Barth Syndrome: Psychosocial Impact and Quality of Life Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Health-Related Quality of Life
2.3. Depression and Anxiety
2.4. Analysis Plan
3. Results
3.1. Descriptive Statistics
3.2. Pearson Correlations
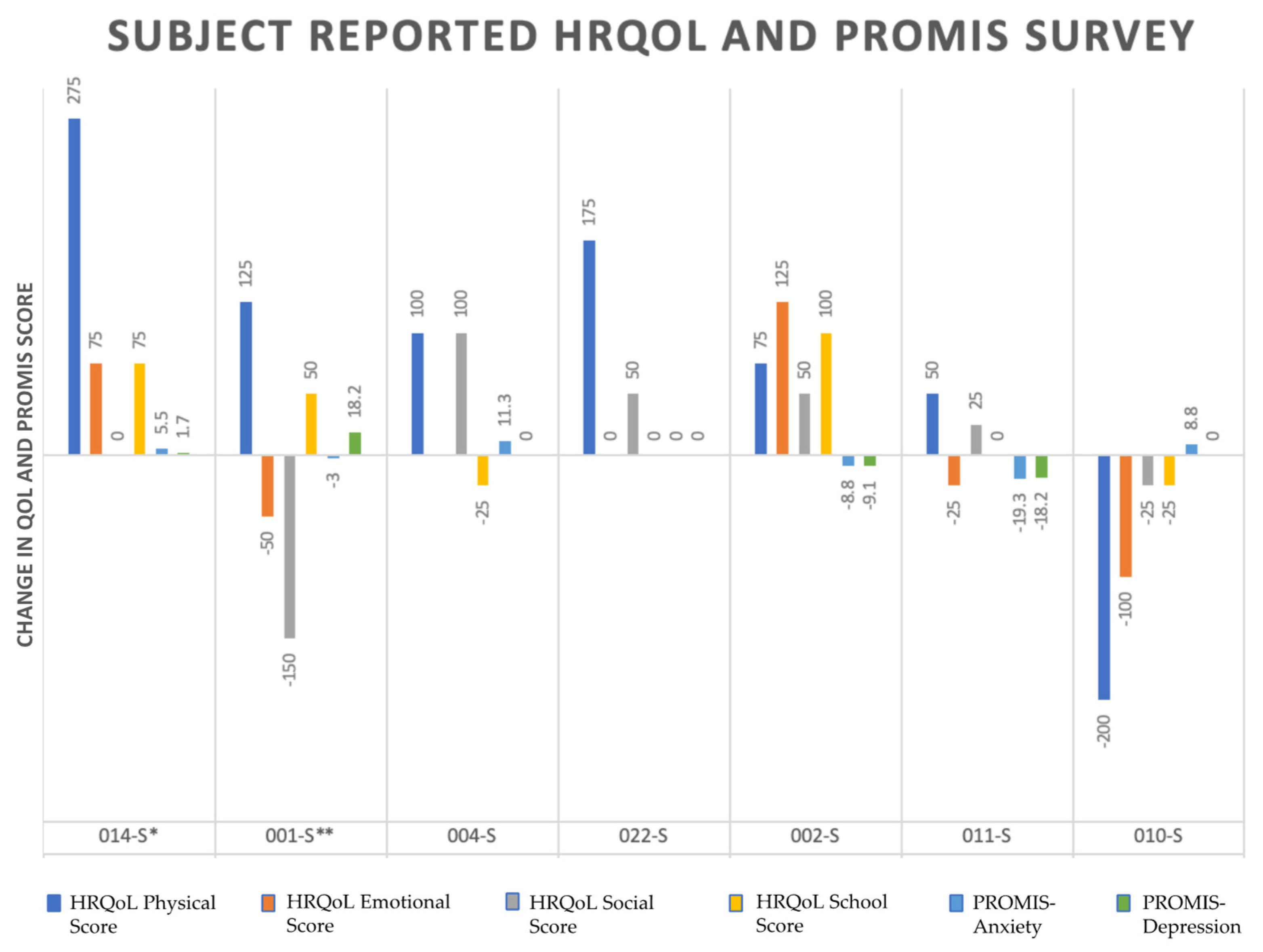
3.3. Score Change over Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barth Syndrome Foundation the Externally-Led Patient-Focused Drug Development Meeting. In Voice of Patient PFDD Meeting Barth Syndrome, Proceedings of the Externally-Led Public Meeting. 8 March 2019. Available online: https://fda.report/media/130562/EL-PFDD+Meeting+on+Barth+Syndrome+Voice+of+the+Patient+Report.pdf (accessed on 9 October 2022).
- Jefferies, J.L. Barth syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Neustein, H.B.; Lurie, P.R.; Dahms, B.; Takahashi, M. An X-Linked Recessive Cardiomyopathy with Abnormal Mitochondria. Pediatrics 1979, 64, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.G.; Scholte, H.R.; Berden, J.A.; Van der Klei-Van Moorsel, J.M.; Luyt-Houwen, I.E.M.; Veer-Korthof, E.T.V.T.; Sobotka-Plojhar, M.A. An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 1983, 62, 327–355. [Google Scholar] [CrossRef] [PubMed]
- Bione, S.; D’Adamo, P.; Maestrini, E.; Gedeon, A.K.; Bolhuis, P.A.; Toniolo, D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 1996, 12, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Zegallai, H.M.; Hatch, G.M. Barth syndrome: Cardiolipin, cellular pathophysiology, management, and novel therapeutic targets. Mol. Cell. Biochem. 2021, 476, 1605–1629. [Google Scholar] [CrossRef]
- Bertero, E.; Nickel, A.; Kohlhaas, M.; Hohl, M.; Sequeira, V.; Brune, C.; Schwemmlein, J.; Abeßer, M.; Schuh, K.; Kutschka, I.; et al. Loss of Mitochondrial Ca 2+ Uniporter Limits Inotropic Reserve and Provides Trigger and Substrate for Arrhythmias in Barth Syndrome Cardiomyopathy. Circulation 2021, 144, 1694–1713. [Google Scholar] [CrossRef]
- Koshkin, V.; Greenberg, M.L. Cardiolipin prevents rate-dependent uncoupling and provides osmotic stability in yeast mitochondria. Biochem. J. 2002, 364, 317–322. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Gottlieb, E. Cardiolipin: Setting the beat of apoptosis. Apoptosis 2007, 12, 877–885. [Google Scholar] [CrossRef]
- Klingenberg, M. Cardiolipin and Mitochondrial Carriers. Biochimica et Biophysica Acta BBA Biomembranes; Elsevier: Amsterdam, The Netheland, 2009; Volume 1788, pp. 2048–2058. [Google Scholar] [CrossRef]
- Acehan, D.; Xu, Y.; Stokes, D.L.; Schlame, M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab. Investig. 2007, 87, 40–48. [Google Scholar] [CrossRef]
- Kiebish, M.A.; Han, X.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008, 49, 2545–2556. [Google Scholar] [CrossRef]
- Vaz, F.M.; van Lenthe, H.; Vervaart, M.A.; Stet, F.S.; Klinkspoor, J.H.; Vernon, H.J.; Wanders, R.J. An improved functional assay in blood spot to diagnose Barth syndrome using the monolysocardi-olipin/cardiolipin ratio. J. Inherit. Metab. Dis. 2022, 45, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kulik, W.; van Lenthe, H.; Stet, F.S.; Houtkooper, R.H.; Kemp, H.; Stone, J.E.; Steward, C.G.; Wanders, R.J.; Vaz, F.M. Bloodspot Assay Using HPLC–Tandem Mass Spectrometry for Detection of Barth Syndrome. Clin. Chem. 2008, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Storch, E.A.; Keeley, M.; Merlo, L.J.; Amant, J.B.S.; Jacob, M.; Storch, J.F.; Spencer, C.; Byrne, B.J. Psychosocial Functioning in Youth with Barth Syndrome. Child. Health Care 2009, 38, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.L.; Johnco, C.; Dane, B.F.; Collier, A.; Storch, E.A. Psychosocial functioning in Barth syndrome: Assessment of individual and parental adjustment. Child. Health Care 2017, 46, 66–92. [Google Scholar] [CrossRef]
- Raches, D.; Mazzocco, M.M.M. Emergence and Nature of Mathematical Difficulties in Young Children with Barth Syndrome. J. Dev. Behav. Pediatr. 2012, 33, 328–335. [Google Scholar] [CrossRef]
- Manning , W.G., Jr.; Wells, K.B. The Effects of Psychological Distress and Psychological Well-being on Use of Medical Services. In Medical Care; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1992; Volume 30, pp. 541–553. [Google Scholar]
- Simon, G.; Ormel, J.; Vonkorff, M.; Barlow, W. Health care costs associated with depressive and anxiety disorders in primary care. Am. J. Psychiatry 1995, 152, 352–357. [Google Scholar] [CrossRef]
- Seigel, W.M.; Golden, N.H.; Gough, J.W.; Lashley, M.S.; Sacker, I.M. Depression, self-esteem, and life events in adolescents with chronic diseases. J. Adolesc. Health Care 1990, 11, 501–504. [Google Scholar] [CrossRef]
- Klerman, G.L. Panic attacks in the community. Social morbidity and health care utilization. JAMA 1991, 265, 742–746. [Google Scholar] [CrossRef]
- Mazar, I.; Moorman, S.M. “I Want That Life a Lot…How on Earth Do I Get That?” Examining Challenges for Men with Barth Syndrome in Their Transitions to Adulthood. J. Patient Exp. 2021, 8. [Google Scholar] [CrossRef]
- Lim, Y.; Kreider, C.M.; Alvarez, M.; Bendixen, R.M. HRQoL in Barth Syndrome: Agreement between Child Self-reports and Parent Proxy-reports and Its Relationship to Parental HRQoL. J. Hum. Clin. Genet. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Chapman, D.P.; Perry, G.S.; Strine, T.W. Peer reviewed: The vital link between chronic disease and depressive disorders. Prev. Chronic Dis. 2005, 2, 15670467. [Google Scholar]
- Varni, J.W.; Limbers, C.A. The PedsQL™ 4.0 generic core scales young adult version: Feasibility, reliability and validity in a university student population. J. Health Psychol. 2009, 14, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Rose, M. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med. Care 2007, 45, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef]
- Uzark, K.; Jones, K.; Slusher, J.; Limbers, C.A.; Burwinkle, T.M.; Varni, J.W. Quality of Life in Children with Heart Disease as Perceived by Children and Parents. Pediatrics 2008, 121, e1060–e1067. [Google Scholar] [CrossRef] [PubMed]

| n (%) | Mean (SD; Range) | |
|---|---|---|
| Sex | ||
| Male | 16 (100%) | |
| Female | 0 (0%) | |
| Age | 21.25 (12.66; 7–51) | |
| ≤14 | 5 (31.25%) | |
| 15–19 | 5 (31.25%) | |
| 20–29 | 2 (12.5%) | |
| 30–39 | 3 (18.75%) | |
| 40–49 | 0 (0%) | |
| 50+ | 1 (6.25%) | |
| Follow-up Period | ||
| Enrollment/Baseline | 26 | |
| 6 months | 7 | |
| 12 months | 7 | |
| 18 months | 3 | |
| 24 months | 2 | |
| 30 months | 3 | |
| 36 months | 8 | |
| HRQoL (Baseline caregiver-report) | ||
| Physical Score | 8 | 365.63 (262.18; 50–775) |
| Emotional Score | 8 | 356.25 (101.55; 225–500) |
| Social Score | 8 | 306.25 (117.83; 175–500) |
| School Score | 8 | 315.63 (195.37; 200–500) |
| HRQoL (Baseline self-report) | ||
| Physical Score | 14 | 426.79 (210.19; 75–775) |
| Emotional Score | 13 | 371.15 (87.11; 225–500) |
| Social Score | 14 | 346.43 (97.01; 200–500) |
| School Score | 14 | 316.07 (115.86; 150–500) |
| PROMIS (Baseline caregiver-report) | ||
| Anxiety | 5 | 45.90 (15.60; 34.60–65.60) |
| Depression | 5 | 36.20 (0; 36.20–36.20) |
| PROMIS (Baseline self-report) | ||
| Anxiety | 14 | 46.26 (8.60; 33.50–59.40) |
| Depression | 14 | 43.11 (7.80; 35.20–59.70) |
| ID | Subject Age at Enrolment (years) | Earliest Follow Up (months) | |
|---|---|---|---|
| Parent | 005-p | 8 | 30 |
| 019-p | 8 | 24 | |
| 014-p * | 16 | 6 | |
| 001-p ** | 17 | 6 | |
| 007-p | 18 | 12 | |
| Subjects | 014-s * | 16 | 6 |
| 001-s ** | 17 | 6 | |
| 004-s | 29 | 6 | |
| 022-S | 29 | 30 | |
| 002-s | 30 | 6 | |
| 011-s | 34 | 12 | |
| 010-s | 38 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bath, A.; Akbilgic, O.; Wilbanks, D.; Patel, J.; Wallen, M.; Haji, S.; Das, A.; Alexander, J.; Pour-Ghaz, I.; Alkhatib, D.; et al. Barth Syndrome: Psychosocial Impact and Quality of Life Assessment. J. Cardiovasc. Dev. Dis. 2022, 9, 448. https://doi.org/10.3390/jcdd9120448
Bath A, Akbilgic O, Wilbanks D, Patel J, Wallen M, Haji S, Das A, Alexander J, Pour-Ghaz I, Alkhatib D, et al. Barth Syndrome: Psychosocial Impact and Quality of Life Assessment. Journal of Cardiovascular Development and Disease. 2022; 9(12):448. https://doi.org/10.3390/jcdd9120448
Chicago/Turabian StyleBath, Anandbir, Oguz Akbilgic, David Wilbanks, Jay Patel, Morgan Wallen, Shereen Haji, Arnab Das, John Alexander, Issa Pour-Ghaz, Deya Alkhatib, and et al. 2022. "Barth Syndrome: Psychosocial Impact and Quality of Life Assessment" Journal of Cardiovascular Development and Disease 9, no. 12: 448. https://doi.org/10.3390/jcdd9120448
APA StyleBath, A., Akbilgic, O., Wilbanks, D., Patel, J., Wallen, M., Haji, S., Das, A., Alexander, J., Pour-Ghaz, I., Alkhatib, D., Huang, Y., Lontok, E., & Jefferies, J. (2022). Barth Syndrome: Psychosocial Impact and Quality of Life Assessment. Journal of Cardiovascular Development and Disease, 9(12), 448. https://doi.org/10.3390/jcdd9120448








