Left Atrial Remodeling after Mitral Valve Repair for Primary Mitral Regurgitation: Evolution over Time and Prognostic Significance
Abstract
:1. Introduction
2. Methods
2.1. Patient Population
2.2. Echocardiography
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Population
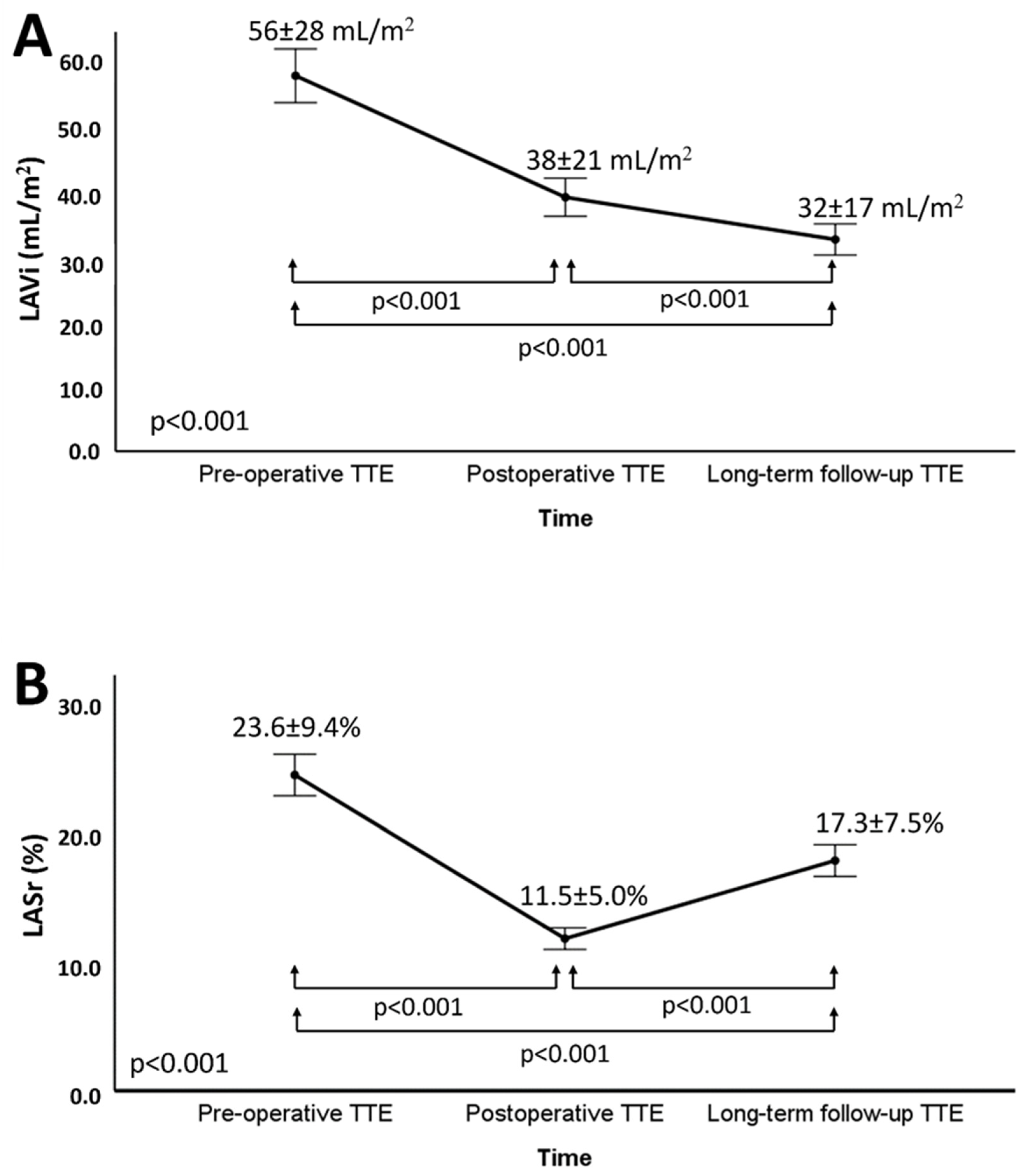
3.2. Changes in LA Volume and Function after Mitral Valve Repair
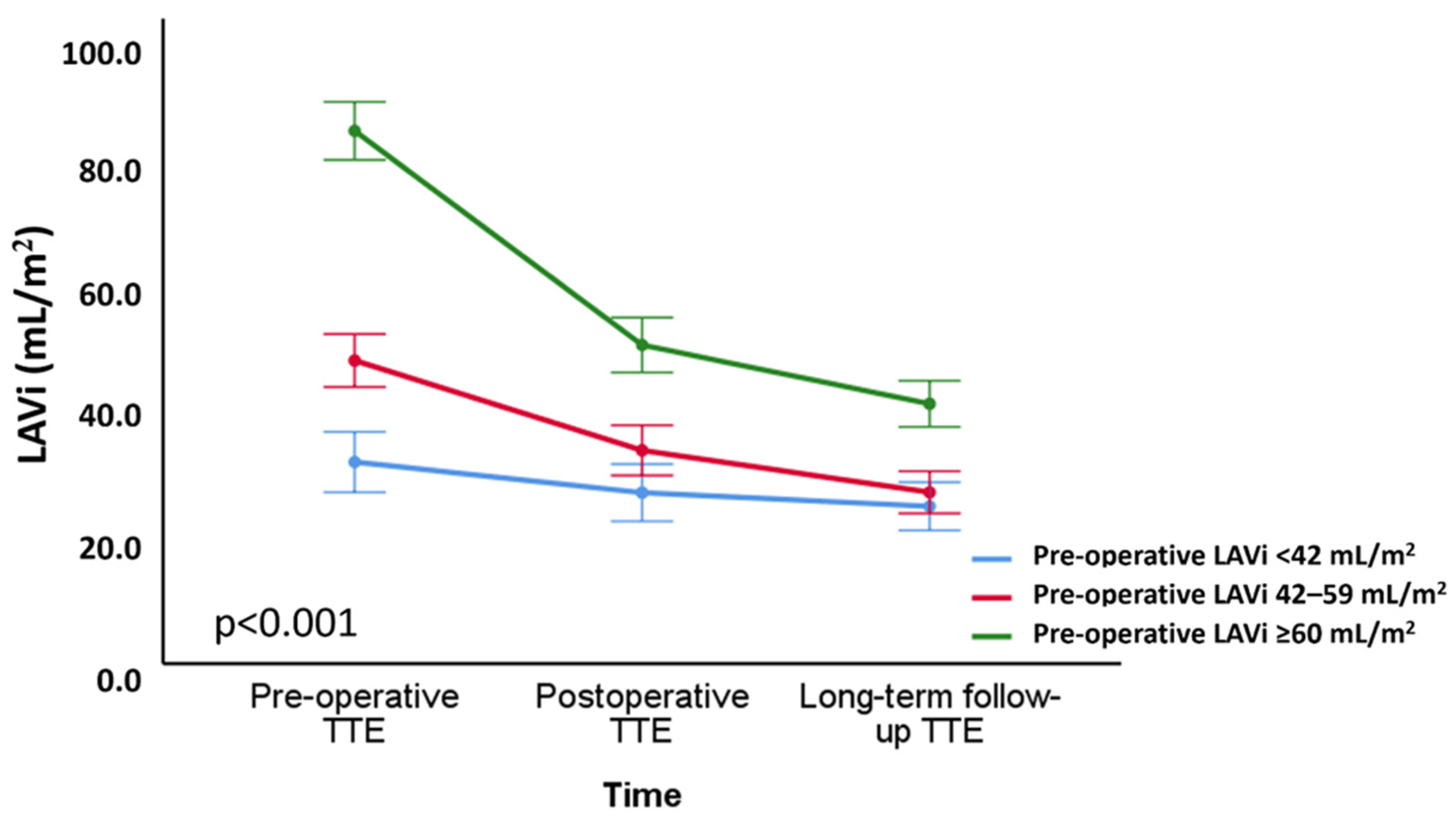
3.3. Correlates of Changes in LA Volume and Function
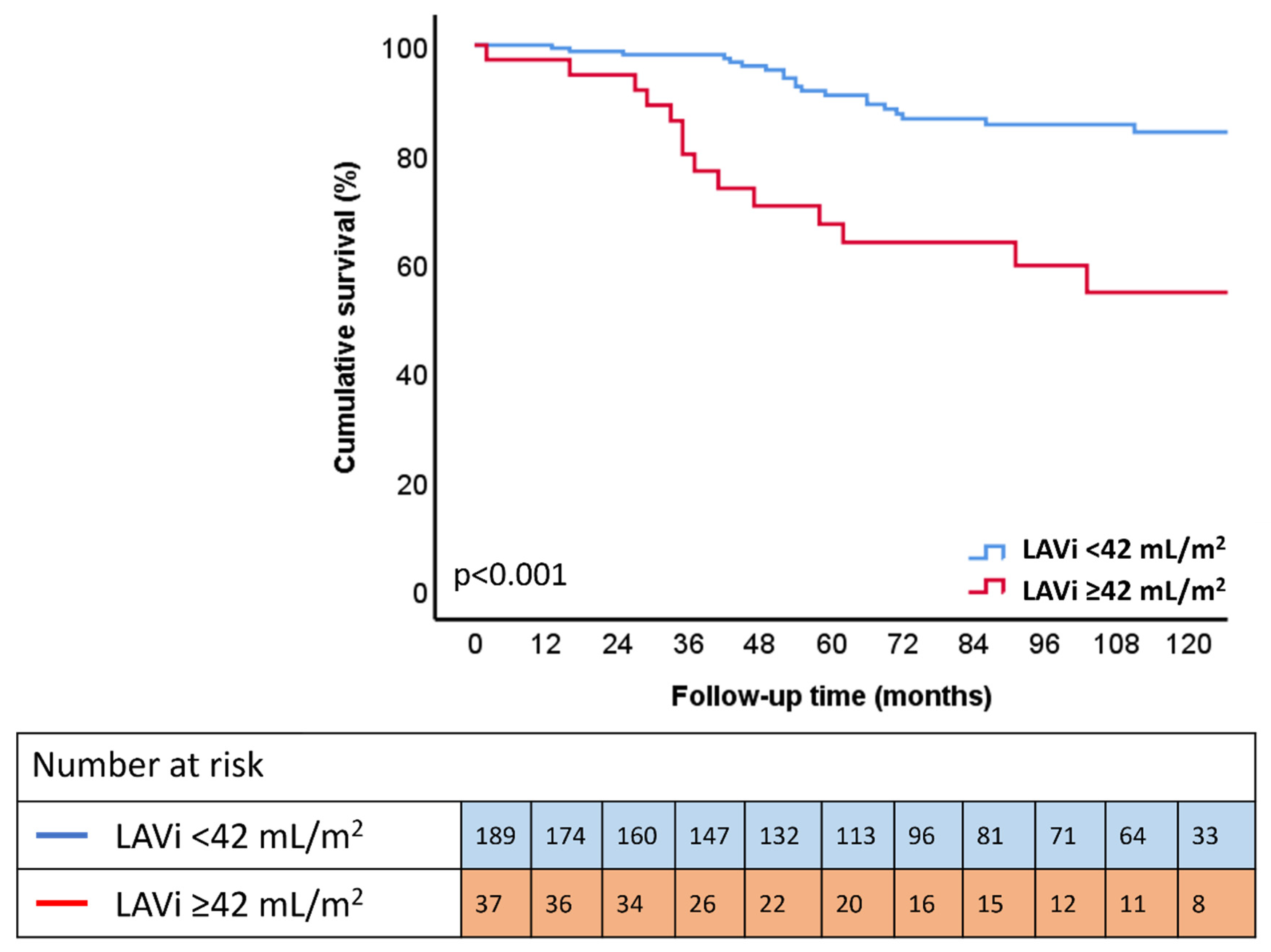
3.4. Outcome
4. Discussion
4.1. LA Reverse Remodeling after MV Repair
4.2. Predictors of LA Reverse Remodeling after MV Repair
4.3. Prognostic Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LA | left atrial |
| LASr | left atrial reservoir strain |
| LAVi | left atrial volume index |
| LV | left ventricular |
| MR | mitral regurgitation |
| MV | mitral valve |
References
- Savage, D.D.; Garrison, R.J.; Devereux, R.B.; Castelli, W.P.; Anderson, S.J.; Levy, D.; McNamara, P.M.; Stokes, J., 3rd; Kannel, W.B.; Feinleib, M. Mitral valve prolapse in the general population. 1. Epidemiologic features: The Framingham Study. Am. Heart J. 1983, 106, 571–576. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef]
- Lazam, S.; Vanoverschelde, J.L.; Tribouilloy, C.; Grigioni, F.; Suri, R.M.; Avierinos, J.F.; de Meester, C.; Barbieri, A.; Rusinaru, D.; Russo, A.; et al. Twenty-Year Outcome after Mitral Repair versus Replacement for Severe Degenerative Mitral Regurgitation: Analysis of a Large, Prospective, Multicenter, International Registry. Circulation 2017, 135, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Suri, R.M.; Clavel, M.A.; Mantovani, F.; Michelena, H.I.; Pislaru, S.; Mahoney, D.W.; Schaff, H.V. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 150, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Dziadzko, V.; Clavel, M.A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and undertreatment of mitral regurgitation: A community cohort study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef]
- Kihara, Y.; Sasayama, S.; Miyazaki, S.; Onodera, T.; Susawa, T.; Nakamura, Y.; Fujiwara, H.; Kawai, C. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ. Res. 1988, 62, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Le Tourneau, T.; Messika-Zeitoun, D.; Russo, A.; Detaint, D.; Topilsky, Y.; Mahoney, D.W.; Suri, R.; Enriquez-Sarano, M. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J. Am. Coll. Cardiol. 2010, 56, 570–578. [Google Scholar] [CrossRef] [Green Version]
- Essayagh, B.; Antoine, C.; Benfari, G.; Messika-Zeitoun, D.; Michelena, H.; Le Tourneau, T.; Mankad, S.; Tribouilloy, C.M.; Thapa, P.; Enriquez-Sarano, M. Prognostic Implications of Left Atrial Enlargement in Degenerative Mitral Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 858–870. [Google Scholar] [CrossRef]
- Stassen, J.; van Wijngaarden, A.L.; Butcher, S.C.; Palmen, M.; Herbots, L.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Prognostic value of left atrial reservoir function in patients with severe primary mitral regurgitation undergoing mitral valve repair. Eur. Heart J. Cardiovasc. Imaging 2022, jeac058. [Google Scholar] [CrossRef]
- van Wijngaarden, A.L.; Mantegazza, V.; Hiemstra, Y.L.; Volpato, V.; van der Bijl, P.; Pepi, M.; Palmen, M.; Delgado, V.; Ajmone Marsan, N.; Tamborini, G.; et al. Prognostic Impact of Extra-Mitral Valve Cardiac Involvement in Patients with Primary Mitral Regurgitation. JACC Cardiovasc. Imaging 2022, 15. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Tajik, A.J.; Schaff, H.V.; Orszulak, T.A.; McGoon, M.D.; Bailey, K.R.; Frye, R.L. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: Results and clinical implications. J. Am. Coll. Cardiol. 1994, 24, 1536–1543. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Griffin, B.P.; Stewart, W.J.; Cosgrove, D.M., 3rd; Thomas, J.D.; Marwick, T.H. Left ventricular function after valve repair for chronic mitral regurgitation: Predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J. Am. Coll. Cardiol. 1996, 28, 1198–1205. [Google Scholar] [CrossRef] [Green Version]
- Tomšič, A.; Hiemstra, Y.L.; van Hout, F.M.A.; van Brakel, T.J.; Versteegh, M.I.M.; Marsan, N.A.; Klautz, R.J.M.; Palmen, M. Long-term results of mitral valve repair for severe mitral regurgitation in asymptomatic patients. J. Cardiol. 2018, 72, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 1, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Antonini-Canterin, F.; Beladan, C.C.; Popescu, B.A.; Ginghina, C.; Popescu, A.C.; Piazza, R.; Leiballi, E.; Zingone, B.; Nicolosi, G.L. Left atrial remodelling early after mitral valve repair for degenerative mitral regurgitation. Heart 2008, 94, 759–764. [Google Scholar] [CrossRef]
- Marsan, N.A.; Maffessanti, F.; Tamborini, G.; Gripari, P.; Caiani, E.; Fusini, L.; Muratori, M.; Zanobini, M.; Alamanni, F.; Pepi, M. Left atrial reverse remodeling and functional improvement after mitral valve repair in degenerative mitral regurgitation: A real-time 3-dimensional echocardiography study. Am. Heart J. 2011, 161, 314–321. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Burgon, N.S.; Badger, T.J.; Kholmovski, E.G.; Vijayakumar, S.; Rao, S.N.; Blauer, J.; Fish, E.N.; Dibella, E.V.; et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging 2010, 3, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stassen, J.; Butcher, S.C.; Namazi, F.; Ajmone Marsan, N.; Bax, J.J.; Delgado, V. Left Atrial Deformation Imaging and Atrial Fibrillation in Patients with Rheumatic Mitral Stenosis. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2022, 35, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Keren, G.; Etzion, T.; Sherez, J.; Zelcer, A.A.; Megidish, R.; Miller, H.I.; Laniado, S. Atrial fibrillation and atrial enlargement in patients with mitral stenosis. Am. Heart J. 1987, 114, 1146–1155. [Google Scholar] [CrossRef]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
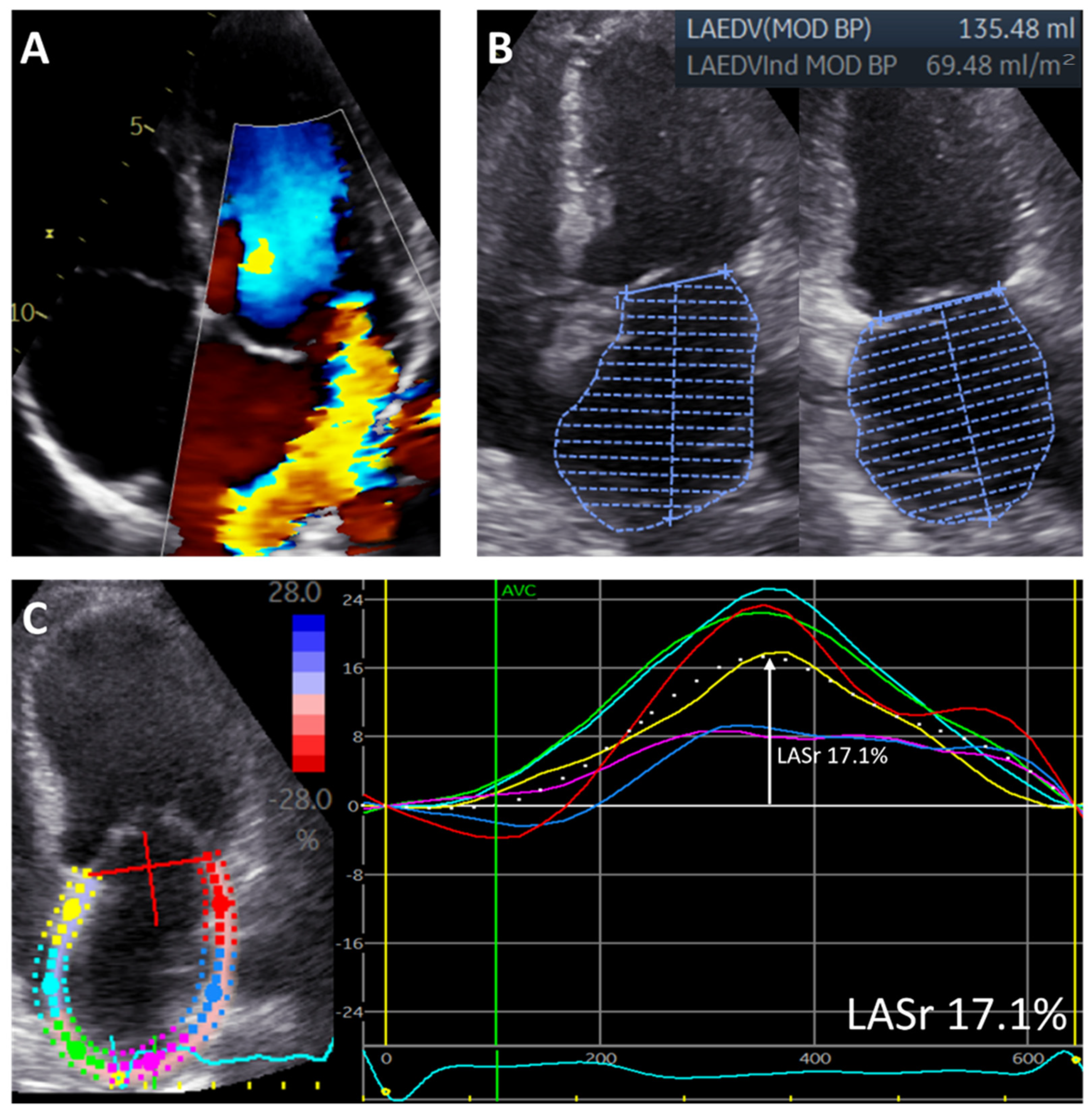
| Study Population (n = 226) | |
|---|---|
| Clinical characteristics | |
| Age, years | 62.2 ± 12.6 |
| Male sex | 149 (65.9%) |
| Heart rate, bpm | 76 ± 23 |
| Systolic BP, mmHg | 134 ± 20 |
| Diastolic BP, mmHg | 78 ± 11 |
| BMI, kg/m2 | 25.2 ± 3.5 |
| Arterial hypertension | 86 (38.1%) |
| Diabetes mellitus | 4 (1.8%) |
| (ex)smoker | 69 (32.9%) |
| Coronary artery disease | 44 (20.0%) |
| COPD | 15 (6.8%) |
| eGFR, mL/min/1.73 m2 | 81.5 ± 25.2 |
| CKD (eGFR < 60 mL/min/1.73 m2) | 44 (19.6%) |
| Atrial fibrillation | 75 (33.2%) |
| NYHA class ≥ II | 165 (73.0%) |
| Mitral valve lesion and associated surgical procedures | |
| Prolapsing leaflet | |
| Anterior | 26 (11.5%) |
| Posterior | 161 (71.%2) |
| Both | 39 (17.3%) |
| Associated surgical procedures | |
| CABG | 35 (15.5%) |
| TVP | 113 (50.0%) |
| MAZE | 64 (28.3%) |
| LAA occlusion | 30 (13.3%) |
| Echocardiographic characteristics | |
| LVEDD, mm | 54.5 ± 6.6 |
| LVESD, mm | 33.4 ± 7.0 |
| LVEDVi, mL/m2 | 71 ± 19 |
| LVESVi, mL/m2 | 24 (19–31) |
| LVEF, % | 65 ± 8 |
| sPAP, mmHg | 32 (25–45) |
| MR EROA, mm2 | 41 (29–55) |
| MR vena contracta, mm | 7.2 ± 1.8 |
| MR Rvol, mL | 55 ± 23 |
| LAVi, mL/m2 | 56 ± 28 |
| LASr, % | 23.6 ± 9.4 |
| Pre-Operative | Long-Term Follow-Up | p Value | |
|---|---|---|---|
| LVEDD, mm | 54.5 ± 6.6 | 49.0 ± 6.3 | <0.001 |
| LVESD, mm | 33.4 ± 7.0 | 35.6 ± 7.6 | <0.001 |
| LVEDVi mL/m2 | 71 ± 19 | 57 ± 17 | <0.001 |
| LVESVi, mL/m2 | 24 (19–31) | 25 (18–31) | 0.315 |
| LVEF, % | 65 ± 8 | 55 ± 11 | <0.001 |
| sPAP, mmHg | 32 (25–45) | 27 (23–33) | <0.001 |
| LAVi, mL/m2 | 56 ± 28 | 32 ± 17 | <0.001 |
| LASr, % | 23.6 ± 9.4 | 17.3 ± 7.5 | <0.001 |
| Change in LAVi at Long-Term Follow-Up | Change in LASr at Long-Term Follow-Up | |||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient | p Value | Spearman’s Correlation Coefficient | p Value | |
| Demographic characteristics | ||||
| Age, years | −0.139 | 0.037 | −0.364 | <0.001 |
| BMI, kg/m2 | −0.124 | 0.062 | 0.095 | 0.192 |
| Systolic BP, mmHg | 0.002 | 0.978 | −0.102 | 0.173 |
| Diastolic BP, mmHg | −0.057 | 0.402 | −0.057 | 0.448 |
| eGFR, mL/min/1.73 m2 | 0.062 | 0.358 | 0.236 | 0.001 |
| Pre-operative AF | 0.134 | 0.094 | 0.062 | 0.239 |
| Echocardiographic characteristics | ||||
| Preoperative LVEDVi, mL/m2 | 0.199 | 0.003 | 0.016 | 0.826 |
| Preoperative LVESVi, mL/m2 | 0.060 | 0.369 | −0.040 | 0.585 |
| Preoperative LVEF, % | 0.069 | 0.302 | 0.109 | 0.137 |
| Preoperative LAVi, mL/m2 | 0.498 | <0.001 | −0.319 | <0.001 |
| Preoperative LASr, % | 0.008 | 0.904 | 0.569 | <0.001 |
| Preoperative EROA, mm2 | 0.205 | 0.004 | 0.003 | 0.967 |
| Preoperative Rvol, ml | 0.222 | 0.002 | −0.171 | 0.030 |
| Postoperative TMPG immediate after intervention, mmHg | −0.150 | 0.026 | −0.068 | 0.361 |
| Univariable Analysis | Multivariable Analysis Adjusting for Age, Sex, CAD | Multivariable Analysis Adjusting for Age, Sex, LVEF | Multivariable Analysis Adjusting for Age, Sex, AF | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| LAVi, mL/m2 (continuous) | 1.021 (1.012–1.031) | <0.001 | 1.014 (1.001–1.027) | 0.033 | 1.014 (1.003–1.024) | 0.012 | 1.012 (1.001–1.023) | 0.027 |
| LAVi < 42 mL/m2 | Reference | Reference | Reference | Reference | ||||
| LAVi ≥ 42 mL/m2 | 3.729 (2.019–6.890) | <0.001 | 2.494 (1.292–4.815) | 0.006 | 2.518 (1.320–4.806) | 0.005 | 2.468 (1.295–4.704) | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stassen, J.; van Wijngaarden, A.L.; Wu, H.W.; Palmen, M.; Tomsic, A.; Delgado, V.; Bax, J.J.; Marsan, N.A. Left Atrial Remodeling after Mitral Valve Repair for Primary Mitral Regurgitation: Evolution over Time and Prognostic Significance. J. Cardiovasc. Dev. Dis. 2022, 9, 230. https://doi.org/10.3390/jcdd9070230
Stassen J, van Wijngaarden AL, Wu HW, Palmen M, Tomsic A, Delgado V, Bax JJ, Marsan NA. Left Atrial Remodeling after Mitral Valve Repair for Primary Mitral Regurgitation: Evolution over Time and Prognostic Significance. Journal of Cardiovascular Development and Disease. 2022; 9(7):230. https://doi.org/10.3390/jcdd9070230
Chicago/Turabian StyleStassen, Jan, Aniek L. van Wijngaarden, Hoi W. Wu, Meindert Palmen, Anton Tomsic, Victoria Delgado, Jeroen J. Bax, and Nina Ajmone Marsan. 2022. "Left Atrial Remodeling after Mitral Valve Repair for Primary Mitral Regurgitation: Evolution over Time and Prognostic Significance" Journal of Cardiovascular Development and Disease 9, no. 7: 230. https://doi.org/10.3390/jcdd9070230
APA StyleStassen, J., van Wijngaarden, A. L., Wu, H. W., Palmen, M., Tomsic, A., Delgado, V., Bax, J. J., & Marsan, N. A. (2022). Left Atrial Remodeling after Mitral Valve Repair for Primary Mitral Regurgitation: Evolution over Time and Prognostic Significance. Journal of Cardiovascular Development and Disease, 9(7), 230. https://doi.org/10.3390/jcdd9070230







