Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients
Abstract
1. Introduction
2. Materials and Methods
3. Common Pathophysiological Aspects Explaining the Link between Hypertension and Cardiac Arrhythmias
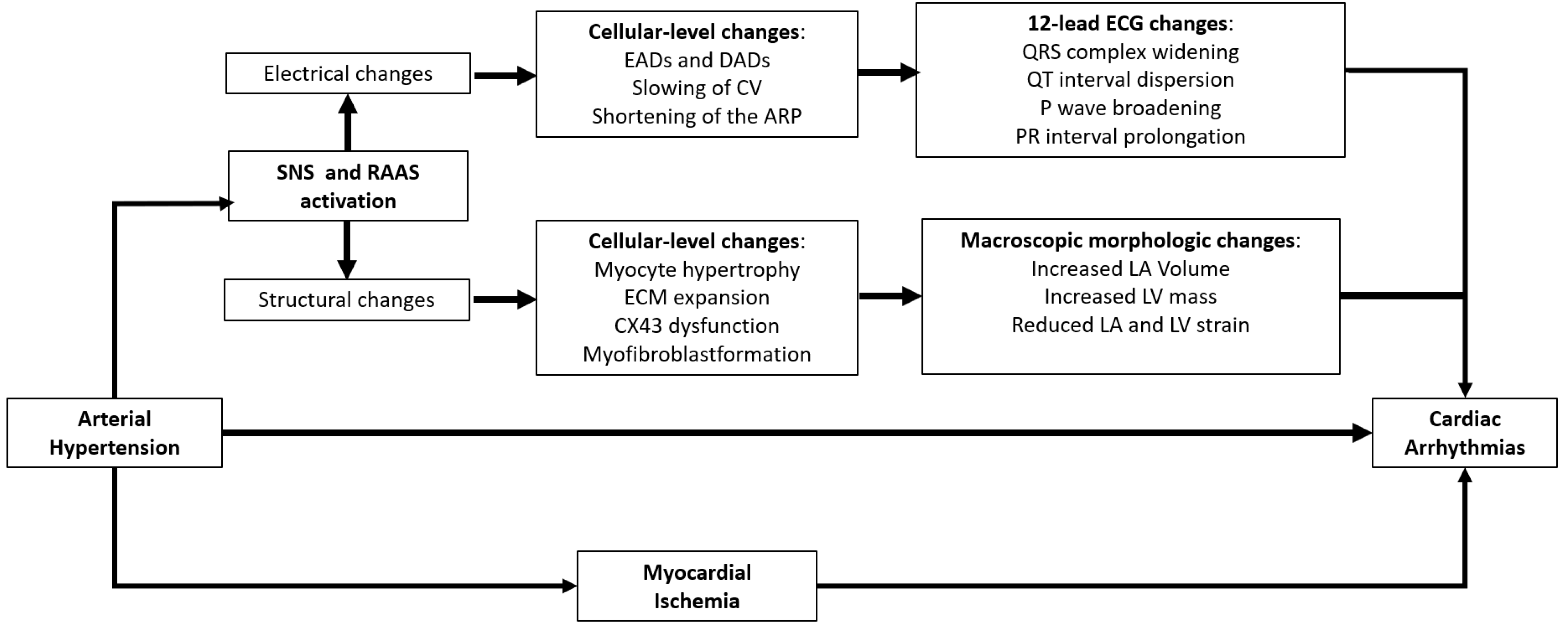
3.1. Myocardial Electro-Pathological Remodelling in Arterial Hypertension: The Key Role of Renin–Angiotensin–Aldosterone and Sympathetic Nervous Systems
3.2. The Role of Myocardial Ischemia
4. Arterial Hypertension and Atrial Fibrillation: Pathophysiology-Based Strategies to Prevent a Hazardous Association
4.1. Clinical Implications of High Blood Pressure in Patients with Atrial Fibrillation
4.2. Primary Prevention of Atrial Fibrillation: A Pathophysiology-Based Approach in Patients with Essential Hypertension
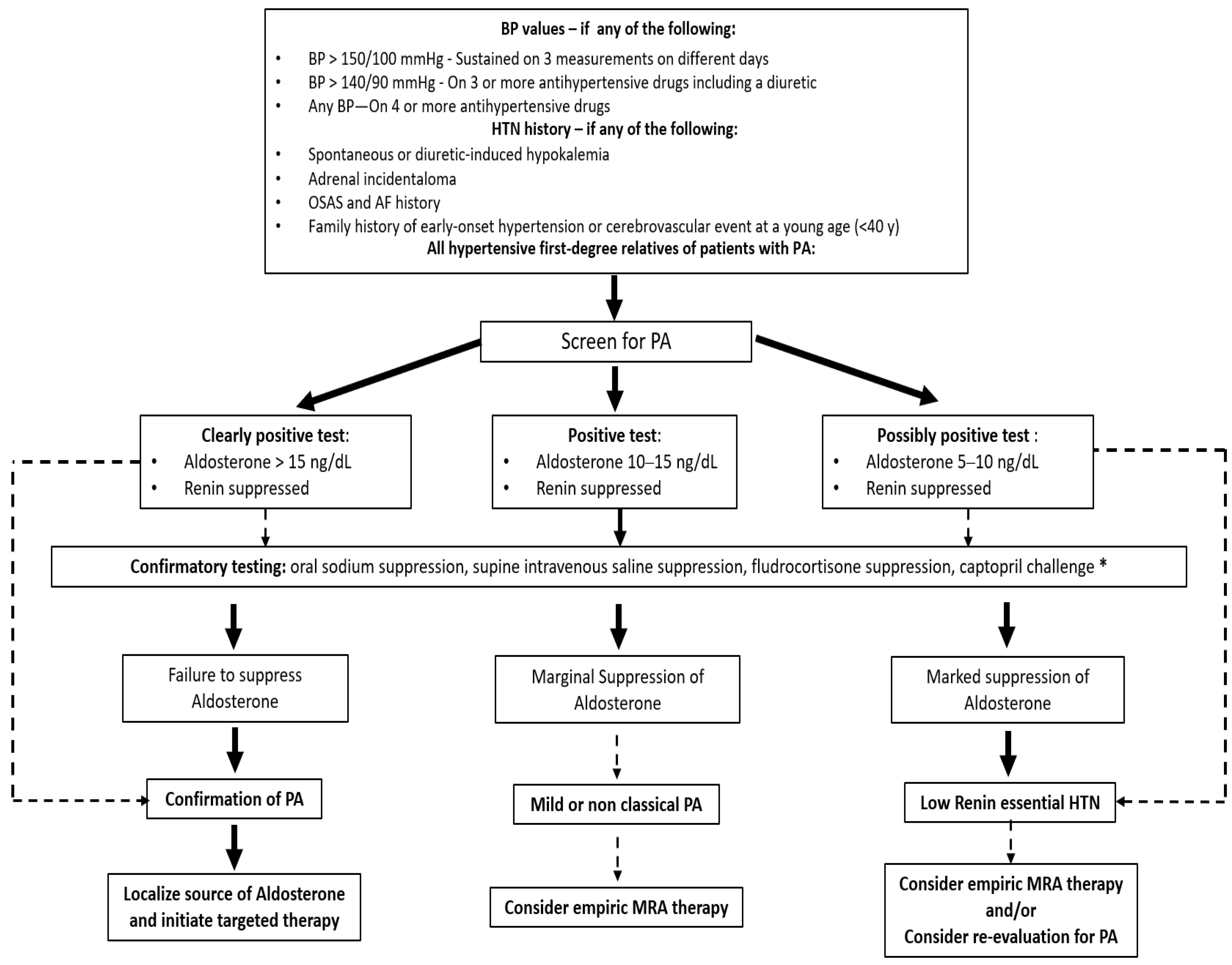
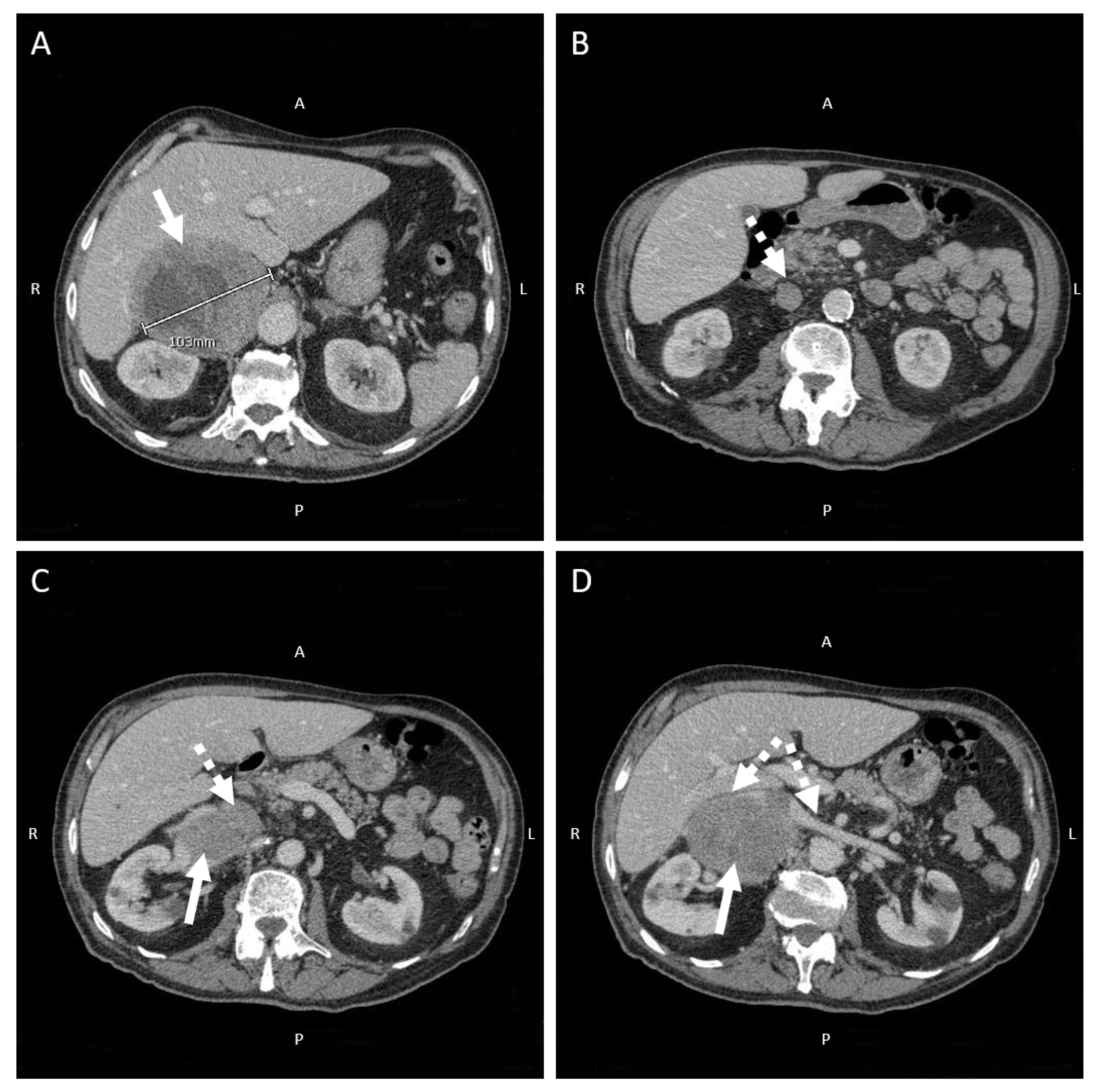
4.3. Focus on Primary Aldosteronism: An Under-Recognized Cause of Secondary Hypertension Prompting a Targeted Medical and Surgical Treatment
5. Early Detection of Atrial Fibrillation in Hypertensive Patients: A Proposed Algorithm
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC/ESH Guidelines for the Management of Arterial Hypertension, The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension, The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. PURE Study Investigators. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Coca, A.; Kahan, T.; Boriani, G.; Manolis, A.S.; Olsen, M.H.; Oto, A.; Potpara, T.S.; Steffel, J.; Marín, F.; et al. Hypertension and cardiac arrhythmias, A consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017, 19, 891–911. [Google Scholar] [PubMed]
- Laukkanen, J.A.; Khan, H.; Kurl, S.; Willeit, P.; Karppi, J.; Ronkainen, K.; Di Angelantonio, E. Left ventricular mass and the risk of sudden cardiac death, A population based study. J. Am. Heart Assoc. 2014, 3, e001285. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.; Doumas, M.; Poulimenos, L.; Kallistratos, M.; Mancia, G. The unappreciated importance of blood pressure in recent and older atrial fibrillation trials. J. Hypertens. 2013, 31, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Tedrow, U.B.; Koplan, B.A.; Glynn, R.J.; Buring, J.E.; Albert, C.M. Influence of systolic and diastolic blood pressure on the risk of incident atrial fibrillation in women. Circulation 2009, 119, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Grundvold, I.; Skretteberg, P.T.; Liestøl, K.; Erikssen, G.; Kjeldsen, S.E.; Arnesen, H.; Erikssen, J.; Bodegard, J. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men, A 35-year follow-up study. Hypertension 2012, 59, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Nalliah, C.J.; Sanders, P.; Kalman, J.M. The impact of diet and lifestyle on atrial fibrillation. Curr. Cardiol. Rep. 2018, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Dzeshka, M.S.; Shantsila, A.; Shantsila, E.; Lip, G.Y.H. Atrial fibrillation and hypertension. Hypertension 2017, 70, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Andreotti, F.; Fauchier, L.; Huber, K.; Hylek, E.; Knight, E.; Lane, D.; Levi, M.; Marín, F.; Palareti, G.; et al. European Heart Rhythm Association. Bleeding risk assessment and management in atrial fibrillation patients. Executive summary of a position document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] working group on thrombosis. Thromb. Haemost. 2011, 106, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Cosmi, B. Bleeding with anticoagulation therapy—who is at risk, and how best to identify such patients. Thromb. Haemost. 2009, 102, 268–278. [Google Scholar] [CrossRef]
- Boycott, H.E.; Barbier, C.S.; Eichel, C.A.; Costa, K.D.; Martins, R.P.; Louault, F.; Dilanian, G.; Coulombe, A.; Hatem, S.N.; Balse, E. Shear stress triggers insertion of voltage-gated potassium channels from intracellular compartments in atrial myocytes. Proc. Natl. Acad. Sci. USA 2013, 110, E3955–E3964. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Risius, T.; Morad, M. Modulation of local Ca2+ release sites by rapid fluid puffing in rat atrial myocytes. Cell Calcium 2007, 41, 397–403. [Google Scholar] [CrossRef]
- Fialova, M.; Dlugosova, K.; Okruhlicová, L.; Kristek, F.; Manoach, M.; Tribulová, N. Adaptation of the heart to hypertension is associated with maladaptive gap junction connexin-43 remodeling. Physiol. Res. 2008, 57, 7–11. [Google Scholar] [CrossRef]
- Lau, D.H.; Mackenzie, L.; Kelly, D.J.; Psaltis, P.J.; Brooks, A.G.; Worthington, M.; Rajendram, A.; Kelly, D.R.; Zhang, Y.; Kuklik, P.; et al. Hypertension and atrial fibrillation, Evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. 2010, 7, 1282–1290. [Google Scholar] [CrossRef]
- Tribulova, N.; Bacova, B.S.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Andelova, K.; Bacova, B.S.; Sykora, M.; Hlivak, P.; Barancik, M.; Tribulova, N. Mechanisms Underlying Antiarrhythmic Properties of Cardioprotective Agents Impacting Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 1416. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Hypertension and Atrial Fibrillation. Doubts and Certainties from Basic and Clinical Studies. Circ Res. 2018, 122, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.R.; Savona, S.; Mohamed, O.; Mohamed-Osman, A.; Kalbfleisch, S.J. Hypertension and Arrhythmias. Heart Failure Clin. 2019, 15, 543–550. [Google Scholar] [CrossRef]
- De Mello, W.C. Intracellular angiotensin II regulates the inward calcium current in cardiac myocytes. Hypertension 1998, 32, 976–982. [Google Scholar] [CrossRef][Green Version]
- Ferron, L.; Capuano, V.; Ruchon, Y.; Deroubaix, E.; Coulombe, A.; Renaud, J.F. Angiotensin II signaling pathways mediate expression of cardiac T-type calcium channels. Circ. Res. 2003, 93, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Daleau, P.; Turgeon, J. Angiotensin II modulates the delayed rectifier potassium current of guinea pig ventricular myocytes. Pflugers Arch. 1994, 427, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Ouvrard-Pascaud, A.; Sainte-Marie, Y.; Bénitah, J.P.; Perrier, R.; Soukaseum, C.; Nguyen Dinh Cat, A.; Royer, A.; Le Quang, K.; Charpentier, F.; Demolombe, S.; et al. Conditional mineralocorticoid receptor expression in the heart leads to life-threatening arrhythmias. Circulation 2005, 111, 3025–3033. [Google Scholar] [CrossRef]
- Tsai, C.T.; Chiang, F.T.; Tseng, C.D.; Hwang, J.J.; Kuo, K.T.; Wu, C.K.; Yu, C.C.; Wang, Y.C.; Lai, L.P.; Lin, J.L. Increased expression of mineralo-corticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J. Am. Coll. Cardiol. 2010, 55, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Rueda, A.; Sainte-Marie, Y.; Pereira, L.; Zissimopoulos, S.; Zhu, X.; Schaub, R.; Perrier, E.; Perrier, R.; Latouche, C.; et al. Mineralocorticoid modulation of cardiac ryanodine receptor activity is associated with downregulation of FK506-binding proteins. Circulation 2009, 119, 2179–2187. [Google Scholar] [CrossRef]
- Pluteanu, F.; Heß, J.; Plackic, J.; Nikonova, Y.; Preisenberger, J.; Bukowska, A.; Schotten, U.; Rinne, A.; Kienitz, M.C.; Schäfer, M.K.; et al. Early subcellular Ca2+ remodelling and increased propensity for Ca2+ alternans in left atrial myocytes from hypertensive rats. Cardiovasc. Res. 2015, 106, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy, A translational perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Kahan, T.; Bergfeldt, L. Left ventricular hypertrophy in hypertension, Its arrhythmogenic potential. Heart 2005, 91, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Lopez, B.; Ravassa, S.; San José, G.; Díez, J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Biophys. Acta Mol. Cell. Res. 2019, 1866, 1421–1432. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/ HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies, Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M. Heart failure with preserved ejection fraction. N. Engl. J. Med. 2016, 375, 1868–1877. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Tentolouris, K.; Androulakis, A.; Trikas, A.; Toutouzas, K.; Kyriakidis, M.; Gialafos, J.; Toutouzas, P. Left atrial mechanical function in the healthy elderly, New insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J. Am. Soc. Echocardiogr. 1995, 8, 801–809. [Google Scholar] [CrossRef]
- Ravelli, F.; Allessie, M. Effects of atrial dilatation on refractory period and vulnerability to atrial fibrillation in the isolated Langendorff perfused rabbit heart. Circulation 1997, 96, 1686–1695. [Google Scholar] [CrossRef]
- Satoh, T.; Zipes, D.P. Unequal atrial stretch in dogs increases dispersion of refractoriness conductive to developing atrial fibrillation. J. Cardiovasc. Electrophysiol. 1996, 7, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Jais, P.; Peng, J.T.; Shah, D.C.; Garrigue, S.; Hocini, M.; Yamane, T.; Haïssaguerre, M.; Barold, S.S.; Roudaut, R.; Clémenty, J. Left ventricular diastolic dysfunction in patients with so-called lone atrial fibrillation. J. Cardiovasc. Electrophysiol. 2000, 11, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.S.M.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left Ventricular Diastolic Dysfunction as a Predictor of the First Diagnosed Nonvalvular Atrial Fibrillation in 840 Elderly Men and Women. JACC 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Verdecchia, P.; Reboldi, G.; Gattobigio, R.; Bentivoglio, M.; Borgioni, C.; Angeli, F.; Carluccio, E.; Sardone, M.G.; Porcellati, C. Atrial fibrillation in hypertension, Predictors and outcome. Hypertension 2003, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ciaroni, S.; Cuenoud, L.; Bloch, A. Clinical study to investigate the predictive parameters for the onset of atrial fibrillation in patients with essential hypertension. Am. Heart J. 2000, 139, 814–819. [Google Scholar] [CrossRef]
- Duncker, D.J.; Zhang, J.; Bache, R.J. Coronary pressure-flow relation in left ventricular hypertrophy. Importance of changes in back pressure versus changes in minimum resistance. Circ. Res. 1993, 72, 579–587. [Google Scholar] [CrossRef]
- Duncker, D.J.; Ishibashi, Y.; Bache, R.J. Effect of treadmill exercise on transmural distribution of blood flow in hypertrophied left ventricle. Am. J. Physiol. 1998, 275, H1274–H1282. [Google Scholar] [CrossRef]
- Vatner, S.F.; Hittinger, L. Coronary vascular mechanisms involved in decompensation from hypertrophy to heart failure. J. Am. Coll. Cardiol. 1993, 22, 34A–40A. [Google Scholar] [CrossRef]
- Kyriakidis, M.; Barbetseas, J.; Antonopoulos, A.; Skouros, C.; Tentolouris, C.; Toutouzas, P. Early atrial arrhythmias in acute myocardial infarction. Role of the sinus node artery. Chest 1992, 101, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Hod, H.; Lew, A.S.; Keltai, M.; Cercek, B.; Geft, I.L.; Shah, P.K.; Ganz, W. Early atrial fibrillation during evolving myocardial infarction, A consequence of impaired left atrial perfusion. Circulation 1987, 75, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Lu Marvin, L.R.; De Venecia, T.; Patnaik, S.; Figueredo, V.M. Atrial myocardial infarction, A tale of the forgotten chamber. Int. J. Cardiol. 2015, 202, 904–909. [Google Scholar] [CrossRef]
- Alasady, M.; Shipp, N.J.; Brooks, A.G.; Lim, H.S.; Lau, D.H.; Barlow, D.; Kuklik, P.; Worthley, M.I.; Roberts-Thomson, K.C.; Saint, D.A.; et al. Myocardial infarction and atrial fibrillation, Importance of atrial ischemia. Circ. Arrhythm. Electrophysiol. 2013, 6, 738–745. [Google Scholar] [CrossRef]
- Kolvekar, S.; D’Souza, A.; Akhtar, P.; Reek, C.; Garratt, C.; Spyt, T. Role of atrial ischaemia in development of atrial fibrillation following coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 1997, 11, 70–75. [Google Scholar] [CrossRef][Green Version]
- Ciulla, M.; Astuti, M.; Carugo, S. The atherosclerosis of the sinus node artery is associated with an increased history of supra-ventricular arrhythmias, A retrospective study on 541 standard coronary angiograms. PeerJ 2015, 3, e1156. [Google Scholar] [CrossRef][Green Version]
- Bikou, O.; Kho, C.; Ishikawa, K. Atrial stretch and arrhythmia after myocardial infarction. Aging 2018, 11, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Feistritzer, H.J.; Desch, S.; Zeymer, U.; Fuernau, G.; de Waha-Thiele, S.; Dudek, D.; Huber, K.; Stepinska, J.; Schneider, S.; Ouarrak, T.; et al. Prognostic Impact of Atrial Fibrillation in Acute Myocardial Infarction and Cardiogenic Shock. Circ. Cardiovasc. Interv. 2019, 12, e007661. [Google Scholar] [CrossRef]
- Jebberi, Z.; Marazzato, J.; De Ponti, R.; Bagliani, G.; Leonelli, F.M.; Boveda, S. Polymorphic Wide QRS Complex Tachycardia, Differential Diagnosis. Card. Electrophysiol. Clin. 2019, 11, 333–344. [Google Scholar] [CrossRef]
- Marazzato, J.; Angeli, F.; De Ponti, R.; Di Pasquale, G.; Verdecchia, P. Atrial fibrillation and sudden cardiac death, A mystery to unravel? GIC 2021, 22, 544–553. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. ESC Scientific Document Group, 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD, The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Prystowsky, E.N.; Halperin, J.; Kowey, P. Atrial fibrillation, atrial flutter and atrial tachycardia. In Hurst’s the Heart, 14e; McGraw-Hill: New York, NY, USA, 2017; pp. 1950–1966. [Google Scholar]
- De Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef]
- Potpara, T.S.; Stankovic, G.R.; Beleslin, B.D.; Polovina, M.M.; Marinkovic, J.M.; Ostojic, M.C.; Lip, G.Y.H.A. 12-year follow-up study of patients with newly diagnosed lone atrial fibrillation, Implications of arrhythmia progression on prognosis, The Belgrade Atrial Fibrillation study. Chest 2012, 141, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Potpara, T.S.; Polovina, M.M.; Marinkovic, J.M.; Lip, G.Y. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation, The Belgrade Atrial Fibrillation study. Int. J. Cardiol. 2013, 168, 4744–4749. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases, Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef]
- Li, C.; Engström, G.; Hedblad, B.; Hedblad, B.; Berglund, G.; Janzon, L. Blood pressure control and risk of stroke, A population-based prospective cohort study. Stroke 2005, 36, 725–730. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation, A major contributor to stroke in the elderly. The Framingham Study. Arch. Intern. Med. 1987, 147, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Rosenqvist, M.; Lip, G.Y. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation, The Swedish Atrial Fibrillation cohort study. Eur. Heart J. 2012, 33, 1500–1510. [Google Scholar] [CrossRef]
- Paciaroni, M.; Agnelli, G.; Ageno, W.; Caso, V. Timing of anticoagulation therapy in patients with acute ischaemic stroke and atrial fibrillation. Thromb. Haemost. 2016, 116, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.F.; Waterman, A.D.; Shannon, W.; Boechler, M.; Rich, M.W.; Radford, M.J. Validation of clinical classification schemes for predicting stroke, Results from the national registry of atrial fibrillation. JAMA 2001, 285, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Ogawa, H.; Unoki, T.; An, Y.; Iguchi, M.; Masunaga, N.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; et al. Relationship of Hypertension and Systolic Blood Pressure with the Risk of Stroke or Bleeding in Patients with Atrial Fibrillation, The Fushimi AF Registry. Am. J. Hypertens. 2017, 30, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Kodani, E.; Inoue, H.; Atarashi, H.; Okumura, K.; Yamashita, T.; Otsuka, T.; Origasa, H. Impact of Blood Pressure Visit-to-Visit Variability on Adverse Events in Patients With Nonvalvular Atrial Fibrillation, Subanalysis of the J-RHYTHM Registry. J. Am. Heart Assoc. 2021, 10, e018585. [Google Scholar] [CrossRef] [PubMed]
- Parcha, V.; Patel, N.; Kalra, R.; Kim, J.; Gutiérrez, O.M.; Arora, G.; Arora, P. Incidence and Implications of Atrial Fibrillation/Flutter in Hypertension Insights From the SPRINT Trial. Hypertension 2020, 75, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Zabalgoitia, M.; Halperin, J.L.; Pearce, L.A.; Blackshear, J.L.; Asinger, R.W.; Hart, R.G. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J. Am. Coll. Cardiol. 1998, 31, 1622–1626. [Google Scholar] [CrossRef]
- Goldman, M.E.; Pearce, L.A.; Hart, R.G.; Zabalgoitia, M.; Asinger, R.W.; Safford, R.; Halperin, J.L. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation, I. reduced flow velocity in the left atrial appendage (the Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 1999, 12, 1080–1087. [Google Scholar] [CrossRef]
- Bukowska, A.; Zacharias, I.; Weinert, S.; Skopp, K.; Hartmann, C.; Huth, C.; Goette, A. Coagulation factor Xa induces an inflammatory signalling by activation of protease-activated receptors in human atrial tissue. Eur. J. Pharmacol. 2013, 718, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, A.; Rocken, C.; Erxleben, M.; Röhl, F.W.; Hammwöhner, M.; Huth, C.; Ebert, M.P.; Lendeckel, U.; Goette, A. Atrial expression of endothelial nitric oxide synthase in patients with and without atrial fibrillation. Cardiovasc. Pathol. 2010, 19, e51–e60. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Bukowska, A.; Lendeckel, U.; Erxleben, M.; Hammwöhner, M.; Strugala, D.; Pfeiffenberger, J.; Röhl, F.W.; Huth, C.; Ebert, M.P.; et al. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation 2008, 117, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Hammwohner, M.; Ittenson, A.; Dierkes, J.; Bukowska, A.; Klein, H.U.; Lendeckel, U.; Goette, A. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp. Biol. Med. 2007, 232, 581–589. [Google Scholar]
- Fiebeler, A.; Schmidt, F.; Müller, D.N.; Park, J.K.; Dechend, R.; Bieringer, M.; Shagdarsuren, E.; Breu, V.; Haller, H.; Luft, F.C. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 2001, 37, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Keidar, S.; Kaplan, M.; Pavlotzky, E.; Coleman, R.; Hayek, T.; Hamoud, S.; Aviram, M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development, A possible role for angiotensin-convert-ing enzyme and the receptors for angiotensin II and aldosterone. Circulation 2004, 109, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J.; Lu, L.; Chen, S.S.; Quinn, M.T.; Weber, K.T. Aldosterone-induced inflammation in the rat heart, Role of oxidative stress. Am. J. Pathol. 2002, 161, 1773–1781. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Kim, T.H.; Jang, E.; Shin, H.; Kim, H.Y.; Yu, H.T.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; et al. Ideal blood pressure in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2018, 72, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Ito, M.; Tomita, M.; Hoyano, M.; Obata, H.; Ding, L.; Chinushi, M.; Hanawa, H.; Kodama, M.; Aizawa, Y. Role of mineralocorticoid receptor on atrial structural remodeling and inducibility of atrial fibrillation in hypertensive rats. Hypertens. Res. 2011, 34, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, N.; Tsutsumi, T.; Kubota, N.; Nakajima, T.; Suzuki, H.; Takeyama, Y. Direct action of an angiotensin II receptor blocker on angiotensin II-induced left atrial conduction delay in spontaneously hypertensive rats. Hypertens. Res. 2009, 32, 721–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fogari, R.; Zoppi, A.; Maffioli, P.; Mugellini, A.; Preti, P.; Perrone, T.; Derosa, G. Effect of telmisartan on paroxysmal atrial fibrillation recurrence in hypertensive patients with normal or increased left atrial size. Clin. Cardiol. 2012, 35, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wachtell, K.; Lehto, M.; Gerdts, E.; Olsen, M.H.; Hornestam, B.; Dahlöf, B.; Ibsen, H.; Julius, S.; Kjeldsen, S.E.; Lindholm, L.H.; et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol, The Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J. Am. Coll. Cardiol. 2005, 45, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Tognoni, G. Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Ducharme, A.; Swedberg, K.; Pfeffer, M.A.; Cohen-Solal, A.; Granger, C.B.; Maggioni, A.P.; Michelson, E.L.; McMurray, J.J.V.; Olsson, L.; Rouleau, J.L.; et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure, Assessment of Reduction in Mortality and morbidity (CHARM) program. Am. Heart J. 2006, 152, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vermes, E.; Tardif, J.C.; Bourassa, M.G.; Racine, N.; Levesque, S.; White, M.; Guerra, P.G.; Ducharme, A. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction, Insight from the Studies of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003, 107, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Schaer, B.A.; Schneider, C.; Jick, S.S.; Conen, D.; Osswald, S.; Meier, C.R. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs, A nested case-control study. Ann. Intern. Med. 2010, 152, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Shi, H.; Vincent, J.; Pitt, B. Eplerenone and atrial fibrillation in mild systolic heart failure, Results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and SurvIval Study in Heart Failure) study. J. Am. Coll. Cardiol. 2012, 59, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Al-Gobariet, M.; El Khatib, C.; Pillon, F.; Gueyffier, F. Beta-blockers for the prevention of sudden cardiac death in heart failure patients, A meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2013, 13, 52. [Google Scholar] [CrossRef]
- Disertori, M.; Latini, R.; Barlera, S.; Franzosi, M.G.; Staszewsky, L.; Maggioni, A.P.; Lucci, D.; Di Pasquale, G.; Tognoni, G. Valsartan for prevention of recurrent atrial fibrillation. N. Engl. J. Med. 2009, 360, 1606–1617. [Google Scholar] [PubMed]
- Goette, A.; Schon, N.; Kirchhof, P.; Breithardt, G.; Fetsch, T.; Häusler, K.G.; Klein, H.U.; Steinbeck, G.; Wegscheider, K.; Meinertz, T. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ. Arrhythm. Electrophysiol. 2012, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tveit, A.; Grundvold, I.; Olufsen, M.; Seljeflot, I.; Abdelnoor, M.; Arnesen, H.; Smith, P. Candesartan in the prevention of relapsing atrial fibrillation. Int. J. Cardiol. 2007, 120, 85–91. [Google Scholar] [CrossRef]
- Schirpenbach, C.; Reincke, M. Primary aldosteronism, Current knowledge and controversies in Conn’s syndrome. Nat. Rev. Endocrinol. 2007, 3, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism, Case detection, diagnosis, and treatment, An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism, A retrospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 51–59. [Google Scholar] [CrossRef]
- Milliez, P.; Girerd, X.; Plouin, P.F.; Blacher, J.; Safar, M.E.; Mourad, J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005, 45, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Born-Frontsberg, E.; Reincke, M.; Rump, L.C.; Hahner, S.; Diederich, S.; Lorenz, R.; Allolio, B.; Seufert, J.; Schirpenbach, C.; Beuschlein, F.; et al. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism, Results of the German Conn’s Registry. J. Clin. Endocrinol. Metab. 2009, 94, 1125–1130. [Google Scholar] [CrossRef]
- Pan, C.-T.; Tsai, C.; Chen, Z.W.; Chang, Y.Y.; Wu, V.C.; Hung, C.S.; Lin, Y.H. Atrial Fibrillation in Primary Aldosteronism. Horm. Metab. Res. 2020, 52, 357–365. [Google Scholar] [CrossRef]
- Oestreicher, E.M.; Martinez-Vasquez, D.; Stone, J.R.; Jonasson, L.; Roubsanthisuk, W.; Mukasa, K.; Adler, G.K. Aldosterone and not plasminogen activator inhibitor-1 is a critical mediator of early angiotensin II/NG-nitro-L-arginine methyl ester-induced myocardial injury. Circulation 2003, 108, 2517–2523. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Heeringa, J.; Kors, J.A.; Hofman, A.; Franco, O.H.; Witteman, J.C.; Stricker, B.H. Serum potassium levels and the risk of atrial fibrillation, The Rotterdam Study. Int. J. Cardiol. 2013, 168, 5411–5415. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Weber, T.; Berent, R.; Lamm, G.; Eber, B. Serum potassium level and risk of postoperative atrial fibrillation in patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 2004, 44, 938–939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, J.N.; Qu, Z.; Shivkumar, K. Electrophysiology of hypokalemia and hyperkalemia. Circ. Arrhyth. Electrophysiol. 2017, 10, e004667. [Google Scholar] [CrossRef]
- Vaidya, A.; Mulatero, P.; Baudrand, R.; Adler, G.K. The Expanding Spectrum of Primary Aldosteronism, Implications for Diagnosis, Pathogenesis, and Treatment. Endocr. Rev. 2018, 39, 1057–1088. [Google Scholar] [CrossRef]
- Wang, K.; Hu, J.; Yang, J.; Song, Y.; Fuller, P.J.; Hashimura, H.; He, W.; Feng, Z.; Cheng, Q.; Du, Z.; et al. Development and Validation of Criteria for Sparing Confirmatory Tests in Diagnosing Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2020, 105, dgaa282. [Google Scholar] [CrossRef] [PubMed]
- Hundemer, G.L.; Curhan, G.C.; Yozamp, N.; Wang, M.; Vaidya, A. Incidence of Atrial Fibrillation and Mineralocorticoid Receptor Activity in Patients With Medically and Surgically Treated Primary Aldosteronism. JAMA Cardiol. 2018, 3, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Maiolino, G.; Flego, A.; Belfiore, A.; Bernini, G.; Fabris, B.; Ferri, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. PAPY Study Investigators. Adrenalectomy Lowers Incident Atrial Fibrillation in Primary Aldosteronism Patients at Long Term. Hypertension 2018, 71, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Imberti, J.F.; Vitolo, M. The challenge to improve knowledge on the interplay between subclinical atrial fibrillation, atrial cardiomyopathy, and atrial remodeling. J. Cardiovasc. Electrophysiol. 2021, 32, 1364–1366. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Moe, G. The case against using hypertension as the only criterion for oral anticoagulation in atrial fibrillation. Can. J. Cardiol. 2015, 31, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.; Camm, J.; Calkins, H.; Healey, J.S.; Rosenqvist, M.; Wang, J.; Albert, C.M.; Anderson, C.S.; Antoniou, S.; Benjamin, E.J.; et al. AF-Screen Collaborators. Screening for atrial fibrillation, A report of the AF-SCREEN International Collaboration. Circulation 2017, 135, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Alings, M.; Ha, A.; Leong-Sit, P.; Birnie, D.H.; de Graaf, J.J.; Freericks, M.; Verma, A.; Wang, J.; Leong, D.; et al. ASSERT-II Investigators. Subclinical atrial fibrillation in older patients. Circulation 2017, 136, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129, Erratum in N. Engl. J. Med. 2016, 374, 998. [Google Scholar] [CrossRef]
- Svendsen, J.H.; Diederichsen, S.Z.; Højberg, S.; Krieger, D.W.; Graff, C.; Kronborg, C.; Olesen, M.S.; Nielsen, J.B.; Holst, A.G.; Brandes, A.; et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study), A randomised controlled trial. Lancet 2021, 398, 1507–1516, Erratum in Lancet 2021, 398, 1486. [Google Scholar] [CrossRef]
- Gorenek, B.; Bax, J.; Boriani, G.; Chen, S.A.; Dagres, N.; Glotzer, T.V.; Healey, J.S.; Israel, C.W.; Kudaiberdieva, G.; Levin, L.Å.; et al. ESC Scientific Document Group. Device-detected subclinical atrial tachyarrhythmias, Definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017, 19, 1556–1578, Erratum in Europace 2017, 19, 1507; Erratum in Europace 2018, 20, 658. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.G.M.; Wang, J.; Hohnloser, S.H.; Gold, M.R.; Capucci, A.; Lau, C.P.; Morillo, C.A.; Hobbelt, A.H.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur. Heart J. 2017, 38, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; Alings, M.; Connolly, S.J.; Beresh, H.; Granger, C.B.; Mazuecos, J.B.; Boriani, G.; Nielsen, J.C.; Conen, D.; Hohnloser, S.H.; et al. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am. Heart J. 2017, 189, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Paulus, K.; Blank, B.F.; Calvert, M.; Camm, A.J.; Chlouverakis, G.; Diener, H.C.; Goette, A.; Huening, A.; Lip, G.Y.H.; Simantirakis, E.; et al. Probing oral anticoagulation in patients with atrial high rate episodes. Rationale and design of the Non vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH—AFNET 6) trial. Am. Heart J. 2017, 190, 12–18. [Google Scholar] [CrossRef]
- Diederichsen, S.; Haugan, K.J.; Brandes, A.; Graff, C.; Krieger, D.; Kronborg, C.; Holst, A.G.; Nielsen, J.B.; Køber, L.; Højberg, S.; et al. Incidence and predictors of atrial fibrillation episodes as detected by implantable loop recorder in patients at risk From the LOOP study. Am. Heart J. 2019, 219, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; Pereira-Rejálaga, L.E.; Nikus, K.; Shenasa, M. Electrocardiographic and Echocardiographic Abnormalities in Patients with Risk Factors for Atrial Fibrillation. Card. Electrophysiol. Clin. 2021, 13, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.R.; Taylor, C.J.; Hobbs, F.D.R.; Bowman, L.; Casadei, B. Screening for atrial fibrillation, A call for evidence. Eur. Heart J. 2020, 41, 1075–1085. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marazzato, J.; Blasi, F.; Golino, M.; Verdecchia, P.; Angeli, F.; De Ponti, R. Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 110. https://doi.org/10.3390/jcdd9040110
Marazzato J, Blasi F, Golino M, Verdecchia P, Angeli F, De Ponti R. Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients. Journal of Cardiovascular Development and Disease. 2022; 9(4):110. https://doi.org/10.3390/jcdd9040110
Chicago/Turabian StyleMarazzato, Jacopo, Federico Blasi, Michele Golino, Paolo Verdecchia, Fabio Angeli, and Roberto De Ponti. 2022. "Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients" Journal of Cardiovascular Development and Disease 9, no. 4: 110. https://doi.org/10.3390/jcdd9040110
APA StyleMarazzato, J., Blasi, F., Golino, M., Verdecchia, P., Angeli, F., & De Ponti, R. (2022). Hypertension and Arrhythmias: A Clinical Overview of the Pathophysiology-Driven Management of Cardiac Arrhythmias in Hypertensive Patients. Journal of Cardiovascular Development and Disease, 9(4), 110. https://doi.org/10.3390/jcdd9040110









