A Propensity Score Analysis of Early and Long-Term Outcomes of Retrograde Arterial Perfusion for Endoscopic and Minimally Invasive Heart Valve Surgery in Both Young and Elderly Patients
Abstract
1. Introduction
2. Materials and Methods
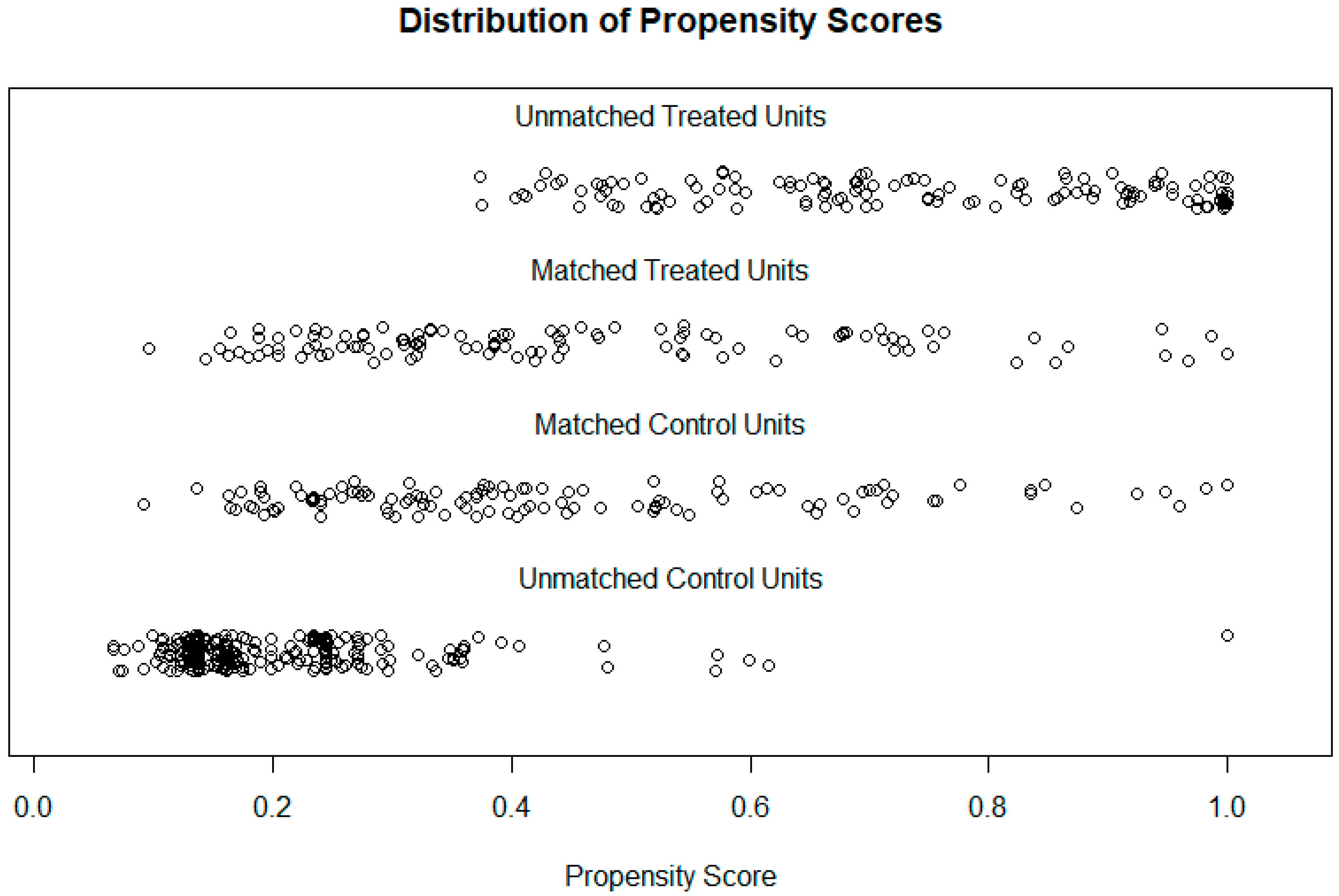
3. Surgical Technique
4. Data Analysis
5. Results
5.1. Patient Characteristics and Operative Data
5.2. Risk-Adjusted In-Hospital Outcomes
5.3. Primary Endpoint: Post-Operative Complications
5.4. Secondary Endpoint: Survival Rates
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soltesz, E.G.; Cohn, L.H. Minimally invasive valve surgery. Cardiol. Rev. 2007, 15, 109–115. Available online: https://journals.lww.com/cardiologyinreview/Fulltext/2007/05000/Minimally_Invasive_Valve_Surgery.1.aspx (accessed on 16 August 2021). [CrossRef] [PubMed][Green Version]
- Cheng, D.C.; Martin, J.; Lal, A.; Diegeler, A.; Folliguet, T.A.; Nifong, L.W.; Perier, P.; Raanani, E.; Smith, J.M.; Seeburger, J.; et al. Minimally invasive versus conventional open mitral valve surgery: A meta-analysis and systematic review. Innovations 2011, 6, 84–103. Available online: https://journals.sagepub.com/doi/10.1097/IMI.0b013e3182167feb?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed on 16 August 2021). [CrossRef] [PubMed]
- Glower, D.D.; Landolfo, K.P.; Clements, F.; Debruijn, N.P.; Stafford-Smith, M.; Smith, P.K.; Duhaylongsod, F. Mitral valve operation via Port Access versus median sternotomy. Eur. J. Cardio-Thorac. Surg. 1998, 14, S143–S147. Available online: https://academic.oup.com/ejcts/article/14/Supplement_1/S143/408260 (accessed on 16 December 2021). [CrossRef]
- Chitwood, J.; Wixon, C.L.; Elbeery, J.R.; Moran, J.F.; Chapman, W.H.H.; Lust, R.M. Video-assisted minimally invasive mitral valve surgery. J. Thorac. Cardiovasc. Surg. 1997, 114, 773–782. [Google Scholar] [CrossRef]
- Felger, J.E.; Chitwood, W.R.; Nifong, L.W.; Holbert, D. Evolution of mitral valve surgery: Toward a totally endoscopic approach. Ann. Thorac. Surg. 2001, 72, 1203–1209. [Google Scholar] [CrossRef]
- Grossi, E.A.; Loulmet, D.F.; Schwartz, C.F.; Ursomanno, P.; Zias, E.A.; Dellis, S.L.; Galloway, A.C. Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J. Thorac. Cardiovasc. Surg. 2012, 143, S68–S70. Available online: http://www.jtcvs.org/article/S0022522312000128/fulltext (accessed on 16 August 2021). [CrossRef]
- Grossi, E.A.; Galloway, A.C.; LaPietra, A.; Ribakove, G.H.; Ursomanno, P.; Delianides, J.; Culliford, A.T.; Bizekis, C.; Esposito, R.A.; Baumann, F.G.; et al. Minimally invasive mitral valve surgery: A 6-year experience with 714 patients. Ann. Thorac. Surg. 2002, 74, 660–664. Available online: http://www.annalsthoracicsurgery.org/article/S0003497502037542/fulltext (accessed on 16 August 2021). [CrossRef]
- Greco, E.; Mestres, C.A.; Cartañá, R.; Pomar, J.L. Video-Assisted Cardioscopy for Removal of Primary Left Ventricular Myxoma. Eur. J. Cardio-Thorac. Surg. 1999, 16, 677–678. [Google Scholar] [CrossRef]
- Belluschi, I.; Lapenna, E.; Blasio, A.; del Forno, B.; Giacomini, A.; Ruggeri, S.; Schiavi, D.; Castiglioni, A.; Alfieri, O.; de Bonis, M. Excellent Long-Term Results with Minimally Invasive Edge-to-Edge Repair in Myxomatous Degenerative Mitral Valve Regurgitation. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 28–34. [Google Scholar] [CrossRef]
- Grant, S.W.; Hickey, G.L.; Modi, P.; Hunter, S.; Akowuah, E.; Zacharias, J. Propensity-Matched Analysis of Minimally Invasive Approach versus Sternotomy for Mitral Valve Surgery. Heart 2019, 105, 783–789. [Google Scholar] [CrossRef]
- Glauber, M.; Miceli, A.; Canarutto, D.; Lio, A.; Murzi, M.; Gilmanov, D.; Ferrarini, M.; Farneti, P.A.; Quaini, E.L.; Solinas, M. Early and Long-Term Outcomes of Minimally Invasive Mitral Valve Surgery through Right Minithoracotomy: A 10-Year Experience in 1604 Patients. J. Cardiothorac. Surg. 2015, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, J.; Greco, E. The Treatment of Mitral Valve Disease—The Only Thing Constant Is Change. Biomedicines 2021, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Kastengren, M.; Svenarud, P.; Ahlsson, A.; Dalén, M. Minimally Invasive Mitral Valve Surgery Is Associated with a Low Rate of Complications. J. Intern. Med. 2019, 286, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Murzi, M.; Cerillo, A.G.; Miceli, A.; Bevilacqua, S.; Kallushi, E.; Farneti, P.; Solinas, M.; Glauber, M. Antegrade and Retrograde Arterial Perfusion Strategy in Minimally Invasive Mitral-Valve Surgery: A Propensity Score Analysis on 1280 Patients. Eur. J. Cardio-Thorac. Surg. 2013, 43, e167–e172. [Google Scholar] [CrossRef]
- Gammie, J.S.; Zhao, Y.; Peterson, E.D.; O’Brien, S.M.; Rankin, J.S.; Griffith, B.P. Less-Invasive Mitral Valve Operations: Trends and Outcomes From The Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann. Thorac. Surg. 2010, 90, 1401–1410.e1. Available online: http://www.annalsthoracicsurgery.org/article/S000349751001218X/fulltext (accessed on 16 October 2021). [CrossRef]
- Modi, P.; Chitwood, W.R., Jr. Retrograde femoral arterial perfusion and stroke risk during minimally invasive mitral valve surgery: Is there cause for concern? Ann. Cardiothorac. Surg. 2013, 2, E1. [Google Scholar]
- Crooke, G.A.; Schwartz, C.F.; Ribakove, G.H.; Ursomanno, P.; Gogoladze, G.; Culliford, A.T.; Galloway, A.C.; Grossi, E.A. Retrograde Arterial Perfusion, Not Incision Location, Significantly Increases the Risk of Stroke in Reoperative Mitral Valve Procedures. Ann. Thorac. Surg. 2010, 89, 723–730. Available online: http://www.annalsthoracicsurgery.org/article/S0003497509023935/fulltext (accessed on 16 September 2021). [CrossRef]
- Moodley, S.; Schoenhagen, P.; Gillinov, A.M.; Mihaljevic, T.; Flamm, S.D.; Griffin, B.P.; Desai, M.Y. Preoperative multidetector computed tomograpy angiography for planning of minimally invasive robotic mitral valve surgery: Impact on decision making. J. Thorac. Cardiovasc. Surg. 2013, 146, 262–268.e1. Available online: http://www.jtcvs.org/article/S0022522312007313/fulltext (accessed on 16 December 2021). [CrossRef][Green Version]
- Holzhey, D.M.; Seeburger, J.; Misfeld, M.; Borger, M.A.; Mohr, F.W. Learning minimally invasive mitral valve surgery: A cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013, 128, 483–491. Available online: https://pubmed.ncbi.nlm.nih.gov/23804253/ (accessed on 16 September 2021). [CrossRef]
- Tabata, M.; Cohn, L.H. Minimally invasive mitral valve repair with and without robotic technology in the elderly. Am. J. Geriatr. Cardiol. 2006, 15, 306–310. Available online: https://pubmed.ncbi.nlm.nih.gov/16957450/ (accessed on 16 September 2021). [CrossRef]
- Grossi, E.A.; Galloway, A.C.; Ribakove, G.H.; Buttenheim, P.M.; Esposito, R.; Baumann, F.G.; Colvin, S.B. Minimally invasive port access surgery reduces operative morbidity for valve replacement in the elderly. Heart Surg. Forum 1999, 2, 212–215. Available online: https://europepmc.org/article/med/11276477 (accessed on 16 August 2021). [PubMed]
- Schneider, F.; Onnasch, J.F.; Falk, V.; Walther, T.; Autschbach, R.; Mohr, F.W. Cerebral microemboli during minimally invasive and conventional mitral valve operations. Ann. Thorac. Surg. 2000, 70, 1094–1097. Available online: https://pubmed.ncbi.nlm.nih.gov/11016385/ (accessed on 16 August 2021). [CrossRef]
| Variable | Elderly Patients Age ≥ 70 (n = 241) | Adult Patients Age < 70 (n = 355) | p-Value |
|---|---|---|---|
| Age | 76.0 [72, 79] | 58 [47, 65] | p < 0.001 |
| Female | 105 (43.6%) | 116 (32.7%) | p = 0.009 |
| BMI (Body Mass Index) | 26.2 [23.3, 29.2] | 26.5 [23.5, 29.5] | p = 0.41 |
| DM (Diabetes Mellitus) | 25 (10.3%) | 21 (5.9%) | p = 0.065 |
| HTN | 148 (61.4%) | 132 (37.8%) | p < 0.001 |
| Peripheral vascular disease | 13 (5.4%) | 13 (3.7%) | p = 0.42 |
| COPD | 33 (13.7%) | 50 (14.1%) | p = 0.97 |
| CKD (chronic kidney disease) | 4 (1.7%) | 2 (0.6%) | 0.37 |
| Previous stroke: TIA (Transient Ischaemic Attack) CVA (cerebrovascular accident) | 27 (11.2%) 9 (3.7%) | 16 (4.5%) 12 (3.4%) | p = 0.008 |
| Pre-op AF | 55 (22.8%) | 27 (7.6%) | p < 0.001 |
| Urgent operation | 16 (6.6%) | 37 (10.4%) | p = 0.15 |
| NYHA class III/IV | 129 (57.5%) | 151 (46.5%) | p = 0.011 |
| Ejection fraction < 30% | 6 (2.5%) | 10 (2.8%) | p > 0.99 |
| Logistic Euro Score | 7.01 [4.25, 12.25] | 2.4 [1.51, 4.38] | p < 0.001 |
| Variable | Elderly Patients >= 70 | Adult Patients < 70 | p Value |
|---|---|---|---|
| CPB time (mins) | 146 [127, 172] | 152 [131, 185] | p = 0.036 |
| Aortic clamp time | 96 [75, 111] | 103 [82, 127] | p < 0.001 |
| Mitral valve procedure +/− Tricuspid valve procedure +/− other | 177 (73.4%) | 291 (82%) | p = 0.017 |
| Other cardiac procedure +/− valve | 0 (0%) | 23 (6.5%) | |
| Aortic valve procedure | 64 (26.6%) | 61 (17.2%) | p = 0.006 |
| AF ablation | 56 (23.2%) | 40 (11.3%) | p < 0.001 |
| Conversion to sternotomy | 8 (3.3%) | 16 (4.5%) | p = 0.61 |
| Variable | Elderly Patients Age ≥ 70 (n = 112) | Adult Patients Age < 70 (n = 112) | p-Value |
|---|---|---|---|
| Age | 75.0 [71, 79] | 64.0 [55, 68] | p < 0.001 |
| Female | 69 (38.4%) | 57 (49.1%) | p = 0.14 |
| BMI | 26.4 [23.6, 29.2] | 26.4 [23.2, 29.8] | p = 0.85 |
| DM | 6 (5.4%) | 6 (5.4%) | p > 0.99 |
| HTN | 57 (50.9%) | 57 (50.9%) | p > 0.99 |
| Peripheral vascular disease | 3 (2.7%) | 8 (7.1%) | p = 0.22 |
| COPD | 14 (12.5%) | 12 (10.7%) | p = 0.83 |
| CKD | 1 (0.9%) | 2 (1.8%) | p > 0.99 |
| Previous stroke: TIA CVA | 9 (8.0%) 3 (2.7%) | 8 (7.1%) 5 (4.5%) | p = 0.75 |
| Pre-op AF | 17 (15.2%) | 17 (15.2%) | p > 0.99 |
| Urgent operation | 5 (4.5%) | 11 (9.8%) | p = 0.19 |
| NYHA class III/IV | 55 (49.1%) | 52 (46.4%) | p = 0.79 |
| Ejection fraction < 30% | 1 (0.9%) | 7 (6.2%) | p = 0.07 |
| Logistic Euro Score | 4.83 [3.51, 7.46] | 4.70 [3.19, 7.17] | p = 0.36 |
| Variable | Elderly Patients Age ≥ 70 (n = 112) | Adult Patients Age < 70 (n = 112) | p-Value |
|---|---|---|---|
| Stroke: transient permanent | 1 (0.9%) 2 (1.8%) | 3 (2.7%) 2 (1.8%) | p = 0.60 |
| MI | 0 | 0 | p > 0.99 |
| New post-operative AF | 6 (5.4%) | 2 (1.8%) | p = 0.66 |
| Renal failure | 4 (3.6%) | 0 | p = 0.12 |
| Re-operation (any purpose) | 4 (3.6%) | 4 (3.6%) | p > 0.99 |
| Pulmonary complications | 16 (14.3%) | 11 (9.8%) | p = 0.41 |
| ICU LOS (days) | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | p = 0.77 |
| Inotropes | 30 (26.8%) | 34 (30.4%) | p = 0.66 |
| GI complications | 4 (3.6%) | 2 (1.8%) | p = 0.68 |
| Required blood transfusion | 7 (7.1%) | 6 (6.2%) | p = 0.87 |
| Extubation > 12 h | 10 (8.9%) | 14 (12.5%) | p = 0.52 |
| Duration of hospitalisation (days) | 7 [5, 9] | 6 [5, 8] | p = 0.38 |
| Discharge destination (home) | 105 (93.8%) | 107 (95.5%) | p = 0.42 |
| Reintervention | 1 (0.9%) | 3 (2.7%) | p = 0.60 |
| Variable | Elderly Patients Age ≥ 70 | Adult Patients Age < 70 | p-Value | The Relative Risk of Death for Elderly Patients over Adult (CI) |
|---|---|---|---|---|
| Mortality | 4 (3.6%) | 1 (0.9%) | p = 0.37 | 4.0 [0.45, 35.2] |
| One year survival | 104/110 (94.5%) | 99/101 (98.0%) | p = 0.28 | 2.8 [0.57, 13.3] |
| 3 years survival | 76/88 (86.4%) | 85/90 (94.4%) | p = 0.078 | 1.7 [0.58, 5.0] |
| Time Period (Postop) | Mean Restricted Survival in Years (95% Confidence Interval) | Difference in RMST | |
|---|---|---|---|
| Elderly (Age ≥ 70) | Adult (Age < 70) | ||
| 1 year | 0.96 [0.92, 0.99] | 0.98 [0.96, 1.00] | 0.02 [−0.07, 0.02] |
| 3 years | 2.78 [2.65, 2.91] | 2.90 [2.81, 2.99] | 0.12 [−0.27, 0.04] |
| 5 years | 4.46 [4.21, 4.71] | 4.75 [4.57, 4.93] | 0.29 [−0.60, 0.02] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhassan, H.; Abdelbar, A.; Taylor, R.; Laskawski, G.; Saravanan, P.; Knowles, A.; Zacharias, J. A Propensity Score Analysis of Early and Long-Term Outcomes of Retrograde Arterial Perfusion for Endoscopic and Minimally Invasive Heart Valve Surgery in Both Young and Elderly Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 44. https://doi.org/10.3390/jcdd9020044
Elhassan H, Abdelbar A, Taylor R, Laskawski G, Saravanan P, Knowles A, Zacharias J. A Propensity Score Analysis of Early and Long-Term Outcomes of Retrograde Arterial Perfusion for Endoscopic and Minimally Invasive Heart Valve Surgery in Both Young and Elderly Patients. Journal of Cardiovascular Development and Disease. 2022; 9(2):44. https://doi.org/10.3390/jcdd9020044
Chicago/Turabian StyleElhassan, Hind, Abdelrahman Abdelbar, Rebecca Taylor, Grzegorz Laskawski, Palanikumar Saravanan, Andrew Knowles, and Joseph Zacharias. 2022. "A Propensity Score Analysis of Early and Long-Term Outcomes of Retrograde Arterial Perfusion for Endoscopic and Minimally Invasive Heart Valve Surgery in Both Young and Elderly Patients" Journal of Cardiovascular Development and Disease 9, no. 2: 44. https://doi.org/10.3390/jcdd9020044
APA StyleElhassan, H., Abdelbar, A., Taylor, R., Laskawski, G., Saravanan, P., Knowles, A., & Zacharias, J. (2022). A Propensity Score Analysis of Early and Long-Term Outcomes of Retrograde Arterial Perfusion for Endoscopic and Minimally Invasive Heart Valve Surgery in Both Young and Elderly Patients. Journal of Cardiovascular Development and Disease, 9(2), 44. https://doi.org/10.3390/jcdd9020044






