Reduction of Hospitalization and Mortality by Echocardiography-Guided Treatment in Advanced Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
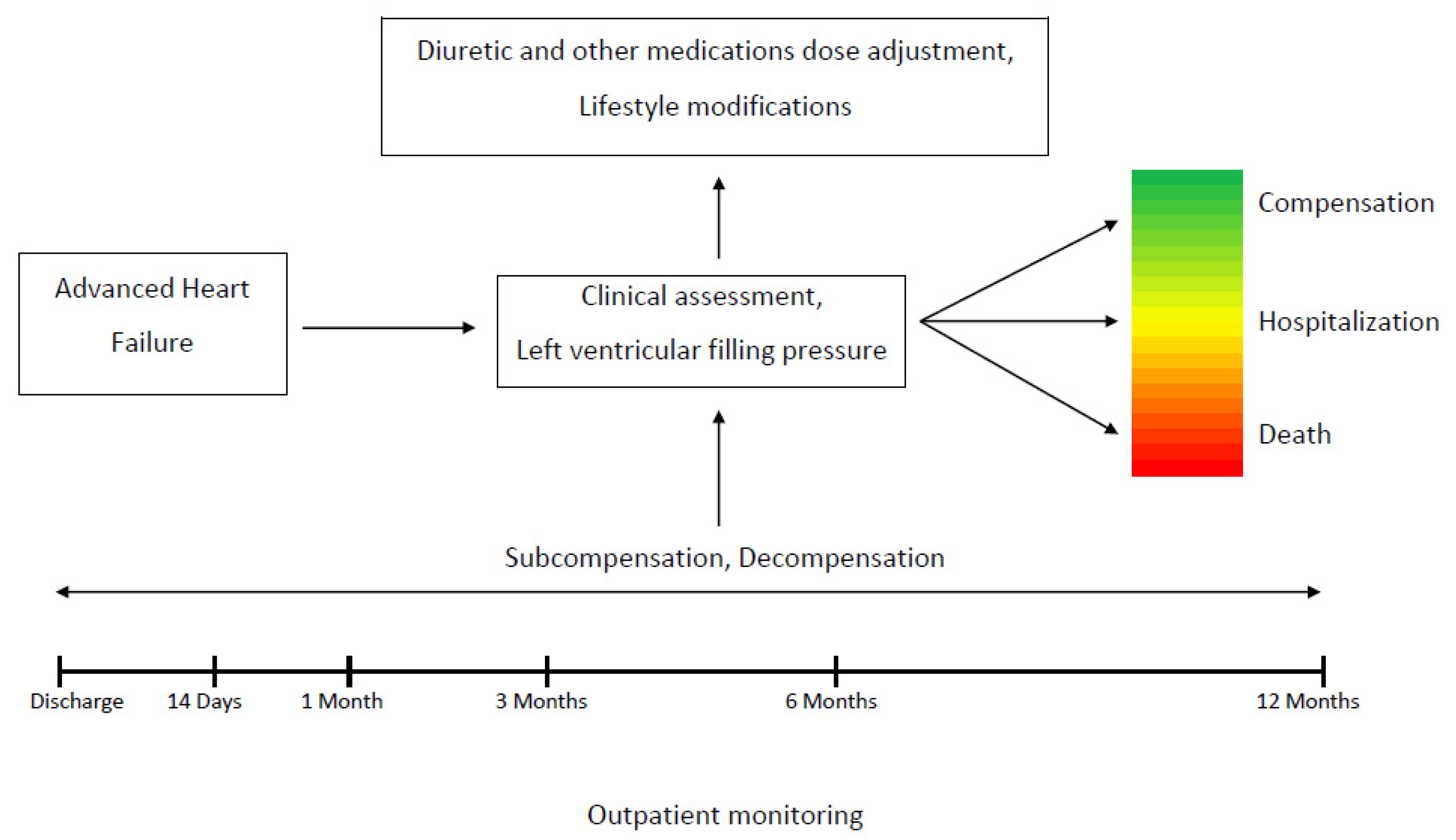
2.2. Clinical Data and Study Protocol
2.3. Echocardiographic Evaluation
2.4. Study Endpoints
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
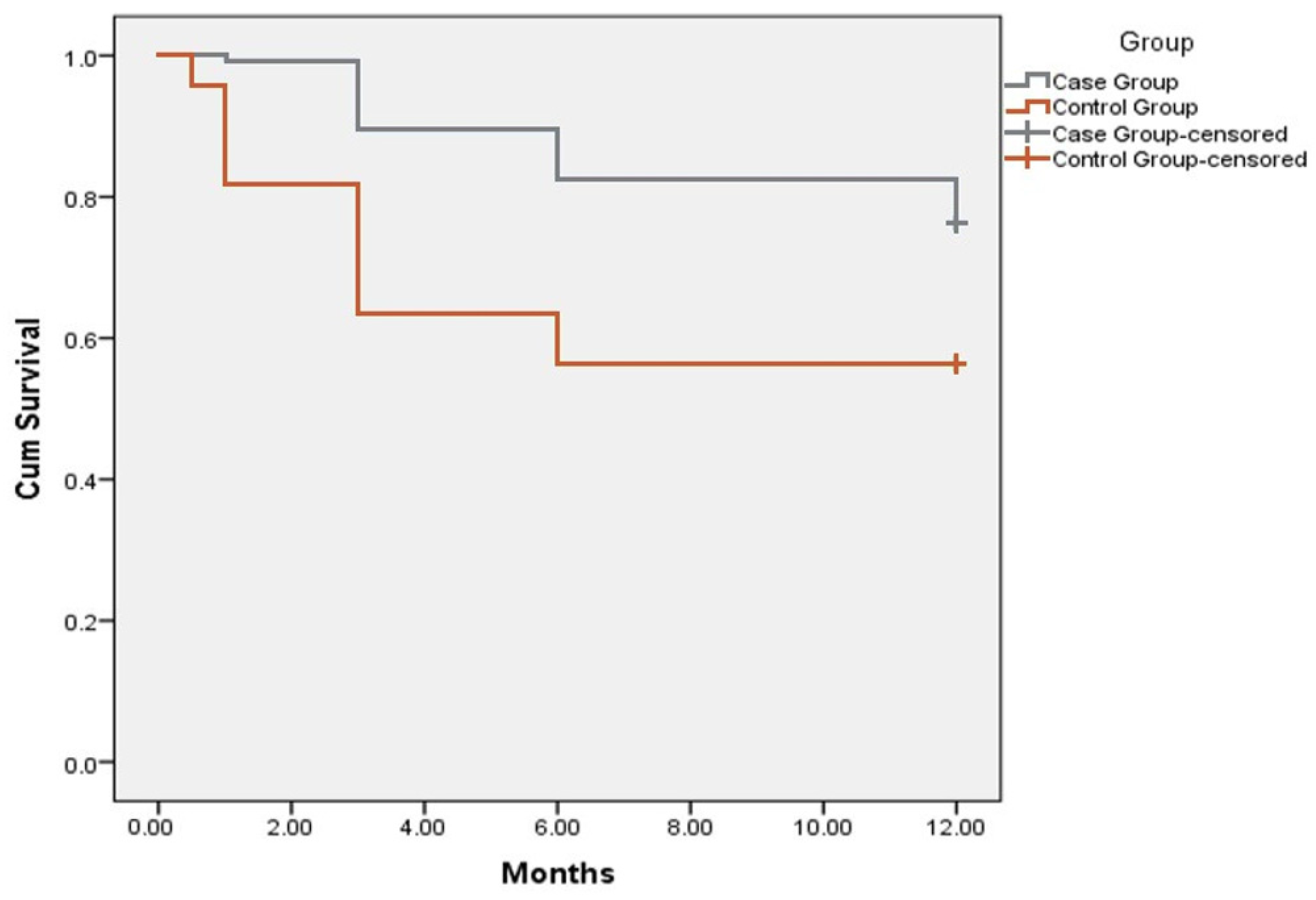
3.2. Outcomes
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology Founda-tion/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Metra, M.; Dinatolo, E.; Dasseni, N. Advanced Heart Failure the New Heart Failure Association Definition of Advanced Heart Failure Advanced Heart Failure. Card. Fail. Rev. 2019, 5, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Metra, M.; Ponikowski, P.; Dickstein, K.; McMurray, J.J.; Gavazzi, A.; Bergh, C.-H.; Fraser, A.G.; Jaarsma, T.; Pitsis, A.; Mohacsi, P.; et al. Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2007, 9, 684–694. [Google Scholar] [CrossRef]
- Ziaeian, B.; Fonarow, G.C. The Prevention of Hospital Readmissions in Heart Failure. Prog. Cardiovasc. Dis. 2016, 58, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Damy, T.; Kallvikbacka-Bennett, A.; Zhang, J.; Goode, K.; Buga, L.; Hobkirk, J.; Yassin, A.; Dubois-Randé, J.-L.; Hittinger, L.; Cleland, J.G.; et al. Does the physical examination still have a role in patients with suspected heart failure? Eur. J. Heart Fail. 2011, 13, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.; Lewis, E.F.; Uno, H.; Peck, J.; Pivetta, E.; Merz, A.; Hempel, D.; Wilson, C.; Frasure, S.E.; Jhund, P.; et al. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur. Heart J. 2016, 37, 1244–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lampert, B.C.; Emani, S. Remote hemodynamic monitoring for ambulatory left ventricular assist device patients. J. Thorac. Dis. 2015, 7, 2165–2171. [Google Scholar] [CrossRef]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr. Heart Fail. Rep. 2009, 6, 287–292. [Google Scholar] [CrossRef]
- Mangi, M.A.; Rehman, H.; Rafique, M.; Illovsky, M. Ambulatory Heart Failure Monitoring: A Systemic Review. Cureus 2017, 9, e1174. [Google Scholar] [CrossRef] [Green Version]
- Vazir, A.; Cowie, M.R. Assessing Acute Decompensated Heart Failure—Strategies and Tools. Eur. Cardiol. Rev. 2012, 8, 128. [Google Scholar] [CrossRef]
- Vignon, P. Cardiovascular failure and weaning. Ann. Transl. Med. 2018, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Dokainish, H.; Nguyen, J.S.; Bobek, J.; Goswami, R.; Lakkis, N.M. Assessment of the American Society of Echocardiography-European Association of Echocardiography guidelines for diastolic function in patients with depressed ejection fraction: An echocardiographic and invasive haemodynamic study. Eur. J. Echocardiogr. 2011, 12, 857–864. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Shrestha, K.; Mullens, W.; Borowski, A.G.; Martin, M.G.; Troughton, R.W.; Klein, A.L. Impact of Left Ventricular Remodeling on Diagnostic and Prognostic Value of Tissue Doppler Indices in Chronic Systolic Heart Failure. J. Card. Fail. 2011, 17, 128–134. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200, Erratum in 2018, 39, 860. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, A.F. Relationship Between Early Physician Follow-up and 30-Day Readmission Among Medicare Beneficiaries Hospitalized for Heart Failure. JAMA J. Am. Med. Assoc. 2010, 303, 1716–1722. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and grading congestion in acute heart failure: A scientific statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef]
- Zymliński, R.; Sokolski, M.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Sokolska, J.; Dudkowiak, M.; Marciniak, D.; Todd, J.; Jankowska, E.A.; et al. Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur. J. Heart Fail. 2019, 21, 744–750. [Google Scholar] [CrossRef]
- Lucas, C.; Johnson, W.; Hamilton, M.A.; Fonarow, G.; Woo, M.A.; Flavell, C.M.; Creaser, J.A.; Stevenson, L.W. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am. Heart J. 2000, 140, 840–847. [Google Scholar] [CrossRef]
- Murphy, N.; Shanks, M.; Alderman, P. Management of Heart Failure with Outpatient Technology. J. Nurse Pract. 2018, 15, 12–18. [Google Scholar] [CrossRef]
- Angermann, C.E.; Störk, S.; Gelbrich, G.; Faller, H.; Jahns, R.; Frantz, S.; Loeffler, M.; Ertl, G. Competence Network Heart Failure. Mode of Action and Effects of Standardized Collaborative Disease Management on Mortality and Morbidity in Patients with Systolic Heart Failure: The Interdisciplinary Network for Heart Failure (INH) study. Circ. Heart Fail. 2012, 5, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in Patients with Heart Failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, J.G.; Louis, A.A.; Rigby, A.S.; Janssens, U.; Balk, A.H. Noninvasive Home Telemonitoring for Patients with Heart Failure at High Risk of Recurrent Admission and Death: The Trans-European Network-Home-Care Management System (TEN-HMS) study. J. Am. Coll. Cardiol. 2005, 45, 1654–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of Remote Telemedical Management on Mortality and Hospitalizations in Ambulatory Patients with Chronic Heart Failure. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; Sutton, M.S.J.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.J.M.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from Chronic Compensated to Acute Decompensated Heart Failure. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B.; CHAMPION Trial Study Group. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Binanay, C.; Califf, R.M.; Hasselblad, V.; O’Connor, C.M.; Shah, M.R.; Sopko, G.; Stevenson, L.W.; Francis, G.S.; Leier, C.V.; Miller, L.W.; et al. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness. JAMA J. Am. Med. Assoc. 2005, 294, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Vaduganathan, M.; DeFilippis, E.M.; Fonarow, G.C.; Butler, J.; Mehra, M.R. Postmarketing Adverse Events Related to the CardioMEMS HF System. JAMA Cardiol. 2017, 2, 1277–1279. [Google Scholar] [CrossRef]
- Desai, A.S.; Bhimaraj, A.; Bharmi, R.; Jermyn, R.; Bhatt, K.; Shavelle, D.; Redfield, M.M.; Hull, R.; Pelzel, J.; Davis, K.; et al. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real-World” Clinical Practice. J. Am. Coll. Cardiol. 2017, 69, 2357–2365. [Google Scholar] [CrossRef]
- Öhman, J.; Harjola, V.-P.; Karjalainen, P.; Lassus, J. Focused echocardiography and lung ultrasound protocol for guiding treatment in acute heart failure. ESC Heart Fail. 2018, 5, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Lasarte, M.; Alvarez-Garcia, J.; Fernández-Martínez, J.; Maestro, A.; López-López, L.; Solé-González, E.; Pirla, M.J.; Mesado, N.; Mirabet, S.; Fluvià, P.; et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 2019, 21, 1605–1613. [Google Scholar] [CrossRef]
- Marini, C.; Fragasso, G.; Italia, L.; Sisakian, H.; Tufaro, V.; Ingallina, G.; Stella, S.; Ancona, F.; LoIacono, F.; Innelli, P.; et al. Lung ultrasound-guided therapy reduces acute decompensation events in chronic heart failure. Heart 2020, 106, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.S.; Russell, F.M.; Ehrman, R.; Ferre, R.; Gargani, L.; Levy, P.D.; Noble, V.; Lane, K.A.; Li, X.; Collins, S.P. Lung Ultrasound–Guided Emergency Department Management of Acute Heart Failure (BLUSHED-AHF). JACC Heart Fail. 2021, 9, 638–648. [Google Scholar] [CrossRef]

| Variable | Intensive Monitoring Group (n = 143) | Standard Monitoring Group (n = 71) | p Value |
|---|---|---|---|
| Age (years) | 66.6 ± 10.1 | 64.6 ± 10.1 | 0.186 |
| Women (n, %) | 30 (21) | 16 (23) | 0.794 |
| Body mass (kg) | 84.6 ± 14.2 | 80.5 ± 13.3 | 0.043 |
| Coronary artery disease (n, %) | 100 (70) | 64 (90) | 0.001 |
| Diabetes mellitus (n, %) | 37 (26) | 18 (25) | 0.934 |
| CKD (n, %) | 51 (36) | 16 (23) | 0.051 |
| Heart rate (beats per minute) | 84.8 ± 15.0 | 79.2 ± 17.4 | 0.016 |
| Sinus rhythm (n, %) | 93 (65) | 54 (76) | 0.102 |
| Atrial fibrillation (n, %) | 48 (34) | 17 (24) | 0.149 |
| Pacemaker (n, %) | 2 (1) | 0 (0) | 0.317 |
| ICD (n, %) | 3 (2) | 2 (3) | 0.743 |
| CRT (n, %) | 4 (3) | 0 (0) | 0.155 |
| Systolic blood pressure (mmHg) | 117.2 ± 17.9 | 119.7 ± 17.4 | 0.326 |
| Diastolic blood pressure (mmHg) | 71.2 ± 10.5 | 75.3 ± 11.9 | <0.001 |
| Creatinine (mmol/L) | 103.9 ± 38.3 | 130 ± 39.4 | <0.001 |
| Potassium (mmol/L) | 4.6 ± 0.5 | 4.2 ± 0.6 | 0.027 |
| NYHA class | |||
| III | 83 (58%) | 49 (69%) | 0.120 |
| IV | 60 (42%) | 22 (31%) | 0.120 |
| Echocardiographic parameters | |||
| LV ejection fraction (%) | 20.1 ± 5.2 | 22.5 ± 4.0 | 0.001 |
| LA volume index (mL/m2) | 51.7 ± 19.1 | 46 ± 10.4 | 0.020 |
| E/e’ ratio | 24.1 ± 6.9 | 15.8 ± 2.2 | <0.001 |
| Medical Treatment | Intensive Monitoring Group (n = 143) | Standard Monitoring Group (n = 71) | p Value |
|---|---|---|---|
| Beta-blocker (n, %) | 131 (91.6) | 62 (87.3) | 0.321 |
| ACEi/ARB (n, %) | 123 (86) | 63 (88.7) | 0.014 |
| MRA (n, %) | 136 (95.1) | 62 (87.3) | 0.042 |
| Furosemide, oral (n, %) | 138 (96.5) | 66 (93) | 0.247 |
| Digoxin (n, %) | 36 (25.2) | 12 (16.9) | 0.172 |
| ARNI (n, %) | 16 (11.2) | 1 (1.4) | 0.013 |
| Inotropes (in-hospital) (n, %) | 81 (57) | 24 (33.4) | 0.002 |
| Vasodilators (n, %) | 14 (9.7) | 32 (45) | <0.0001 |
| Furosemide, Oral | at Discharge | 3 Months | 6 Months | 12 Months | |
| IMG | Study group (n) | 142 | 133 | 128 | 126 |
| Patient number (n, %) | 141 (99.3%) | 126 (94.7%) | 123 (96.1%) | 122 (96.8%) | |
| Mean dose (mg) | 44.96 ± 16.76 | 59.52 ± 32.64 | 63.74 ± 35.61 | 65.74 ± 39.29 | |
| SMG | Control Group (n) | 71 | 57 | 51 | 51 |
| Patient number (n, %) | 66 (93%) | 55 (96.5%) | 36 (70.6%) | 13 (25.5%) | |
| Mean dose (mg) | 64.55 ± 23.48 | 58.91 ± 30.65 | 64.44 ± 34.84 | 61.54 ± 35.08 | |
| Furosemide, i.v. | at discharge | 3 months | 6 months | 12 months | |
| IMG | Patient number (n, %) | 28 (19.7%) | 62 (46.6%) | 54 (42.2%) | 35 (27.8%) |
| Mean dose (mg) | 25.71 ± 10.69 | 36.45 ± 21.89 | 43.70 ± 25.50 | 62.29 ± 36.23 | |
| SMG | Patient number (n, %) | 25 (35.2%) | 4 (7.0%) | 3 (5.9%) | 0 |
| Mean dose (mg) | 64.8 ± 20.23 | 65.00 ± 25.17 | 86.67 ± 30.55 | 0 | |
| Torasemide, Oral | at discharge | 3 months | 6 months | 12 months | |
| IMG | Patient number (n, %) | 61 (43.0%) | 81 (60.9%) | 83 (64.8%) | 82 (65.1%) |
| Mean dose (mg) | 8.28 ± 3.28 | 10.43 ± 4.89 | 10.18 ± 4.58 | 10.06 ± 4.87 | |
| SMG | Patient number (n, %) | 2 (2.8%) | 3 (5.3%) | 2 (3.9%) | 2 (3.9%) |
| Mean dose (mg) | 10 | 10 | 10 | 10 | |
| Author (Year) | Study Type | Number of Patients | Patient Characteristics | Methodology | Outcomes | Limitations and Pitfalls |
|---|---|---|---|---|---|---|
| Ohman J., Harjola V-P., 2018 [35] | Pilot, prospective | 20 | Acute HF, E/e’ > 15, pulmonary congestion | E/e’, IVC index, LUS | Decrease of all-cause death and acute HF rehospitalisation. Better decongestion of patients | Small pilot study with unequal population of patients in two groups |
| Rivas-Lasarte M., Alvarez-Garcia J., 2019 (LUS-HF) [36] | Randomized trial | 123 | HF, high NT-proBNP, pulmonary congestion | LUS | LUS-guided strategy reduced hospitalisations and mortality at 6-month follow-up | Treatment protocol was not exclusively based on LUS findings |
| Marini C., Fragasso G., 2020 [37] | Multicentre, randomized | 244 | Chronic HF outpatient | LUS | Mid-term reduction of hospitalisations with LUS-guided managements | Mid-term (90 days) follow-up |
| Pang P., Russel F., 2021 (BLUSHED-AHF) [38] | Multicentre, randomized | 130 | Acute HF | LUS | No benefit of LUS-guided strategy compared to usual care at 90 days. No benefit of B-lines < 15 after 6 h decongestion, however faster resolution of congestion after 48 h | No assessment of long-term rehospitalisation |
| Sisakian H., Shahnazaryan S., 2022 | Prospective | 214 | Advanced chronic HF | E/e, LV filling pressure | Decrease of hospitalisations and mortality in echo-guided group by intensive monitoring at 12-month follow-up | Exclusion of patients with severe valvular disease. Preliminary un-blinded selection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisakian, H.; Shahnazaryan, S.; Pepoyan, S.; Minasyan, A.; Martirosyan, G.; Hovhannisyan, M.; Maghaqelyan, A.; Melik-Stepanyan, S.; Chopikyan, A.; Lopatin, Y. Reduction of Hospitalization and Mortality by Echocardiography-Guided Treatment in Advanced Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 74. https://doi.org/10.3390/jcdd9030074
Sisakian H, Shahnazaryan S, Pepoyan S, Minasyan A, Martirosyan G, Hovhannisyan M, Maghaqelyan A, Melik-Stepanyan S, Chopikyan A, Lopatin Y. Reduction of Hospitalization and Mortality by Echocardiography-Guided Treatment in Advanced Heart Failure. Journal of Cardiovascular Development and Disease. 2022; 9(3):74. https://doi.org/10.3390/jcdd9030074
Chicago/Turabian StyleSisakian, Hamayak, Syuzanna Shahnazaryan, Sergey Pepoyan, Armine Minasyan, Gor Martirosyan, Mariam Hovhannisyan, Ashkhen Maghaqelyan, Sona Melik-Stepanyan, Armine Chopikyan, and Yury Lopatin. 2022. "Reduction of Hospitalization and Mortality by Echocardiography-Guided Treatment in Advanced Heart Failure" Journal of Cardiovascular Development and Disease 9, no. 3: 74. https://doi.org/10.3390/jcdd9030074
APA StyleSisakian, H., Shahnazaryan, S., Pepoyan, S., Minasyan, A., Martirosyan, G., Hovhannisyan, M., Maghaqelyan, A., Melik-Stepanyan, S., Chopikyan, A., & Lopatin, Y. (2022). Reduction of Hospitalization and Mortality by Echocardiography-Guided Treatment in Advanced Heart Failure. Journal of Cardiovascular Development and Disease, 9(3), 74. https://doi.org/10.3390/jcdd9030074






