Desynchronization Strain Patterns and Contractility in Left Bundle Branch Block through Computer Model Simulation
Abstract
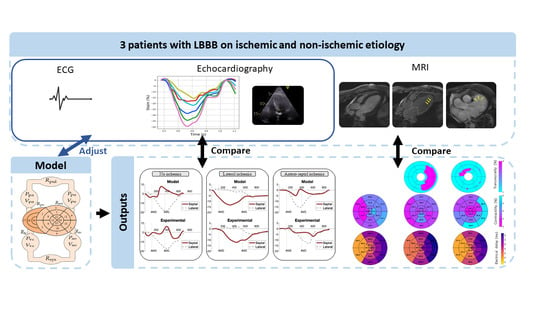
:1. Introduction
2. Materials and Methods
2.1. Experimental Data
2.1.1. Study Population
2.1.2. Echocardiography
2.1.3. Cardiac Magnetic Resonance Imaging
2.2. Computational Model
2.2.1. Electrical Activity
2.2.2. Right and Left Atria
2.2.3. Multi-Segment Representation of the Right and the Left Ventricles
2.2.4. Systemic and Pulmonary Circulations
3. Results
3.1. Baseline Model Simulations
3.2. LBBB Strain Patterns
4. Discussion
4.1. Strain Patterns and Scar Localization in LBBB
4.2. Modelling and Personalized Medicine
4.3. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the predictors of response to crt (prospect) trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lumens, J.; Tayal, B.; Walmsley, J.; Delgado-Montero, A.; Huntjens, P.R.; Schwartzman, D.; Althouse, A.D.; Delhaas, T.; Prinzen, F.W.; Gorcsan, J. Differentiating Electromechanical from Non-Electrical Substrates of Mechanical Discoordination to Identify Responders to Cardiac Resynchronization Therapy. Circ. Cardiovasc. Imaging 2015, 8, e003744. [Google Scholar] [CrossRef] [Green Version]
- Le Rolle, V.; Galli, E.; Danan, D.; El Houari, K.; Hubert, A.; Donal, E.; Hernández, A.I. Sensitivity Analysis of a Left Ventricle Model in the Context of Intraventricular Dyssynchrony. Acta Biotheor. 2020, 68, 45–59. [Google Scholar] [CrossRef]
- Constantino, J.; Hu, Y.; Trayanova, N.A. A computational approach to understanding the cardiac electromechanical activation sequence in the normal and failing heart, with translation to the clinical practice of CRT. Prog. Biophys. Mol. Biol. 2012, 110, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Crozier, A.; Blazevic, B.; Lamata, P.; Plank, G.; Ginks, M.; Duckett, S.; Sohal, M.; Shetty, A.; Rinaldi, C.A.; Razavi, R.; et al. The relative role of patient physiology and device optimisation in cardiac resynchronisation therapy: A computational modelling study. J. Mol. Cell. Cardiol. 2016, 96, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Van Everdingen, W.M.; Walmsley, J.; Cramer, M.J.; van Hagen, I.; De Boeck, B.W.L.; Meine, M.; Delhaas, T.; Doevendans, P.A.; Prinzen, F.W.; Lumens, J.; et al. Echocardiographic Prediction of Cardiac Resynchronization Therapy Response Requires Analysis of Both Mechanical Dyssynchrony and Right Ventricular Function: A Combined Analysis of Patient Data and Computer Simulations. J. Am. Soc. Echocardiogr. 2017, 30, 1012–1020. [Google Scholar] [CrossRef]
- Lumens, J.; Leenders, G.E.; Cramer, M.J.; De Boeck, B.W.L.; Doevendans, P.A.; Prinzen, F.W.; Delhaas, T. Mechanistic evaluation of echocardiographic dyssynchrony indices patient data combined with multiscale computer simulations. Circ. Cardiovasc. Imaging 2012, 5, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, G.; Lerolle, V.; Loiodice, C.; Amblard, A.; Pepin, J.-L.; Hernandez, A.I. Modeling patient-specific desaturation patterns in sleep apnea. IEEE Trans. Biomed. Eng. 2021. [Google Scholar] [CrossRef]
- Gallard, A.; Hubert, A.; Smiseth, O.; Voigt, J.U.; Le Rolle, V.; Leclercq, C.; Bidaut, A.; Galli, E.; Donal, E.; Hernandez, A.I. Prediction of response to cardiac resynchronization therapy using a multi-feature learning method. Int. J. Cardiovasc. Imaging 2020, 37, 989–998. [Google Scholar] [CrossRef]
- Le Rolle, V.; Hernández, A.I.; Richard, P.Y.; Donal, E.; Carrault, G. Model-based analysis of myocardial strain data acquired by tissue Doppler imaging. Artif. Intell. Med. 2008, 44, 201–219. [Google Scholar] [CrossRef] [Green Version]
- Owashi, K.; Galli, E.; Hubert, A.; Donal, E.; Hernandez, A.; Le Rolle, V. Patients-Specific Analysis of Myocardial Strains in Left Bundle Branch Block Based on Computational Models. In Proceedings of the 2020 Computing in Cardiology, Rimini, Italy, 13–16 September 2020. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Victor, M.A.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Kachenoura, N.; Redheuil, A.; Herment, A.; Mousseaux, E.; Frouin, F. Robust assessment of the transmural extent of myocardial infarction in late gadolinium-enhanced MRI studies using appropriate angular and circumferential subdivision of the myocardium. Eur. Radiol. 2008, 18, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Baron, N.; Kachenoura, N.; Cluzel, P.; Frouin, F.; Herment, A.; Grenier, P.; Montalescot, G.; Beygui, F. Comparison of various methods for quantitative evaluation of myocardial infarct volume from magnetic resonance delayed enhancement data. Int. J. Cardiol. 2013, 167, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Le Rolle, V.; Romero, D.; Béhar, N.; Gomis, P.; Mabo, P.; Hernández, A.I. Model-based analysis of the autonomic response to head-up tilt testing in Brugada syndrome. Comput. Biol. Med. 2018, 103, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Owashi, K.P.; Hubert, A.; Galli, E.; Donal, E.; Hernández, A.I.; Rolle, V.L. Model-based estimation of left ventricular pressure and myocardial work in aortic stenosis. PLoS ONE 2020, 15, e0229609. [Google Scholar] [CrossRef] [Green Version]
- Rolle, V.L.; Hernández, A.I.; Richard, P.-Y.; Donal, E.; Carrault, G. A Tissue-Level Model of the Left Ventricle for the Analysis of Regional Myocardial Function. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007. [Google Scholar] [CrossRef] [Green Version]
- Hernández, A.I.; Carrault, G.; Mora, F. Model-based interpretation of cardiac beats by evolutionary algorithms. Comput. Cardiol. 2002, 29, 93–96. [Google Scholar] [CrossRef]
- Ve Koon, K.T.; Thebault, C.; Le Rolle, V.; Donal, E.; Hernández, A.I. Atrioventricular delay optimization in cardiac resynchronization therapy assessed by a computer model. Comput. Cardiol. 2010, 37, 333–336. [Google Scholar]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.J.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J. Cardiovasc. Magn. Reson. 2002, 4, 203–210. [Google Scholar] [CrossRef]
- Rolle, V.L.; Ojeda, D.; Hernández, A.I. Embedding a cardiac pulsatile model into an integrated model of the cardiovascular regulation for heart failure followup. IEEE Trans. Biomed. Eng. 2011, 58, 2982–2986. [Google Scholar] [CrossRef] [Green Version]
- Parsai, C.; Bijnens, B.; Sutherland, G.R.; Baltabaeva, A.; Claus, P.; Marciniak, M.; Paul, V.; Scheffer, M.; Donal, E.; Derumeaux, G.; et al. Toward understanding response to cardiac resynchronization therapy: Left ventricular dyssynchrony is only one of multiple mechanisms. Eur. Heart J. 2009, 30, 940–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovic, I.; Prinz, C.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Belmans, A.; Neskovic, A.N.; et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). Eur. Heart J. Cardiovasc. Imaging 2016, 17, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Leenders, G.E.; Lumens, J.; Cramer, M.J.; Delhaas, T.; Prinzen, F.W. Septal Deformation Patterns Delineate Mechanical Dyssynchrony and Regional Differences in Contractility Analysis of Patient Data Using a Computer Model. Circ. Heart Fail. 2011, 5, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Suga, H.; Sagawa, K.; Shoukas, A.A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 1973, 32, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Gorcsan, J.; Anderson, C.P.; Tayal, B.; Sugahara, M.; Walmsley, J.; Starling, R.C.; Lumens, J. Systolic Stretch Characterizes the Electromechanical Substrate Responsive to Cardiac Resynchronization Therapy. JACC Cardiovasc. Imaging 2019, 12, 1741–1752. [Google Scholar] [CrossRef]
- Bohbot, Y.; De Meester de Ravenstein, C.; Chadha, G.; Rusinaru, D.; Belkhir, K.; Trouillet, C.; Pasquet, A.; Marechaux, S.; Vanoverschelde, J.L.; Tribouilloy, C. Relationship Between Left Ventricular Ejection Fraction and Mortality in Asymptomatic and Minimally Symptomatic Patients With Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 38–48. [Google Scholar] [CrossRef]
- Aalen, J.M.; Remme, E.W.; Larsen, C.K.; Andersen, O.S.; Krogh, M.; Duchenne, J.; Hopp, E.; Ross, S.; Beela, A.S.; Kongsgaard, E.; et al. Mechanism of Abnormal Septal Motion in Left Bundle Branch Block: Role of Left Ventricular Wall Interactions and Myocardial Scar. JACC Cardiovasc. Imaging 2019, 12, 2402–2413. [Google Scholar] [CrossRef]
- Khan, F.Z.; Virdee, M.S.; Palmer, C.R.; Pugh, P.J.; O’Halloran, D.; Elsik, M.; Read, P.A.; Begley, D.; Fynn, S.P.; Dutka, D.P. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: The TARGET study: A randomized, controlled trial. J. Am. Coll. Cardiol. 2012, 59, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef]
- Gjesdal, O.; Remme, E.W.; Opdahl, A.; Skulstad, H.; Russell, K.; Kongsgaard, E.; Edvardsen, T.; Smiseth, O.A. Mechanisms of abnormal systolic motion of the interventricular septum during left bundle-branch block. Circ. Cardiovasc. Imaging 2011, 4, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Willemen, E.; Schreurs, R.; Huntjens, P.R.; Strik, M.; Plank, G.; Vigmond, E.; Walmsley, J.; Vernooy, K.; Delhaas, T.; Prinzen, F.W.; et al. The left and right ventricles respond differently to variation of pacing delays in cardiac resynchronization therapy: A combined experimental-computational approach. Front. Physiol. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Age (years) | Etiology | QRS Width (ms) | LVEF (%) | |
|---|---|---|---|---|
| Patient 1 | 77 | Non-ischemic cardiomyopathy | 136 | 33 |
| Patient 2 | 80 | Ischemic cardiomyopathy—lateral scar | 157 | 24 |
| Patient 3 | 82 | Ischemic cardiomyopathy—antero-septal scar | 152 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owashi, K.; Taconné, M.; Courtial, N.; Simon, A.; Garreau, M.; Hernandez, A.; Donal, E.; Le Rolle, V.; Galli, E. Desynchronization Strain Patterns and Contractility in Left Bundle Branch Block through Computer Model Simulation. J. Cardiovasc. Dev. Dis. 2022, 9, 53. https://doi.org/10.3390/jcdd9020053
Owashi K, Taconné M, Courtial N, Simon A, Garreau M, Hernandez A, Donal E, Le Rolle V, Galli E. Desynchronization Strain Patterns and Contractility in Left Bundle Branch Block through Computer Model Simulation. Journal of Cardiovascular Development and Disease. 2022; 9(2):53. https://doi.org/10.3390/jcdd9020053
Chicago/Turabian StyleOwashi, Kimi, Marion Taconné, Nicolas Courtial, Antoine Simon, Mireille Garreau, Alfredo Hernandez, Erwan Donal, Virginie Le Rolle, and Elena Galli. 2022. "Desynchronization Strain Patterns and Contractility in Left Bundle Branch Block through Computer Model Simulation" Journal of Cardiovascular Development and Disease 9, no. 2: 53. https://doi.org/10.3390/jcdd9020053
APA StyleOwashi, K., Taconné, M., Courtial, N., Simon, A., Garreau, M., Hernandez, A., Donal, E., Le Rolle, V., & Galli, E. (2022). Desynchronization Strain Patterns and Contractility in Left Bundle Branch Block through Computer Model Simulation. Journal of Cardiovascular Development and Disease, 9(2), 53. https://doi.org/10.3390/jcdd9020053