1593–1600. [Google Scholar] [CrossRef]
- Pribish, A.M.; Beyer, S.E.; Krawisz, A.K.; Weinberg, I.; Carroll, B.J.; Secemsky, E.A. Sex differences in presentation, management, and outcomes among patients hospitalized with acute pulmonary embolism. Vasc. Med. 2020, 25, 541–548. [Google Scholar] [CrossRef]
- Tanabe, Y.; Yamamoto, T.; Murata, T.; Mabuchi, K.; Hara, N.; Mizuno, A.; Nozato, T.; Hisatake, S.; Obayashi, T.; Takayama, M.; et al. Gender Differences Among Patients With Acute Pulmonary Embolism. Am. J. Cardiol. 2018, 122, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Rappold, L.; Gerhold-Ay, A.; Hobohm, L.; Hasenfuss, G.; Konstantinides, S.V.; Dellas, C.; Lankeit, M. Sex-specific differences in pulmonary embolism. Thromb. Res. 2019, 178, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Bang, S.M.; Oh, D. Incidence of venous thromboembolism in Korea: From the Health Insurance Review and Assessment Service database. J. Thromb. Haemost. 2011, 9, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kitamukai, O.; Sakuma, M.; Takahashi, T.; Kagaya, Y.; Watanabe, J.; Shirato, K. Incidence and characteristics of pulmonary thromboembolism in Japan 2000. Intern. Med. 2003, 42, 1090–1094. [Google Scholar] [CrossRef]
- Verso, M.; Agnelli, G.; Ageno, W.; Imberti, D.; Moia, M.; Palareti, G.; Pistelli, R.; Cantone, V. Long-term death and recurrence in patients with acute venous thromboembolism: The MASTER registry. Thromb. Res. 2012, 130, 369–373. [Google Scholar] [CrossRef]
- Tagalakis, V.; Kondal, D.; Ji, Y.; Boivin, J.F.; Moride, Y.; Ciampi, A.; Kahn, S.R. Men had a higher risk of recurrent venous thromboembolism than women: A large population study. Gend. Med. 2012, 9, 33–43. [Google Scholar] [CrossRef]
- Chung, W.S.; Peng, C.L.; Lin, C.L.; Chang, Y.J.; Chen, Y.F.; Chiang, J.Y.; Sung, F.C.; Kao, C.H. Rheumatoid arthritis increases the risk of deep vein thrombosis and pulmonary thromboembolism: A nationwide cohort study. Ann. Rheum. Dis. 2014, 73, 1774–1780. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Vostretsova, K.; De Vera, M.A.; Sayre, E.C.; Choi, H.K. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: A general population-based study. Semin. Arthritis Rheum. 2015, 45, 195–201. [Google Scholar] [CrossRef]
- You, H.; Zhao, J.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Characteristics and risk factors of pulmonary embolism in patients with systemic lupus erythematosus: A case control study. Clin. Exp. Rheumatol. 2020, 38, 940–948. [Google Scholar]
- Arthes, F.G. An epidemiologic survey of hospitalized cases of venous thrombosis and pulmonary embolism in young women. Milbank Mem. Fund Q. 1972, 50 (Suppl. 2), 233–243. [Google Scholar] [CrossRef]
- Berghaus, T.M.; Haeckel, T.; Behr, W.; Wehler, M.; von Scheidt, W.; Schwaiblmair, M. Central thromboembolism is a possible predictor of right heart dysfunction in normotensive patients with acute pulmonary embolism. Thromb. Res. 2010, 126, e201–e205. [Google Scholar] [CrossRef] [PubMed]
- Haskins, I.N.; Amdur, R.; Sarani, B.; Vaziri, K. Congestive heart failure is a risk factor for venous thromboembolism in bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- de-Miguel-Diez, J.; López-de-Andrés, A.; Hernandez-Barrera, V.; Jimenez, D.; Monreal, M.; López-Herranz, M.; Ji, Z.; Jiménez-García, R. The significance of heart failure in hospitalised patients with pulmonary embolism. A gender-specific analysis. Int. J. Clin. Pract. 2021, 75, e14558. [Google Scholar] [CrossRef]
- Melgaard, L.; Nielsen, P.B.; Overvad, T.F.; Skjøth, F.; Lip, G.Y.H.; Larsen, T.B. Sex differences in risk of incident venous thromboembolism in heart failure patients. Clin. Res. Cardiol. 2019, 108, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Bito, S.; Nakamura, M.; Miyata, S.; Saito, M.; Kakizaki, H.; Nakayama, Y.; Matsusita, T.; Furuichi, I.; Sasazaki, Y.; et al. Venous thromboembolism after total joint arthroplasty: Results from a Japanese multicenter cohort study. Arthritis Res. Ther. 2014, 16, R154. [Google Scholar] [CrossRef] [PubMed]
- Kabrhel, C.; Varraso, R.; Goldhaber, S.Z.; Rimm, E.; Camargo, C.A., Jr. Physical inactivity and idiopathic pulmonary embolism in women: Prospective study. BMJ 2011, 343, d3867. [Google Scholar] [CrossRef] [PubMed]
- Jové, N.A.; Samaan, S.; Pizzimenti, N.M.; Lincoln, D.; Markel, D.C. Characterization of Pulmonary Emboli in Total Joint Arthroplasty Patients Compared to General Medical Patients. J. Knee Surg. 2020, 33, 1232–1237. [Google Scholar] [CrossRef]
- Ogren, M.; Eriksson, H.; Bergqvist, D.; Sternby, N.H. Subcutaneous fat accumulation and BMI associated with risk for pulmonary embolism in patients with proximal deep vein thrombosis: A population study based on 23 796 consecutive autopsies. J. Intern. Med. 2005, 258, 166–171. [Google Scholar] [CrossRef]
- Kabrhel, C.; Varraso, R.; Goldhaber, S.Z.; Rimm, E.B.; Camargo, C.A. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity 2009, 17, 2040–2046. [Google Scholar] [CrossRef]
- Lian, T.Y.; Lu, D.; Yan, X.X.; Tan, J.S.; Peng, F.H.; Zhu, Y.J.; Wei, Y.P.; Wu, T.; Sun, K.; Jiang, X.; et al. Association between congenital thrombophilia and outcomes in pulmonary embolism patients. Blood Adv. 2020, 4, 5958–5965. [Google Scholar] [CrossRef]
- Obaid, M.; El-Menyar, A.; Asim, M.; Al-Thani, H. Prevalence and Outcomes of Thrombophilia in Patients with Acute Pulmonary Embolism. Vasc. Health Risk Manag. 2020, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, S.; Dzudovic, B.; Rusovic, S.; Subota, V.; Obradovic, D. Gender-related differences in clinical presentation, electrocardiography signs, laboratory markers and outcome in patients with acute pulmonary embolism. Vojn. Pregl. 2016, 73, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Courtney, D.M.; Sasser, H.C.; Pincus, C.L.; Kline, J.A. Pulseless electrical activity with witnessed arrest as a predictor of sudden death from massive pulmonary embolism in outpatients. Resuscitation 2001, 49, 265–272. [Google Scholar] [CrossRef]
- Courtney, D.M.; Kline, J.A. Identification of prearrest clinical factors associated with outpatient fatal pulmonary embolism. Acad. Emerg. Med. 2001, 8, 1136–1142. [Google Scholar] [CrossRef]
- McHugh, K.B.; Visani, L.; DeRosa, M.; Covezzoli, A.; Rossi, E.; Goldhaber, S.Z. Gender comparisons in pulmonary embolism (results from the International Cooperative Pulmonary Embolism Registry [ICOPER]). Am. J. Cardiol. 2002, 89, 616–619. [Google Scholar] [CrossRef]
- Robert-Ebadi, H.; Le Gal, G.; Carrier, M.; Couturaud, F.; Perrier, A.; Bounameaux, H.; Righini, M. Differences in clinical presentation of pulmonary embolism in women and men. J. Thromb. Haemost. 2010, 8, 693–698. [Google Scholar] [CrossRef]
- Stein, P.D.; Beemath, A.; Matta, F.; Weg, J.G.; Yusen, R.D.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Sostman, H.D.; Tapson, V.F.; et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am. J. Med. 2007, 120, 871–879. [Google Scholar] [CrossRef]
- van Mens, T.E.; van der Pol, L.M.; van Es, N.; Bistervels, I.M.; Mairuhu, A.T.A.; van der Hulle, T.; Klok, F.A.; Huisman, M.V.; Middeldorp, S. Sex-specific performance of pre-imaging diagnostic algorithms for pulmonary embolism. J. Thromb. Haemost. 2018, 16, 858–865. [Google Scholar] [CrossRef]
- Stein, P.D.; Fowler, S.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Popovich, J., Jr.; Quinn, D.A.; Sos, T.A.; et al. Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 2006, 354, 2317–2327. [Google Scholar] [CrossRef]
- Chen, Y.A.; Gray, B.G.; Bandiera, G.; MacKinnon, D.; Deva, D.P. Variation in the utilization and positivity rates of CT pulmonary angiography among emergency physicians at a tertiary academic emergency department. Emerg. Radiol. 2015, 22, 221–229. [Google Scholar] [CrossRef]
- de Bruin, S.; van Langevelde, K.; Huisman, M.V.; Cannegieter, S.C. Suspicion of pulmonary embolism: Added value of CT depends on patient characteristics and referring individual. Ned. Tijdschr. Voor Geneeskd. 2012, 156, A4201. [Google Scholar]
- Aggarwal, T.; Eskandari, A.; Priya, S.; Mullan, A.; Garg, I.; Siembida, J.; Mullan, B.; Nagpal, P. Pulmonary embolism rule out: Positivity and factors affecting the yield of CT angiography. Postgrad. Med. J. 2020, 96, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, J.M.; Zander, A.L.; Olson, E.J.; Shackford, S.R.; Dunne, C.E.; Sise, C.B.; Badiee, J.; Schechter, M.S.; Sise, M.J. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis? J. Trauma. Acute. Care. Surg. 2014, 76, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Beemath, A.; Quinn, D.A.; Olson, R.E.; Goodman, L.R.; Gottschalk, A.; Hales, C.A.; Hull, R.D.; Leeper, K.V., Jr.; Sostman, H.D.; et al. Usefulness of multidetector spiral computed tomography according to age and gender for diagnosis of acute pulmonary embolism. Am. J. Cardiol. 2007, 99, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Roggenland, D.; Peters, S.A.; Lemburg, S.P.; Holland-Letz, T.; Nicolas, V.; Heyer, C.M. CT angiography in suspected pulmonary embolism: Impact of patient characteristics and different venous lines on vessel enhancement and image quality. AJR Am. J. Roentgenol. 2008, 190, W351–W359. [Google Scholar] [CrossRef]
- Stein, P.D.; Hull, R.D.; Patel, K.C.; Olson, R.E.; Ghali, W.A.; Alshab, A.K.; Meyers, F.A. Venous thromboembolic disease: Comparison of the diagnostic process in men and women. Arch. Intern. Med. 2003, 163, 1689–1694. [Google Scholar] [CrossRef]
- Morimoto, S.; Kito, G. Rotarod method in young rats and the antidepressive effect: Is the rotarod method capable of evaluating antidepressive effects? Nihon Yakurigaku Zasshi. Folia Pharmacol. Jpn. 1994, 104, 39–49. [Google Scholar] [CrossRef][Green Version]
- Jenab, Y.; Ghaffari-Marandi, N.; Safir, A.; Ejmalian, G.; Zoroufian, A.; Jalali, A.; Sahebjam, M. Sex-related changes in tissue Doppler imaging parameters among patients with acute pulmonary thromboembolism. J. Ultrasound Med. 2013, 32, 1997–2005. [Google Scholar] [CrossRef]
- Shah, T.; Haimi, I.; Yang, Y.; Gaston, S.; Taoutel, R.; Mehta, S.; Lee, H.J.; Zambahari, R.; Baumbach, A.; Henry, T.D.; et al. Meta-Analysis of Gender Disparities in In-hospital Care and Outcomes in Patients with ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2021, 147, 23–32. [Google Scholar] [CrossRef]
- Saad, M.; Nairooz, R.; Pothineni, N.V.K.; Almomani, A.; Kovelamudi, S.; Sardar, P.; Katz, M.; Abdel-Wahab, M.; Bangalore, S.; Kleiman, N.S.; et al. Long-Term Outcomes With Transcatheter Aortic Valve Replacement in Women Compared With Men: Evidence From a Meta-Analysis. JACC Cardiovasc. Interv. 2018, 11, 24–35. [Google Scholar] [CrossRef]
- Werner, N.; Puls, M.; Baldus, S.; Lubos, E.; Bekeredjian, R.; Sievert, H.; Schofer, J.; Kuck, K.H.; Mollmann, H.; Hehrlein, C.; et al. Gender-related differences in patients undergoing transcatheter mitral valve interventions in clinical practice: 1-year results from the German TRAMI registry. Catheter. Cardiovasc. Interv. 2020, 95, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, S.; Li, W.; Zamora, C.; Barakakis, P.A.; Kokkinidis, D.G. Pharmacological and interventional management of pulmonary embolism: Where do we stand? Future Cardiol. 2022, 18, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.; Wiskar, K.; Berger, L.; Griesdale, D.E. The Use of Thrombolysis for Acute Pulmonary Embolism in the United States: National Trends and Patient Characteristics from 2006 to 2011. J. Emerg. Med. 2017, 52, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Geibel, A.; Olschewski, M.; Zehender, M.; Wilsch, M.; Odening, K.; Heinrich, F.; Kasper, W.; Konstantinides, S. Possible gender-related differences in the risk-to-benefit ratio of thrombolysis for acute submassive pulmonary embolism. Am. J. Cardiol. 2007, 99, 103–107. [Google Scholar] [CrossRef]
- Wiegers, H.M.G.; van Es, J.; Pap, A.F.; Lensing, A.W.A.; Middeldorp, S.; Scheres, L.J.J. Sex-specific differences in clot resolution 3 weeks after acute pulmonary embolism managed with anticoagulants-A substudy of the EINSTEIN-PE study. J. Thromb. Haemost. 2021, 19, 1759–1763. [Google Scholar] [CrossRef]
- Masotti, L.; Panigada, G.; Landini, G.; Pieralli, F.; Corradi, F.; Lenti, S.; Migliacci, R.; Arrigucci, S.; Frullini, A.; Bertieri, M.C.; et al. Simplified PESI score and sex difference in prognosis of acute pulmonary embolism: A brief report from a real life study. J. Thromb. Thrombolysis 2016, 41, 606–612. [Google Scholar] [CrossRef]
- McLeod, J.M.; Nandy, S.; Nagraj, S.; Lee, U.; Goldberg, Y.; Murthy, S. Right Heart Reverse Remodeling Correlates with NT-proBNP Outcomes Among Pulmonary Arterial Hypertension Patients on Combination Therapy. J. Heart Lung Transplant. 2022, 41, S143. [Google Scholar] [CrossRef]
- Panigada, G.; Masotti, L.; Rosi, C.; Teghini, L.; Cimolato, B.; Bertieri, M.C.; Angotti, C.; Romagnoli, A.M.; Cascinelli, I.; De Crescenzo, V.; et al. Thromboembolic burden, prognostic assessment and outcomes of females compared to males in acute pulmonary embolism. Acta Clin. Belg. 2016, 71, 142–148. [Google Scholar] [CrossRef]
- White, R.H.; Dager, W.E.; Zhou, H.; Murin, S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb. Haemost. 2006, 96, 267–273. [Google Scholar] [CrossRef]
- Blanco-Molina, A.; Enea, I.; Gadelha, T.; Tufano, A.; Bura-Riviere, A.; Di Micco, P.; Bounameaux, H.; Gonzalez, J.; Villalta, J.; Monreal, M.; et al. Sex differences in patients receiving anticoagulant therapy for venous thromboembolism. Medicine 2014, 93, 309–317. [Google Scholar] [CrossRef]
- Mansour, S.; Alotaibi, G.; Wu, C.; Alsaleh, K.; McMurtry, M.S. Sex disparities in hospitalization and mortality rates for venous thromboembolism. J. Thromb. Thrombolysis 2017, 44, 197–202. [Google Scholar] [CrossRef]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Klok, F.A.; Konstantinides, S.V.; Dartevelle, P.; Fadel, E.; Jenkins, D.; Kim, N.H.; Madani, M.; Matsubara, H.; Mayer, E.; et al. Sex-specific differences in chronic thromboembolic pulmonary hypertension. Results from the European CTEPH registry. J. Thromb. Haemost. 2020, 18, 151–161. [Google Scholar] [CrossRef]
- Yang, Y.L.; Yu, Y.Z.; Yuan, P.; Gong, S.G.; Wang, C.Y.; Li, Y.; Zhao, Q.H.; Jiang, R.; Wu, W.H.; He, J.; et al. Sex differences of hemodynamics during acute vasoreactivity testing to predict the outcomes of chronic thromboembolic pulmonary hypertension. Clin. Respir. J. 2020, 14, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.X.; Pudasaini, B.; Guo, J.; Gong, S.G.; Jiang, R.; Wang, L.; Zhao, Q.H.; Wu, W.H.; Yuan, P.; Liu, J.M. Sex-specific cardiopulmonary exercise testing indices to estimate the severity of inoperable chronic thromboembolic pulmonary hypertension. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, N.; Kimura, A.; Amano, S.; Okada, O.; Kasahara, Y.; Tatsumi, K.; Takahashi, M.; Shibata, H.; Yasunami, M.; Kuriyama, T. Association of clinical features with HLA in chronic pulmonary thromboembolism. Eur. Respir. J. 2005, 25, 131–138. [Google Scholar] [CrossRef]
- Shigeta, A.; Tanabe, N.; Shimizu, H.; Hoshino, S.; Maruoka, M.; Sakao, S.; Tada, Y.; Kasahara, Y.; Takiguchi, Y.; Tatsumi, K.; et al. Gender differences in chronic thromboembolic pulmonary hypertension in Japan. Circ. J. 2008, 72, 2069–2074. [Google Scholar] [CrossRef]
- Fletcher-Sanfeliu, D.; Redón, J.; García-Granero, Á.; Frasson, M.; Barreira, I.; Martínez-León, J.; García-Fuster, M.J. ‘Pulmonary thrombosis in situ’: Risk factors, clinic characteristics and long-term evolution. Blood Coagul. Fibrinolysis 2020, 31, 469–475. [Google Scholar] [CrossRef]
- Zhou, F.L.; Wang, L.H.; Dai, C.Q.; Shentu, G.J.; Xu, G.H. Risk Factors and Outcomes for Preoperative Asymptomatic Pulmonary Embolism in Patients Aged 60 Years and Over with Hip Fracture. Orthop. Surg. 2021, 13, 958–965. [Google Scholar] [CrossRef]
- Siddique, R.M.; Amini, S.B.; Connors, A.F., Jr.; Rimm, A.A. Race and sex differences in long-term survival rates for elderly patients with pulmonary embolism. Am. J. Public Health 1998, 88, 1476–1480. [Google Scholar] [CrossRef]
- Hassanin, I.M.; Shahin, A.Y.; Badawy, M.S.; Karam, K. D-dimer testing versus multislice computed tomography in the diagnosis of postpartum pulmonary embolism in symptomatic high-risk women. Int. J. Gynaecol. Obstet. 2011, 115, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Simcox, L.E.; Ormesher, L.; Tower, C.; Greer, I.A. Pulmonary thrombo-embolism in pregnancy: Diagnosis and management. Breathe 2015, 11, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Diaconescu, M.; Zhe, T.; Mesurolle, B.; Semionov, A. Outcomes of Multidetector Computed Tomography Pulmonary Angiography in Pregnant and Postpartum Women With Suspected Pulmonary Embolism. Can. Assoc. Radiol. J. 2021, 72, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, L.M.; Reiman, R.E.; Yoshizumi, T.T.; Goodman, P.C.; Toncheva, G.; Nguyen, G.; Lowry, C. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: Implications for cancer induction. Radiology 2007, 245, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jimenez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Y.; Liu, W.; Wang, X.; Zhang, S.; Zhang, W.; Zhao, S.; Zhang, M.; Zhang, S.; Jiao, G. Predictive value of D-dimer and analysis of risk factors in pregnant women with suspected pulmonary embolism after cesarean section. BMC Pulm. Med. 2021, 21, 391. [Google Scholar] [CrossRef]
- Bajc, M.; Olsson, B.; Gottsater, A.; Hindorf, C.; Jogi, J. V/P SPECT as a diagnostic tool for pregnant women with suspected pulmonary embolism. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1325–1330. [Google Scholar] [CrossRef]
- Goodacre, S.; Horspool, K.; Shephard, N.; Pollard, D.; Hunt, B.J.; Fuller, G.; Nelson-Piercy, C.; Knight, M.; Thomas, S.; Lecky, F.; et al. Selecting pregnant or postpartum women with suspected pulmonary embolism for diagnostic imaging: The DiPEP diagnostic study with decision-analysis modelling. Health Technol. Assess 2018, 22, 1–230. [Google Scholar] [CrossRef]
- Wang, H.C.; Tsai, P.S.; Li, K.Y.; Fan, Y.C.; Huang, C.J. Perioperative risk factors for postpartum pulmonary embolism in Taiwanese Cesarean section women. Asian J. Anesthesiol. 2017, 55, 35–40. [Google Scholar] [CrossRef]
- Ros, H.S.; Lichtenstein, P.; Bellocco, R.; Petersson, G.; Cnattingius, S. Pulmonary embolism and stroke in relation to pregnancy: How can high-risk women be identified? Am. J. Obstet. Gynecol. 2002, 186, 198–203. [Google Scholar] [CrossRef]
- COVID-19 CORONAVIRUS/DEATH TOLL. Available online: https://www.worldometers.info/coronavirus/coronavirus-death-toll/ (accessed on 6 June 2022).
- Gong, X.; Yuan, B.; Yuan, Y. Incidence and prognostic value of pulmonary embolism in COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263580. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, C.; Weizman, O.; Trimaille, A.; Mika, D.; Pommier, T.; Pace, N.; Douair, A.; Barbin, E.; Fraix, A.; Bouchot, O.; et al. Pulmonary embolism in COVID-19 patients: A French multicentre cohort study. Eur. Heart J. 2020, 41, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, S.; Dev, H.; Behzadi, A.; Kim, J.; Attari, H.; Raza, S.I.; Margolis, D.J.; Jonisch, A.; Megahed, A.; Bamashmos, A.; et al. Pulmonary Embolism in Hospitalized Patients with COVID-19: A Multicenter Study. Radiology 2021, 301, E426–E433. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Nasirian, M.; Keivany, E.; Nasri, P.; Mirenayat, M.S. The demographic, clinical, and medical manifestations of pulmonary thromboembolism development in COVID-19. Blood Res. 2021, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Fernandez, A.; Toledo-Pons, N.; Cosio, B.G.; Millan, A.; Calvo, N.; Ramon, L.; de Mendoza, S.H.; Morell-Garcia, D.; Bauca-Rossello, J.M.; Nunez, B.; et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PLoS ONE 2020, 15, e0238216. [Google Scholar] [CrossRef]
- Abate, B.B.; Kassie, A.M.; Kassaw, M.W.; Aragie, T.G.; Masresha, S.A. Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open 2020, 10, e040129. [Google Scholar] [CrossRef]
- Voci, D.; Fedeli, U.; Farmakis, I.T.; Hobohm, L.; Keller, K.; Valerio, L.; Schievano, E.; Barbiellini Amidei, C.; Konstantinides, S.V.; Kucher, N.; et al. Deaths related to pulmonary embolism and cardiovascular events before and during the 2020 COVID-19 pandemic: An epidemiological analysis of data from an Italian high-risk area. Thromb. Res. 2022, 212, 44–50. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thachil, R.; Nagraj, S.; Kharawala, A.; Sokol, S.I. Pulmonary Embolism in Women: A Systematic Review of the Current Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 234. https://doi.org/10.3390/jcdd9080234
Thachil R, Nagraj S, Kharawala A, Sokol SI. Pulmonary Embolism in Women: A Systematic Review of the Current Literature. Journal of Cardiovascular Development and Disease. 2022; 9(8):234. https://doi.org/10.3390/jcdd9080234
Chicago/Turabian StyleThachil, Rosy, Sanjana Nagraj, Amrin Kharawala, and Seth I. Sokol. 2022. "Pulmonary Embolism in Women: A Systematic Review of the Current Literature" Journal of Cardiovascular Development and Disease 9, no. 8: 234. https://doi.org/10.3390/jcdd9080234
APA StyleThachil, R., Nagraj, S., Kharawala, A., & Sokol, S. I. (2022). Pulmonary Embolism in Women: A Systematic Review of the Current Literature. Journal of Cardiovascular Development and Disease, 9(8), 234. https://doi.org/10.3390/jcdd9080234





