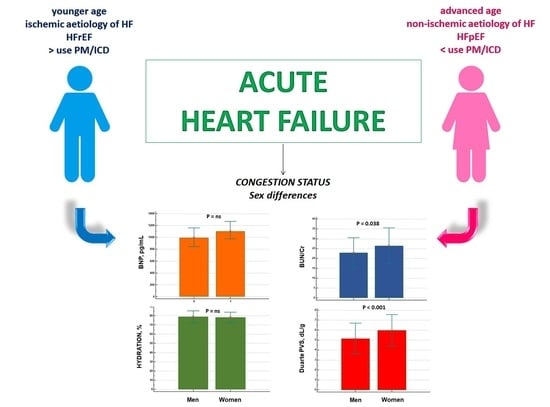
Sex Differences in the Evaluation of Congestion Markers in Patients with Acute Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Estimation of COP, Osmolality and Glomerular Filtration Rate
2.3. Brain Natriuretic Peptide
2.4. BUN to Creatinine Ratio
2.5. Estimated Plasma Volume Status (PVS)
2.6. Bioimpedance Vector Analysis
2.7. Hydra Score
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef] [PubMed]
- Swaraj, S.; Kozor, R.; Arnott, C.; Di Bartolo, B.A.; Figtree, G.A. Heart Failure with Reduced Ejection Fraction-Does Sex Matter? Curr. Heart Fail. Rep. 2021, 18, 345–352. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Yanes, J.M.; Jiménez-García, R.; Hernandez-Barrera, V.; de Miguel-Díez, J.; Muñoz-Rivas, N.; Méndez-Bailón, M.; Pérez-Farinós, N.; López-Herranz, M.; Lopez-de-Andres, A. Sex Differences in the Incidence and Outcomes of Acute Myocardial Infarction in Spain, 2016–2018: A Matched-Pair Analysis. J. Clin. Med. 2021, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Ceia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.; Investigators, E. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Ho, J.E.; Enserro, D.; Brouwers, F.P.; Kizer, J.R.; Shah, S.J.; Psaty, B.M.; Bartz, T.M.; Santhanakrishnan, R.; Lee, D.S.; Chan, C.; et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ. Heart Fail. 2016, 9, e003116. [Google Scholar] [CrossRef]
- Stolfo, D.; Uijl, A.; Vedin, O.; Stromberg, A.; Faxen, U.L.; Rosano, G.M.C.; Sinagra, G.; Dahlstrom, U.; Savarese, G. Sex-based differences in heart failure across the ejection fraction spectrum: Phenotyping, and prognostic and therapeutic implications. JACC Heart Fail. 2019, 7, 505–515. [Google Scholar] [CrossRef]
- Chang, P.P.; Wruck, L.M.; Shahar, E.; Rossi, J.S.; Loehr, L.R.; Russell, S.D.; Agarwal, S.K.; Konety, S.H.; Rodriguez, C.J.; Rosamond, W.D. Trends in hospitalizations and survival of acute decompensated heart failure in four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation 2018, 138, 12–24. [Google Scholar] [CrossRef]
- Scicchitano, P.; Massari, F. Bioimpedance vector analysis in the evaluation of congestion in heart failure. Biomark Med. 2020, 14, 81–85. [Google Scholar] [CrossRef]
- Girerd, N.; Seronde, M.F.; Coiro, S.; Chouihed, T.; Bilbault, P.; Braun, F.; Kenizou, D.; Maillier, B.; Nazeyrollas, P.; Roul, G.; et al. Integrative Assessment of Congestion in Heart Failure Throughout the Patient Journey. JACC Heart Fail. 2018, 6, 273–285. [Google Scholar] [CrossRef]
- Espinosa, B.; Llorens, P.; Gil, V.; Rossello, X.; Jacob, J.; Herrero, P.; Martín-Sánchez, F.J.; Alquézar-Arbé, A.; Masip, J.; Miró, Ò.; et al. Prognosis of acute heart failure based on clinical data of congestion. Rev. Clin. Esp. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Rame, J.E.; Stevenson, L.W.; Dries, D.L. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N. Engl. J. Med. 2001, 345, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.B.; Lippmann, S.J.; DiBello, J.R.; Gorsh, B.; Curtis, L.H.; Sikirica, V.; Hernandez, A.F.; Sprecher, D.L.; Laskey, W.K.; Saini, R.; et al. The Burden of Congestion in Patients Hospitalized with Acute Decompensated Heart Failure. Am. J. Cardiol. 2019, 124, 545–553. [Google Scholar] [CrossRef]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Johnson, W.; Hamilton, M.A.; Fonarow, G.C.; Woo, M.A.; Flavell, C.M.; Creaser, J.A.; Stevenson, L.W. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am. Heart. J. 2000, 140, 840–847. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Evangelista, I.; Nuti, R. Congestion occurrence and evaluation in acute heart failure scenario: Time to reconsider different pathways of volume overload. Heart Fail. Rev. 2020, 25, 119–131. [Google Scholar] [CrossRef]
- Espersen, C.; Campbell, R.T.; Claggett, B.; Lewis, E.F.; Groarke, J.D.; Docherty, K.F.; Lee, M.M.Y.; Lindner, M.; Biering-Sørensen, T.; Solomon, S.D.; et al. Sex differences in congestive markers in patients hospitalized for acute heart failure. ESC Heart Fail. 2021, 8, 1784–1795. [Google Scholar] [CrossRef]
- Dewan, P.; Rørth, R.; Raparelli, V.; Campbell, R.T.; Shen, L.; Jhund, P.S.; Petrie, M.C.; Anand, I.S.; Carson, P.E.; Desai, A.S.; et al. Sex-Related Differences in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e006539. [Google Scholar] [CrossRef]
- Simonavičius, J.; Sanders van-Wijk, S.; Rickenbacher, P.; Maeder, M.T.; Pfister, O.; Kaufmann, B.A.; Pfisterer, M.; Čelutkienė, J.; Puronaitė, R.; Knackstedt, C.; et al. Prognostic Significance of Longitudinal Clinical Congestion Pattern in Chronic Heart Failure: Insights From TIME-CHF Trial. Am. J. Med. 2019, 132, e679–e692. [Google Scholar] [CrossRef]
- Meyer, S.; van der Meer, P.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.A.; Cleland, J.G.; et al. Sex-specific acute heart failure phenotypes and outcomes from PROTECT. Eur. J. Heart Fail. 2013, 15, 1374–1381. [Google Scholar] [CrossRef]
- Pimenta, J.; Paulo, C.; Mascarenhas, J.; Gomes, A.; Azevedo, A.; Rocha-Gonçalves, F.; Bettencourt, P. BNP at discharge in acute heart failure patients: Is it all about volemia? A study using impedance cardiography to assess fluid and hemodynamic status. Int. J. Cardiol. 2010, 145, 209–214. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Valle, R.; Sanasi, M.; Piscopo, A.; Guida, P.; Mastropasqua, F.; Caldarola, P.; Ciccone, M.M. Serum biochemical determinants of peripheral congestion assessed by bioimpedance vector analysis in acute heart failure. Heart Lung 2019, 48, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Purssell, R.A.; Pudek, M.; Brubacher, J.; Abu-Laban, R.B. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann. Emerg. Med. 2001, 38, 653–659. [Google Scholar] [CrossRef]
- Levey, A.S.; Greene, T.; Kusek, J.W.; Beck, G.J. A simplified equation to predict glomerular filtration rate from serum creatinine. J. Am. Soc. Nephrol. 2000, 11, 155A. [Google Scholar]
- Matsue, Y.; van der Meer, P.; Damman, K.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Blood urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart 2017, 103, 407–413. [Google Scholar] [CrossRef]
- Duarte, K.; Monnez, J.M.; Albuisson, E.; Pitt, B.; Zannad, F.; Rossignol, P. Prognostic Value of Estimated Plasma Volume in Heart Failure. JACC Heart Fail. 2015, 3, 886–893. [Google Scholar] [CrossRef]
- Kaplan, A.A. A simple and accurate method for prescribing plasma exchange. ASAIO Trans. 1990, 36, M597–M599. [Google Scholar]
- Fudim, M.; Miller, W.L. Calculated Estimates of Plasma Volume in Patients with Chronic Heart Failure-Comparison with Measured Volumes. J. Card. Fail. 2018, 24, 553–560. [Google Scholar] [CrossRef]
- Massari, F.; Iacoviello, M.; Scicchitano, P.; Mastropasqua, F.; Guida, P.; Riccioni, G.; Speziale, G.; Caldarola, P.; Ciccone, M.M.; Di Somma, S. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung 2016, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Mastropasqua, F.; Guida, P.; De Tommasi, E.; Rizzon, B.; Pontraldolfo, G.; Pitzalis, M.V.; Rizzon, P. Whole-body bioelectrical impedance analysis in patients with chronic heart failure: Reproducibility of the method and effects of body side. Ital. Heart J. 2001, 2, 594–598. [Google Scholar] [PubMed]
- Massari, F.; Scicchitano, P.; Ciccone, M.M.; Caldarola, P.; Aspromonte, N.; Iacoviello, M.; Barro, S.; Pantano, I.; Valle, R. Bioimpedance vector analysis predicts hospital length of stay in acute heart failure. Nutrition 2019, 61, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated with Heart Failure with Preserved and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, K.; Nieves Castro, D.K.; Fu, Q.; Gottlieb, R.A.; Van Eyk, J.E.; Noel Bairey Merz, C. Sex differences in ischemic heart disease and heart failure biomarkers. Biol. Sex Differ. 2018, 9, 43. [Google Scholar] [CrossRef]
- Nakada, Y.; Kawakami, R.; Nakano, T.; Takitsume, A.; Nakagawa, H.; Ueda, T.; Nishida, T.; Onoue, K.; Soeda, T.; Okayama, S.; et al. Sex differences in clinical characteristics and long-term outcome in acute decompensated heart failure patients with preserved and reduced ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H813–H820. [Google Scholar] [CrossRef][Green Version]
- Bassareo, P.P.; Crisafulli, A. Gender Differences in Hemodynamic Regulation and Cardiovascular Adaptations to Dynamic Exercise. Curr Cardiol Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef]
- Shim, C.Y.; Park, S.; Choi, D.; Yang, W.I.; Cho, I.J.; Choi, E.Y.; Chung, N.; Ha, J.W. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J. Am. Coll. Cardiol. 2011, 57, 1226–1233. [Google Scholar] [CrossRef]
- Parrinello, G.; Torres, D.; Testani, J.M.; Almasio, P.L.; Bellanca, M.; Pizzo, G.; Cuttitta, F.; Pinto, A.; Butler, J.; Paterna, S. Blood urea nitrogen to creatinine ratio is associated with congestion and mortality in heart failure patients with renal dysfunction. Intern. Emerg. Med. 2015, 10, 965–972. [Google Scholar] [CrossRef]
- Aronson, D.; Mittleman, M.A.; Burger, A.J. Elevated blood urea nitrogen level a predictor of mortality in patients admitted for decompensated heart failure. Am. J. Med. 2004, 116, 466–473. [Google Scholar] [CrossRef]
- Cleland, J.G.; Chiswell, K.; Teerlink, J.R.; Stevens, S.; Fiuzat, M.; Givertz, M.M.; Davison, B.A.; Mansoor, G.A.; Ponikowski, P.; Voors, A.A.; et al. Predictors of postdischarge outcomes from information acquired shortly after admission for acute heart failure: A report from the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) Study. Circ. Heart Fail. 2014, 7, 76–87. [Google Scholar]
- Testani, J.M.; Coca, S.G.; Shannon, R.P.; Kimmel, S.E.; Cappola, T.P. Influence of renal dysfunction phenotype on mortality in the setting cardiac dysfunction: Analysis of three randomized controlled trials. Eur. J. Heart Fail. 2011, 13, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Nickander, J.; Themudo, R.; Sigfridsson, A.; Xue, H.; Kellman, P.; Ugander, M. Females have higher myocardial perfusion, blood volume and extracellular volume compared to males—an adenosine stress cardiovascular magnetic resonance study. Sci. Rep. 2020, 10, 10380. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Rossignol, P.; Ferreira, J.P.; Aragão, I.; Paku, Y.; Iwasaki, Y.; Watanabe, M.; Fudim, M.; Duarte, K.; Zannad, F.; et al. Prognostic value of estimated plasma volume in acute heart failure in three cohort studies. Clin. Res. Cardiol. 2019, 108, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.R.; Chan, D.; Ng, L.L. Change in plasma volume and prognosis in acute decompensated heart failure: An observational cohort study. J. R Soc. Med. 2016, 109, 337–346. [Google Scholar] [CrossRef]
- Shirakabe, A.; Asai, K.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Goda, H.; Shigihara, S.; Asano, K.; Tani, K.; et al. Prognostic Value of Both Plasma Volume Status and Nutritional Status in Patients with Severely Decompensated Acute Heart Failure. CJC Open 2019, 1, 305–315. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Abe, S.; Sato, Y.; Watanabe, S.; Yokokawa, T.; Miura, S.; Misaka, T.; Sato, T.; Suzuki, S.; Oikawa, M.; et al. Plasma volume status predicts prognosis in patients with acute heart failure syndromes. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 330–338. [Google Scholar] [CrossRef]
- Pellicori, P.; Kaur, K.; Clark, A.L. Fluid Management in Patients with Chronic Heart Failure. Card. Fail. Rev. 2015, 1, 90–95. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef]



| Women (n = 252) | Men (n = 242) | p Level | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, yrs | 79 ± 9 | 77 ± 10 | 0.005 |
| Weight, kg | 69 ± 15 | 74 ± 13 | <0.001 |
| BMI, kg/mq | 28 ± 6 | 27 ± 4 | NS |
| NYHA functional class % | NS | ||
| II | 6 | 9 | |
| III | 35 | 36 | |
| IV | 59 | 55 | |
| Physical examination | |||
| Jugular venous distention (>10 cm), % | 59 | 55 | NS |
| Peripheral oedema, % | 53 | 45 | NS |
| Orthopnoea, % | 39 | 38 | NS |
| Medical history, % | |||
| Coronary artery disease | 65 | 63 | NS |
| Diabetes | 29 | 26 | NS |
| Atrial fibrillation | 57 | 50 | NS |
| PM/ICD | 15 | 27 | 0.001 |
| Prior HF | 65 | 68 | NS |
| Laboratory values | |||
| LVEF, | 45 ± 11 | 38 ± 11 | <0.001 |
| HFrEF, % | 39 | 64 | <0.001 |
| HFmrEF, % | 10 | 10 | NS |
| HFpEF, % | 51 | 26 | <0.001 |
| HFpEF, % | 71 | 47 | <0.001 |
| Haemoglobin, g/dL | 11.4 ± 1.9 | 12.6 ± 2.4 | <0.001 |
| Haematocrit, % | 35 ± 6 | 38 ± 7 | <0.01 |
| Uric acid, mg/dL | 7.4 ± 2.1 | 7.3 ± 2.1 | NS |
| BUN, mg/dL | 38 ± 20 | 36 ± 19 | 0.019 |
| Creatinine, mg/dL | 1.5 ± 1.0 | 1.5 ± 0.9 | NS |
| eCrCl, mL/min per 1.73 m2 | 42 ± 25 | 47 ± 26 | 0.039 |
| Sodium, mmol/L | 139 ± 4 | 139 ± 4 | NS |
| Potassium, mmol/L | 3.9 ± 0.6 | 3.9 ± 0.6 | NS |
| Chloride, mmol/L | 101 ± 5 | 101 ± 6 | NS |
| Serum osmolality, mOsm/kg | 297 ± 21 | 294 ± 12 | NS |
| Serum COP, mmHg | 22 ± 3 | 22 ± 4 | NS |
| Saturation O2, % | 90 ± 6 | 90 ± 6 | NS |
| PaO2, mmHg | 62 ± 13 | 63 ± 12 | NS |
| PaCO2, mmHg | 43 ± 12 | 41 ± 11 | NS |
| pH | 7.43 ± 0.07 | 7.43 ± 0.07 | NS |
| HCO3−, mmol/L | 28 ± 6 | 27 ± 6 | NS |
| Home medications, % | |||
| Diuretic | 83 | 82 | NS |
| Beta-blockers | 56 | 56 | NS |
| ACE inhibitors/ ARBs | 49 | 52 | NS |
| MRAs | 60 | 59 | NS |
| Amiodarone | 28 | 30 | NS |
| Digitalis | 20 | 19 | NS |
| Ivabradine | 5 | 7 | NS |
| Calcium channel blockers | 24 | 23 | NS |
| HFrEF | HFmrEF | HFpEF | p Level | |
|---|---|---|---|---|
| BNP, pg/mL | ||||
| Women | 1708 ± 1366 * | 983 ± 874 | 1084 ± 977 | <0.001 |
| Men | 2147 ± 1349 * | 1441± 1236 | 1139 ± 960 | <0.001 |
| Hydration, % | ||||
| Women | 78 ± 6.0 | 76 ± 3.6 | 79 ± 5.9 | NS |
| Men | 78 ± 5.7 | 80 ± 7.5 | 80 ± 7.2 | NS |
| BUN/Cr | ||||
| Women | 25 ± 9.3 | 24 ± 7.1 | 27 ± 8.9 | NS |
| Men | 23 ± 7.0 | 23 ± 9.3 | 22 ± 8.1 | NS |
| Duarte PVS, dL/gr | ||||
| Women | 6.0 ± 1.4 | 5.6 ± 1.1 | 6.1 ± 1.7 | NS |
| Men | 5.1 ± 1.5 | 5.4 ± 1.3 | 5.3 ± 1.6 | NS |
| Kaplan–Hakim PVS, % | ||||
| Women | 9.9 ± 14 | 3.5 ± 13 | 7.2 ± 13 | NS |
| Men | −7.8 ± 11 | −6.4 ± 12 | −6.6 ± 12 | NS |
| Hydra Score | ||||
| Women | 3.2 ± 1.0 | 2.7 ± 1.5 | 2.9 ± 1.4 | NS |
| Men | 2.1 ± 1.3 | 2.4 ± 1.5 | 2.2 ± 1.4 | NS |
| Variables | Odds Ratio (95% CI) | p | B Coefficient | SE | Wald |
|---|---|---|---|---|---|
| Age, year | 0.98 (0.96–1.01) | NS | |||
| LVEF, % | 1.05 (1.04–1.07) | <0.0001 | 0.055 | 0.009 | 31.3 |
| BUN/Cr ratio | 1.06 (1.03–1.09) | <0.0001 | 0.063 | 0.015 | 17.1 |
| GFR, mL/min | 0.98 (0.97–0.99) | =0.005 | −0.013 | 0.004 | 7.9 |
| KH-PVS, % | 1.10 (1.08–1.13) | <0.0001 | 0.099 | 0.011 | 74.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Paolillo, C.; De Palo, M.; Potenza, A.; Abruzzese, S.; Basile, M.; Cannito, A.; Tangorra, M.; Guida, P.; Caldarola, P.; et al. Sex Differences in the Evaluation of Congestion Markers in Patients with Acute Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 67. https://doi.org/10.3390/jcdd9030067
Scicchitano P, Paolillo C, De Palo M, Potenza A, Abruzzese S, Basile M, Cannito A, Tangorra M, Guida P, Caldarola P, et al. Sex Differences in the Evaluation of Congestion Markers in Patients with Acute Heart Failure. Journal of Cardiovascular Development and Disease. 2022; 9(3):67. https://doi.org/10.3390/jcdd9030067
Chicago/Turabian StyleScicchitano, Pietro, Claudio Paolillo, Micaela De Palo, Angela Potenza, Silvia Abruzzese, Marco Basile, Antonia Cannito, Maria Tangorra, Piero Guida, Pasquale Caldarola, and et al. 2022. "Sex Differences in the Evaluation of Congestion Markers in Patients with Acute Heart Failure" Journal of Cardiovascular Development and Disease 9, no. 3: 67. https://doi.org/10.3390/jcdd9030067
APA StyleScicchitano, P., Paolillo, C., De Palo, M., Potenza, A., Abruzzese, S., Basile, M., Cannito, A., Tangorra, M., Guida, P., Caldarola, P., Ciccone, M. M., & Massari, F. (2022). Sex Differences in the Evaluation of Congestion Markers in Patients with Acute Heart Failure. Journal of Cardiovascular Development and Disease, 9(3), 67. https://doi.org/10.3390/jcdd9030067