Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy and Primary Outcome
- They compared de novo dementia occurrence in AFCA vs. non-AFCA (medical) cohorts, providing adjusted estimates based on matching and/or multivariable analysis statistical techniques; and
- They reported time-to-event risk estimate for the outcome (i.e., hazard ratio, HR).
2.2. Statistical Analysis
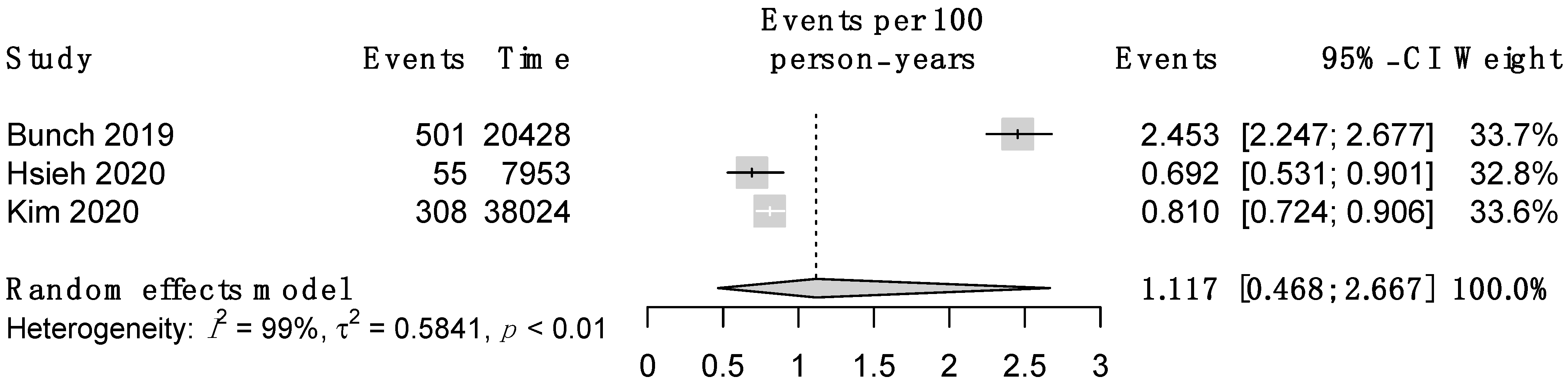
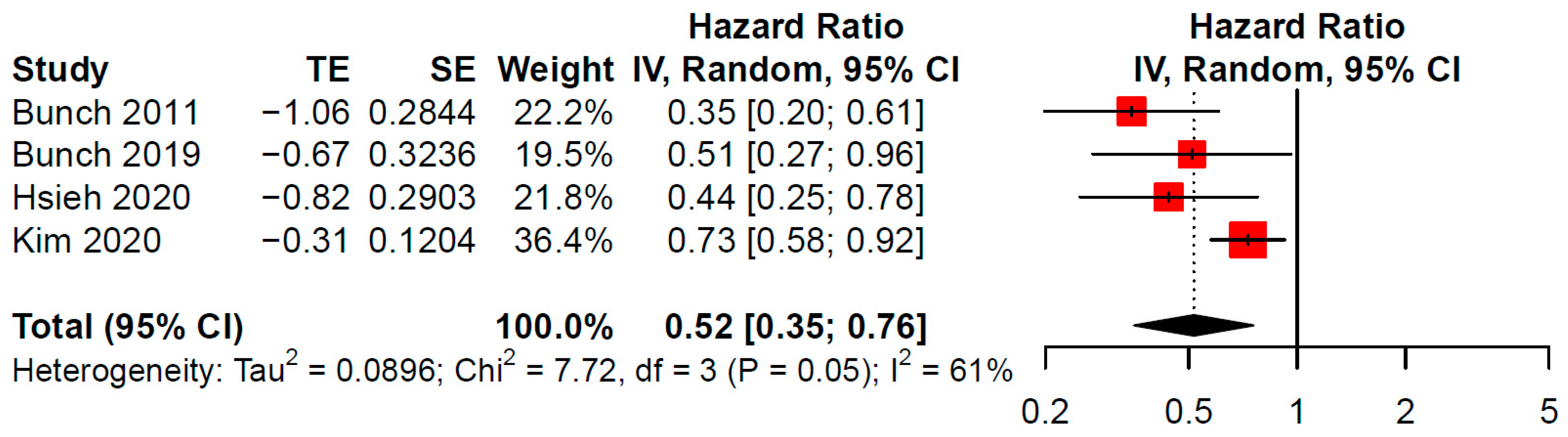
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Carlo, A.; Bellino, L.; Consoli, D.; Mori, F.; Zaninelli, A.; Baldereschi, M.; Cattarinussi, A.; D’Alfonso, M.G.; Gradia, C.; Sgherzi, B.; et al. Prevalence of atrial fibrillation in the Italian elderly population and projections from 2020 to 2060 for Italy and the European Union: The FAI Project. EP Eur. 2019, 21, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef] [PubMed]
- Saglietto, A.; Ballatore, A.; Xhakupi, H.; de Ferrari, G.M.; Anselmino, M. Atrial Fibrillation and dementia: Epidemiological insights on an undervalued association. Medicina 2022, 58, 361. [Google Scholar] [CrossRef]
- Anselmino, M.; Scarsoglio, S.; Saglietto, A.; Gaita, F.; Ridolfi, L. Transient cerebral hypoperfusion and hypertensive events during atrial fibrillation: A plausible mechanism for cognitive impairment. Sci. Rep. 2016, 6, 28635. [Google Scholar] [CrossRef]
- Saglietto, A.; Scarsoglio, S.; Ridolfi, L.; Gaita, F.; Anselmino, M. Higher ventricular rate during atrial fibrillation relates to increased cerebral hypoperfusions and hypertensive events. Sci. Rep. 2019, 9, 3779. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Lopez, F.L.; Gottesman, R.F.; Huxley, R.R.; Agarwal, S.K.; Loehr, L.; Mosley, T.; Alonso, A. Atrial fibrillation and cognitive decline—The role of subclinical cerebral infarcts. Stroke 2014, 45, 2568–2574. [Google Scholar] [CrossRef] [Green Version]
- Gaita, F.; Corsinovi, L.; Anselmino, M.; Raimondo, C.; Pianelli, M.; Toso, E.; Bergamasco, L.; Boffano, C.; Valentini, M.C.; Cesarani, F.; et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J. Am. Coll. Cardiol. 2013, 62, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Junejo, R.T.; Braz, I.D.; Lucas, S.J.E.; Lieshout, J.J.; van Lip, G.Y.H.; Fisher, J.P. Impaired cerebrovascular reactivity in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2019, 73, 1230–1232. [Google Scholar] [CrossRef]
- Conen, D.; Rodondi, N.; Müller, A.; Beer, J.H.; Ammann, P.; Moschovitis, G.; Auricchio, A.; Hayoz, D.; Kobza, R.; Shah, D.; et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2019, 73, 989–999. [Google Scholar] [CrossRef]
- Khan, A.R.; Khan, S.; Sheikh, M.A.; Khuder, S.; Grubb, B.; Moukarbel, G.V. Catheter ablation and antiarrhythmic drug therapy as first- or second-line therapy in the management of atrial fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 853–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saglietto, A.; Gaita, F.; Ponti RDe Ferrari GMDe Anselmino, M. Catheter ablation vs. anti-arrhythmic drugs as first-line treatment in symptomatic paroxysmal atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials. Front. Cardiovasc. Med. 2021, 8, 664647. [Google Scholar] [CrossRef] [PubMed]
- Saglietto, A.; De Ponti, R.; Di Biase, L.; Matta, M.; Gaita, F.; Romero, J.; De Ferrari, G.M.; Anselmino, M. Impact of atrial fibrillation catheter ablation on mortality, stroke, and heart failure hospitalizations: A meta-analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Rivard, L.; Friberg, L.; Conen, D.; Healey, J.S.; Berge, T.; Boriani, G.; Brandes, A.; Calkins, H.; Camm, A.J.; Yee Chen, L.; et al. Atrial fibrillation and dementia: A report from the AF-SCREEN international collaboration. Circulation 2022, 145, 392–409. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Chen, Y.Y.; Chien, K.L.; Chung, F.P.; Lo, L.W.; Chang, S.L.; Chao, T.F.; Hu, Y.F.; Lin, C.Y.; Tuan, T.C.; et al. Catheter ablation of atrial fibrillation reduces the risk of dementia and hospitalization during a very long-term follow-up. Int. J. Cardiol. 2020, 304, 75–81. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; et al. Less dementia after catheter ablation for atrial fibrillation: A nationwide cohort study. Eur. Heart J. 2020, 41, 4483–4493. [Google Scholar] [CrossRef]
- Piccini, J.P.; Todd, D.M.; Massaro, T.; Lougee, A.; Haeusler, K.G.; Blank, B.; De Bono, J.P.; Callans, D.J.; Elvan, A.; Fetsch, T.; et al. Changes in quality of life, cognition and functional status following catheter ablation of atrial fibrillation. Heart 2020, 106, 1919–1926. [Google Scholar] [CrossRef]
- Jin, M.N.; Kim, T.H.; Kang, K.W.; Yu, H.T.; Uhm, J.S.; Joung, B.; Lee, M.H.; Kim, E.; Pak, H.N. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ. Arrhythmia Electrophysiol. 2019, 12, e007197. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; You, S.C.; Sung, J.H.; Jang, E.; Yu, H.T.; Kim, T.H.; Pak, H.N.; Lee, M.H.; Lip, G.Y.; et al. Association of rhythm control with incident dementia among patients with atrial fibrillation: A nationwide population-based cohort study. Age Ageing 2022, 51, afab248. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bunch, T.J.; Crandall, B.G.; Weiss, J.P.; May, H.T.; Bair, T.L.; Osborn, J.S.; Anderson, J.L.; Muhlestein, J.B.; Horne, B.D.; Lappe, D.L.; et al. Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011, 22, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Bunch, T.J.; Bair, T.L.; Crandall, B.G.; Cutler, M.J.; Day, J.D.; Graves, K.G.; Jacobs, V.; Mallender, C.; Osborn, J.S.; Weiss, J.P.; et al. Stroke and dementia risk in patients with and without atrial fibrillation and carotid arterial disease. Heart Rhythm. 2020, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Saglietto, A.; Matta, M.; Gaita, F.; Jacobs, V.; Bunch, T.J.; Anselmino, M. Stroke-independent contribution of atrial fibrillation to dementia: A meta-analysis. Open Heart 2019, 6, e000984. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardarsdottir, M.; Sigurdsson, S.; Aspelund, T.; Rokita, H.; Launer, L.J.; Gudnason, V.; Arnar, D.O. Atrial fibrillation is associated with decreased total cerebral blood flow and brain perfusion. Europace 2018, 20, 1252–1258. [Google Scholar] [CrossRef]
- Saglietto, A.; Scarsoglio, S.; Canova, D.; Roatta, S.; Gianotto, N.; Piccotti, A.; Franzin, S.; Gaita, F.; De Ferrari, G.M.; Ridolfi, L.; et al. Increased beat-to-beat variability of cerebral microcirculatory perfusion during atrial fibrillation: A near-infrared spectroscopy study. Europace 2021, 23, 1219–1226. [Google Scholar] [CrossRef]
- Acute Cognitive Changes during Atrial Fibrillation Episodes, (AFCOG), (NCT04033510). Available online: https://clinicaltrials.gov/ct2/show/study/NCT04033510#wrapper (accessed on 19 April 2022).
- Seligman, W.H.; Das-Gupta, Z.; Jobi-Odeneye, A.O.; Arbelo, E.; Banerjee, A.; Bollmann, A.; Caffrey-Armstrong, B.; Cehic, D.A.; Corbalan, R.; Collins, M.; et al. Development of an international standard set of outcome measures for patients with atrial fibrillation: A report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur. Heart J. 2020, 41, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Dagres, N.; Chao, T.F.; Fenelon, G.; Aguinaga, L.; Benhayon, D.; Benjamin, E.J.; Bunch, T.J.; Chen, L.Y.; Chen, S.A.; Darrieux, F.; et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus on arrhythmias and cognitive function: What is the best practice? Heart Rhythm 2018, 15, e37–e60. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Yang, P.S.; Yu, H.T.; Kim, T.H.; Jang, E.; Sung, J.H.; Pak, H.N.; Lee, M.Y.; Lee, M.H.; Lip, G.Y.; et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: Data from a population-based cohort. Eur. Heart J. 2019, 40, 2313–2323. [Google Scholar] [CrossRef] [Green Version]
- Bunch, T.J.; Jacobs, V.; May, H.; Stevens, S.M.; Crandall, B.; Cutler, M.; Day, J.D.; Mallender, C.; Olson, J.; Osborn, J.; et al. Rationale and design of the impact of anticoagulation therapy on the cognitive decline and dementia in patients with nonvalvular atrial fibrillation (CAF) trial: A vanguard study. Clin. Cardiol. 2019, 42, 506–512. [Google Scholar] [CrossRef]
- Rivard, L.; Khairy, P.; Talajic, M.; Tardif, J.C.; Nattel, S.; Bherer, L.; Black, S.; Healey, J.; Lanthier, S.; Andrade, J.; et al. Blinded randomized trial of anticoagulation to prevent ischemic stroke and neurocognitive impairment in atrial fibrillation (BRAIN-AF): Methods and design. Can. J. Cardiol. 2019, 35, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Trial of Apixaban vs Warfarin in Reducing Rate of Cognitive Decline, Silent Cerebral Infarcts and Cerebral Microbleeds in Patients with Atrial Fibrillation (ARISTA), (NCT03839355). Available online: https://clinicaltrials.gov/ct2/show/NCT03839355 (accessed on 19 April 2022).
- Plug Dementia Trial and MRI Plug Dementia Sub-Study, (NCT03091855). Available online: https://clinicaltrials.gov/ct2/show/NCT03091855 (accessed on 19 April 2022).
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): A randomised controlled trial. Lancet 2021, 398, 1507–1516. [Google Scholar] [CrossRef]
- Svennberg, E.; Friberg, L.; Frykman, V.; Al-Khalili, F.; Engdahl, J.; Rosenqvist, M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): A multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021, 398, 1498–1506. [Google Scholar] [CrossRef]
- Kirchhof, P.; Blank, B.F.; Calvert, M.; Camm, A.J.; Chlouverakis, G.; Diener, H.C.; Goette, A.; Huening, A.; Lip, G.Y.; Simantirakis, E.; et al. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non–vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH–AFNET 6) trial. Am. Heart J. 2017, 190, 12–18. [Google Scholar] [CrossRef]
- Lopes, R.D.; Alings, M.; Connolly, S.J.; Beresh, H.; Granger, C.B.; Mazuecos, J.B.; Boriani, G.; Nielsen, J.C.; Conen, D.; Hohnloser, S.H.; et al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am. Heart J. 2017, 189, 137–145. [Google Scholar] [CrossRef]
- Ballatore, A.; Matta, M.; Saglietto, A.; Desalvo, P.; Bocchino, P.P.; Gaita, F.; De Ferrari, G.M.; Anselmino, M. Subclinical and asymptomatic atrial fibrillation: Current evidence and unsolved questions in clinical practice. Medicina 2019, 55, 497. [Google Scholar] [CrossRef] [Green Version]
- Aronsson, M.; Svennberg, E.; Rosenqvist, M.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman-Kull, V.; Levin, L.Å. Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace 2015, 17, 1023–1029. [Google Scholar] [CrossRef]
- SAFER—Screening for Atrial Fibrillation Study. Available online: https://www.safer.phpc.cam.ac.uk/ (accessed on 14 March 2022).
- Mark, D.B.; Anstrom, K.J.; Sheng, S.; Piccini, J.P.; Baloch, K.N.; Monahan, K.H.; Daniels, M.R.; Bahnson, T.D.; Poole, J.E.; Rosenberg, Y.; et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation. JAMA 2019, 321, 1275. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. EAST-AFNET 4 trial investigators. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef]
- Cognitive Impairment in Atrial Fibrillation (DIAL-F), (NCT01816308). Available online: https://clinicaltrials.gov/ct2/show/NCT01816308 (accessed on 19 April 2022).
- Hurd, M.D.; Martorell, P.; Delavande, A.; Mullen, K.J.; Langa, K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013, 368, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Z.; Yan, X.; Huang, M.; Wu, Y. Radiofrequency and cryoballoon ablation improve cognitive function in patients with atrial fibrillation. Medicine 2021, 100, e26914. [Google Scholar] [CrossRef] [PubMed]
- Tischer, T.S.; Nitschke, D.; Krause, I.; Kundt, G.; Öner, A.; D’Ancona, G.; Şafak, E.; Ince, H.; Ortak, J.; Caglayan, E. Prevalence and Progression of Cognitive Impairment in Atrial Fibrillation Patients after Treatment with Catheter Ablation or Drug Therapy. Cardiol. Res. Pract. 2019, 2019, 7216598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xia, S.-J.; Du, X.; Jiang, C.; Lai, Y.-W.; Wang, Y.-F.; Jia, Z.-X.; He, L.; Tang, R.-B.; Dong, J.-Z.; et al. Incidence and risk factors of post-operative cognitive decline after ablation for atrial fibrillation. BMC Cardiovascular Disorders 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
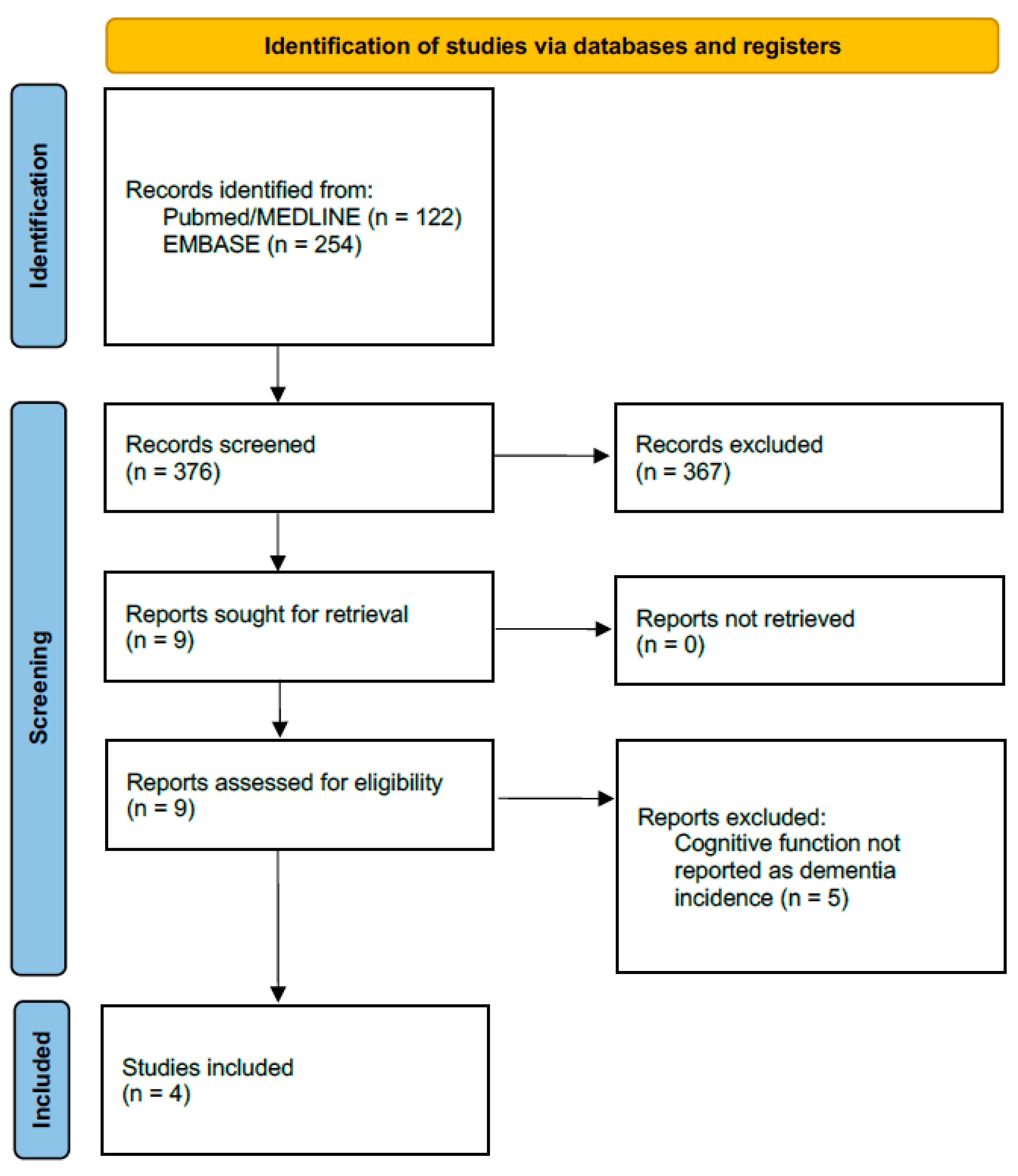
| Study | Database Description | Patients (Ablation Group) | Patients (Non-Ablation Group) | Follow-Up, Years | Dementia Diagnosis Definition | Statistical Analysis to Control Confounding |
|---|---|---|---|---|---|---|
| Bunch 2011 [21] | Intermountain Atrial Fibrillation Study Research Database | 4212 | 16,848 | 4.1 | ICD-9 | Matching based on age and gender; multivariable Cox regression analysis |
| Bunch 2020 [22] | Intermountain Healthcare Registry | 450 | 5336 | 5 | ICD-9 and ICD-10 | Matching based on age, gender and comorbidities *; multivariable Cox regression analysis |
| Hsieh 2020 [15] | Monocentric cohort and control group from National Health Insurance Research Database of Taiwan | 787 | 787 | 9 | ICD-9 | Propensity score matching; multivariable Cox regression analysis |
| Kim 2020 [16] | Korean National Health Insurance Service Database | 5863 | 5863 | 4.3 | ICD-10 and use of dementia drugs | Propensity score matching, matching for health care utilization during follow-up and residential area, multivariable analysis |
| Study | Group | Male Gender (%) | Age (Years) | Hypertension (%) | CHF (%) | CAD (%) | Stroke/TIA (%) | Anticoagulant (%) | CHA2DS2-VASc Score (Median) |
|---|---|---|---|---|---|---|---|---|---|
| Hsieh 2020 | AFCA | 70.1 | 54.1 | 36.8 | 6.5 | 4.8 | 8.4 | 37.0 | 1.0 |
| Hsieh 2020 | Non-AFCA | 70.0 | 54.9 | 36.8 | 6.4 | 2.1 | 3.6 | 56.9 | 0.0 |
| Bunch 2011 | AFCA | 60.8 | 64.8 | 47.8 | 29.5 | 6.4 | 9.1 * | ||
| Bunch 2011 | Non-AFCA | 60.8 | 66.0 | 45.3 | 23.6 | 6.4 | 10.5 * | ||
| Kim 2020 | AFCA | 74.1 | 60.0 | 80.4 | 35.2 | 11.4 | 30.3 † | 64.8 ǂ | 2.0 |
| Kim 2020 | Non-AFCA | 74.8 | 60.0 | 81.1 | 36.4 | 11.1 | 30.3 † | 64.7 ǂ | 2.0 |
| Bunch 2020 | AFCA | 24.2 | 73.7 | 74.7 | 32.0 | 37.1 | 16.4 | 56.0 | 4.5 |
| Bunch 2020 | Non-AFCA | 17.3 | 73.5 | 78.6 | 40.8 | 36.7 | 16.9 | 34.7 | 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saglietto, A.; Ballatore, A.; Xhakupi, H.; De Ferrari, G.M.; Anselmino, M. Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies. J. Cardiovasc. Dev. Dis. 2022, 9, 140. https://doi.org/10.3390/jcdd9050140
Saglietto A, Ballatore A, Xhakupi H, De Ferrari GM, Anselmino M. Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Cardiovascular Development and Disease. 2022; 9(5):140. https://doi.org/10.3390/jcdd9050140
Chicago/Turabian StyleSaglietto, Andrea, Andrea Ballatore, Henri Xhakupi, Gaetano Maria De Ferrari, and Matteo Anselmino. 2022. "Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies" Journal of Cardiovascular Development and Disease 9, no. 5: 140. https://doi.org/10.3390/jcdd9050140
APA StyleSaglietto, A., Ballatore, A., Xhakupi, H., De Ferrari, G. M., & Anselmino, M. (2022). Association of Catheter Ablation and Reduced Incidence of Dementia among Patients with Atrial Fibrillation during Long-Term Follow-Up: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Cardiovascular Development and Disease, 9(5), 140. https://doi.org/10.3390/jcdd9050140








