Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation
Abstract
1. Introduction
2. Methods
2.1. Patients’ Population
2.2. Electrophysiological Study
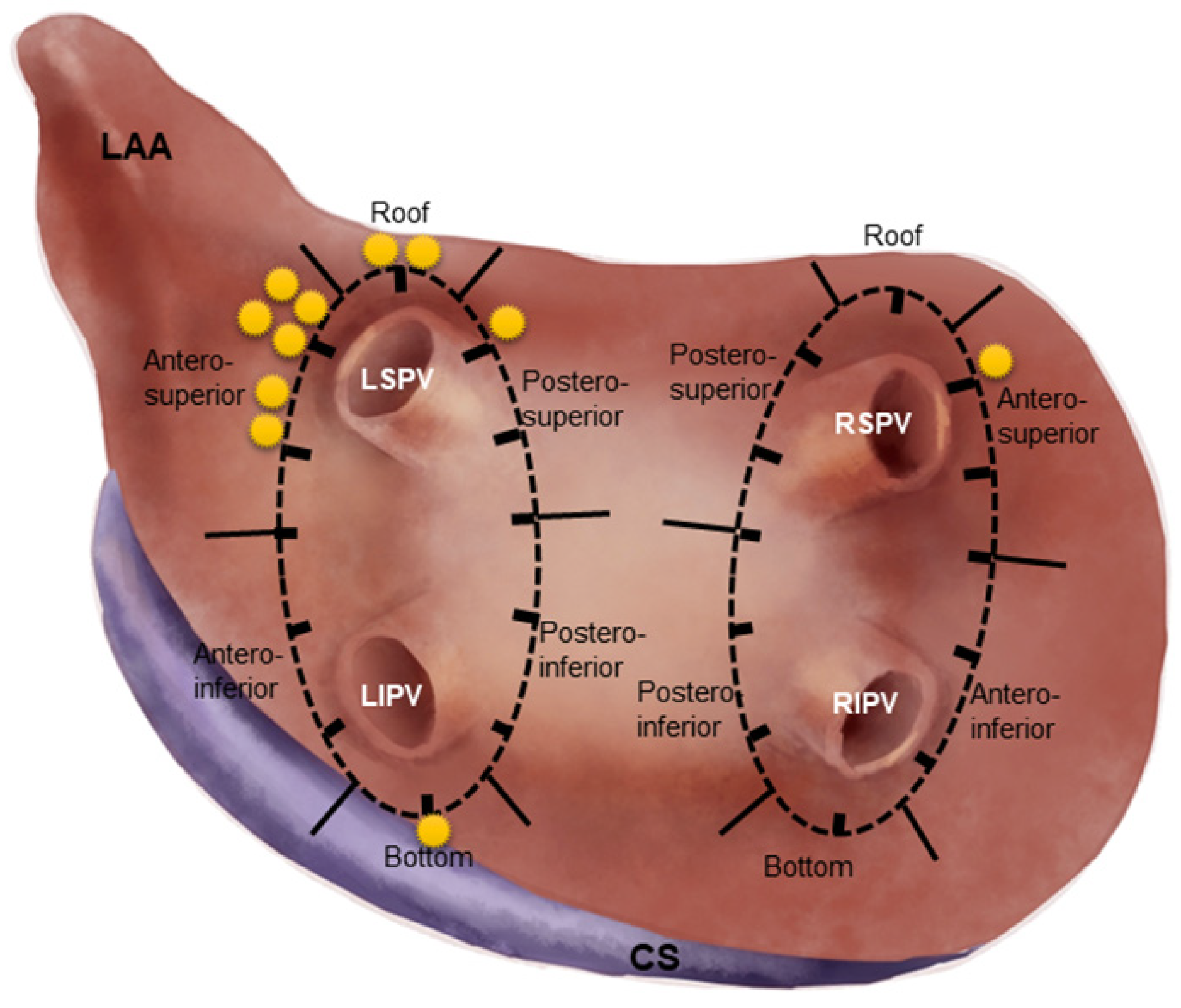
2.3. Segmentation of Circular Lesion Line and Comparison of SP Parameters
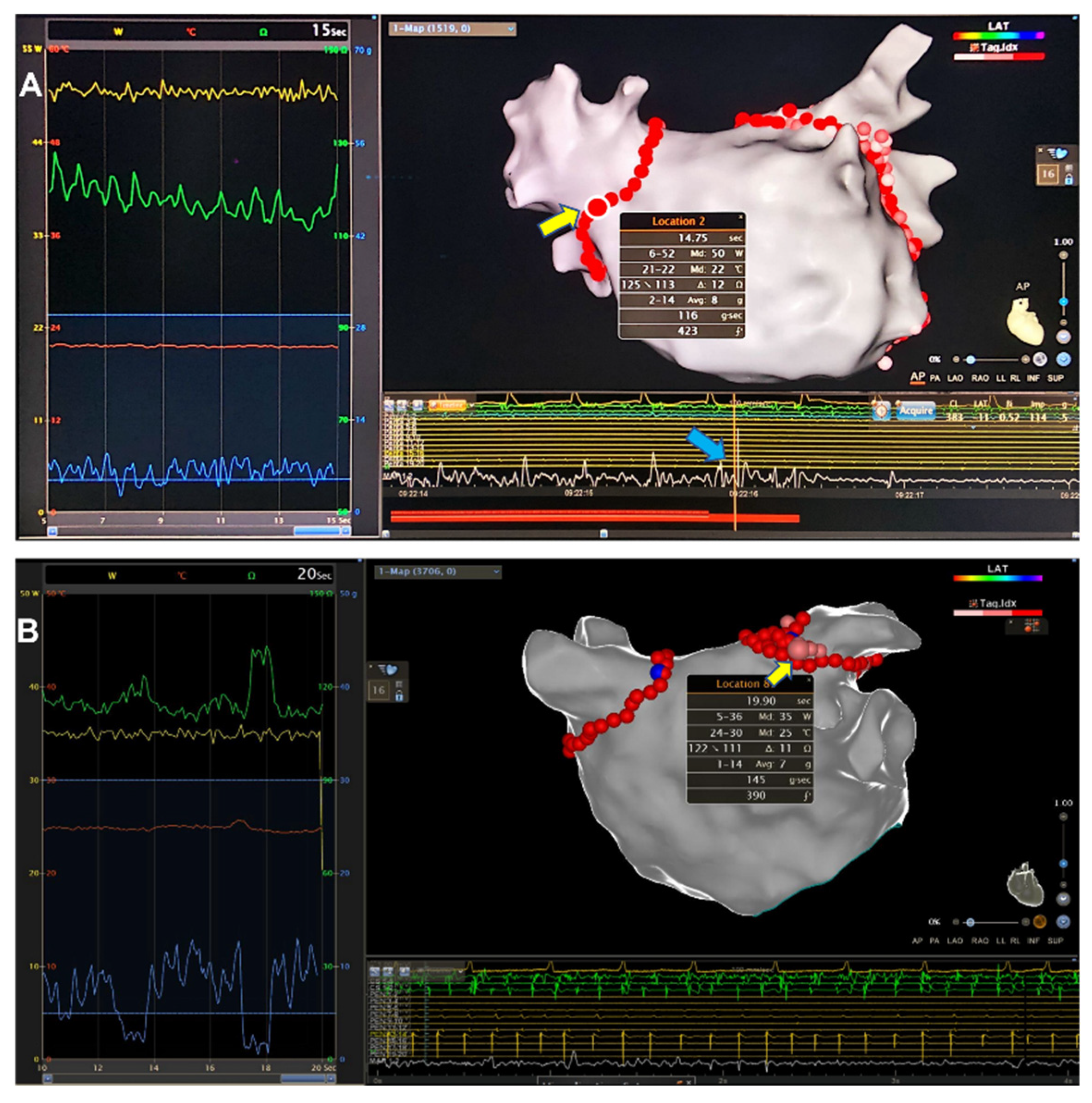
2.4. High-Power CPVI and Management in Case of SP Occurrence
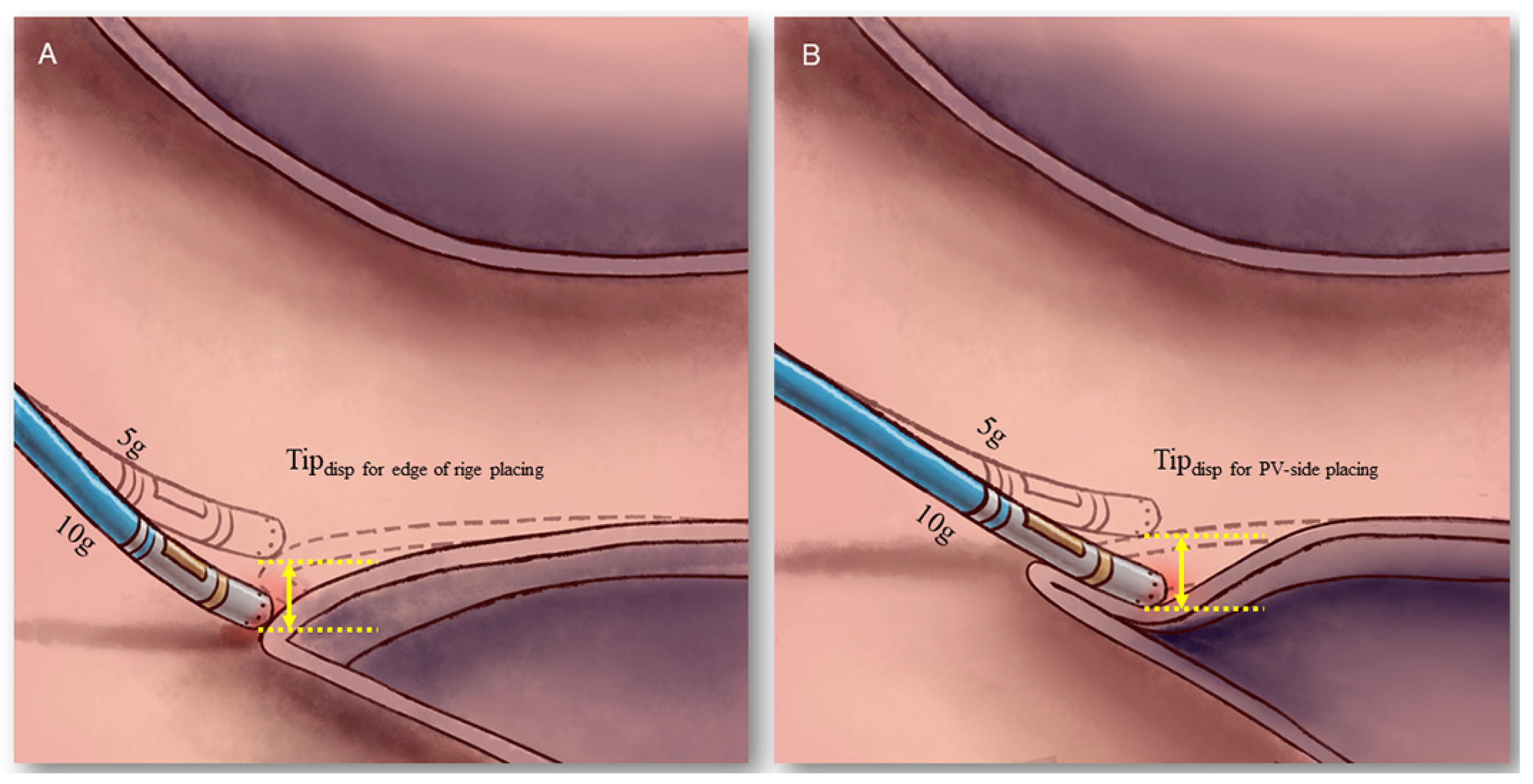
2.5. Comparison of Tip Displacement between the “Edge of Ridge” and the “PV-Side of Ridge” Placement
2.6. Post-Procedural Management and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Prevalence of SP in High Power CPVI
3.2. Distribution of SPs in CPVI
3.3. Comparison of Procedural Parameters
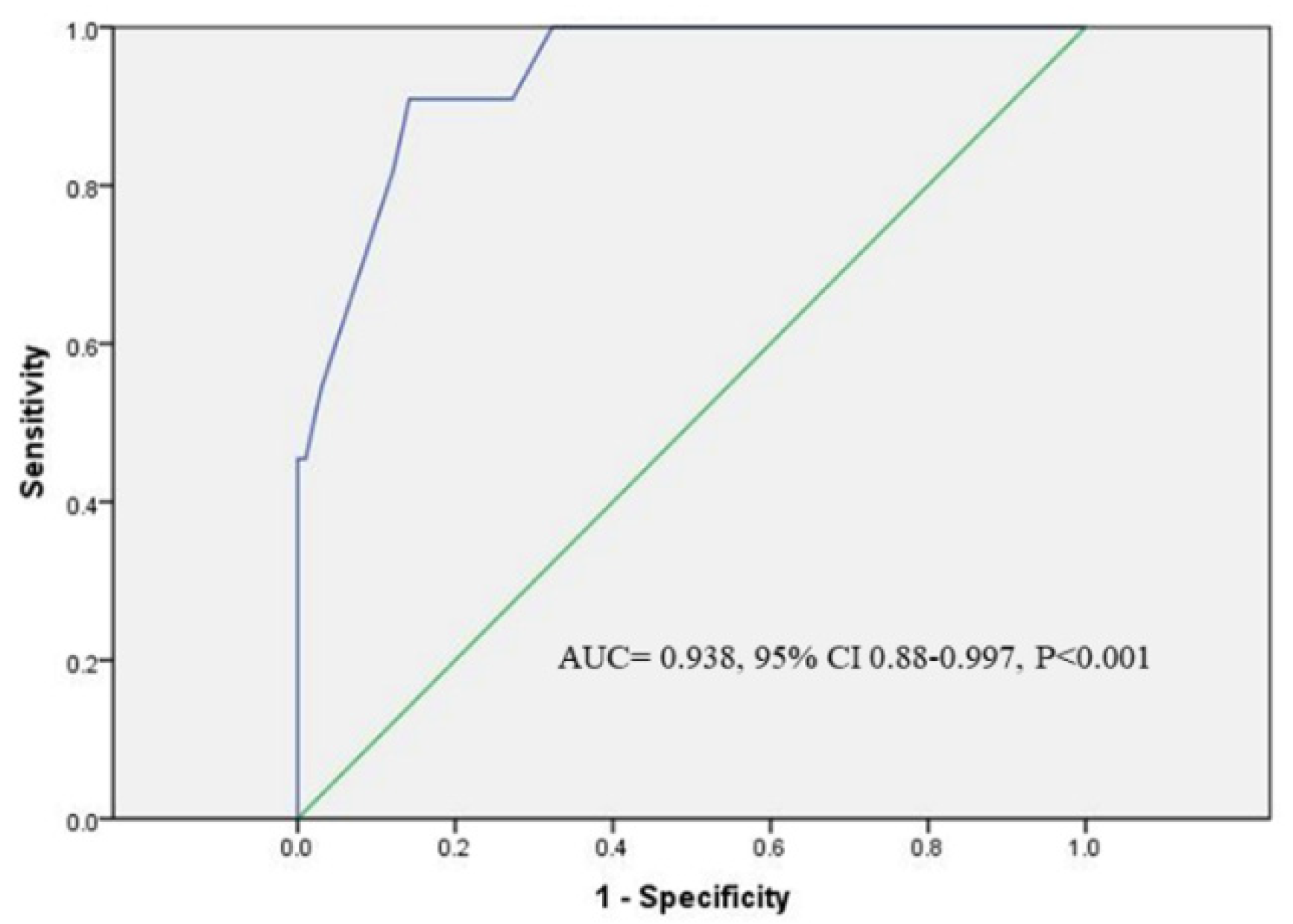
3.4. Predictors for SPs Occurrence
3.5. Results of Catheter Tip Displacement by Two Means of Placement
3.6. Complications
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart. J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Park, C.I.; Lehrmann, H.; Keyl, C.; Weber, R.; Schurr, P.; Schiebeling-Römer, J.; Allgeier, J.; Herrera, C.S.; Kienzle, R.-P.; Shah, D.; et al. Enhanced efficiency of a novel porous tip irrigated RF ablation catheter for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2013, 24, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Chinitz, L.A.; Melby, D.P.; Marchlinski, F.E.; Delaughter, C.; Fishel, R.S.; Monir, G.; Patel, A.M.; Gibson, D.N.; Athill, C.A.; Boo, L.M.; et al. Safety and efficiency of porous-tip contact-force catheter for drug-refractory symptomatic paroxysmal atrial fibrillation ablation: Results from the SMART SF trial. Europace 2018, 20, f392–f400. [Google Scholar] [CrossRef] [PubMed]
- Natale, A.; Reddy, V.Y.; Monir, G.; Wilber, D.J.; Lindsay, B.D.; McElderry, H.T.; Kantipudi, C.; Mansour, M.C.; Melby, D.P.; Packer, D.L.; et al. Paroxysmal AF catheter ablation with a CF sensing catheter: Results of the prospective, multicenter SMART-AF Trial. J. Am. Coll. Cardiol. 2014, 64, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Francke, A.; Taha, N.S.; Scharfe, F.; Schoen, S.; Wunderlich, C.; Christoph, M. Procedural efficacy and safety of standardized, ablation index guided fixed 50 W high-power short-duration pulmonary vein isolation and substrate modification using the CLOSE protocol. J. Cardiovasc. Electrophysiol. 2021, 32, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Kottmaier, M.; Popa, M.; Bourier, F.; Reents, T.; Cifuentes, J.; Semmler, V.; Telishevska, M.; Otgonbayar, U.; Koch-Büttner, K.; Lennerz, C.; et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020, 22, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Schmidt, B.; Bordignon, S.; Urbanek, L.; Tohoku, S.; Bologna, F.; Angelkov, L.; Garvanski, I.; Tsianakas, N.; Konstantinou, A.; et al. Ablation index-guided 50 W ablation for pulmonary vein isolation in patients with atrial fibrillation: Procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J. Cardiovasc. Electrophysiol. 2019, 3, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.G.; Ahn, J.; Han, S.J.; Lim, H.E. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: A prospective randomized controlled trial. Europace 2020, 22, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Theis, C.; Rostock, T.; Mollnau, H.; Sonnenschein, S.; Himmrich, E.; Kämpfner, D.; Ocete, B.Q.; Bock, K.; Münzel, T.; Konrad, T. The Incidence of audible steam pops is increased and unpredictable with the ThermoCool® surround flow catheter during left atrial catheter ablation: A prospective observational study. J. Cardiovasc. Electrophysiol. 2015, 26, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Köktürk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Bourier, F.; Popa, M.; Kottmaier, M.; Maurer, S.; Bahlke, F.; Telishevska, M.; Lengauer, S.; Koch-Büttner, K.; Kornmayer, M.; Risse, E.; et al. RF electrode-tissue coverage significantly influences steam pop incidence and lesion size. J. Cardiovasc. Electrophysiol. 2021, 32, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kato, R.; Sumitomo, N.; Ikeda, Y.; Goto, K.; Tanaka, S.; Asano, S.; Tahara, M.; Nagase, T.; Iwanaga, S.; et al. Relationship between the ablation index, lesion formation, and incidence of steam pops. J. Arrhythm. 2019, 35, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Seiler, J.; Roberts-Thomson, K.C.; Raymond, J.M.; Vest, J.; Delacretaz, E.; Stevenson, W.G. Steam pops during irrigated radiofrequency ablation: Feasibility of impedance monitoring for prevention. Heart Rhythm. 2008, 5, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Strickberger, S.A.; Ravi, S.; Daoud, E.; Niebauer, M.; Man, K.C.; Morady, F. Relation between impedance and temperature during radiofrequency ablation of accessory pathways. Am. Heart J. 1995, 130, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, F.; Zhu, H.; Su, H.; Wu, Y.; Zhu, J.; Zhang, C.; Xu, J. Electro-characteristics of Myocardial Pouches and Reduction of the Frequency of Steam Pops During Radiofrequency Ablation. Front. Physiol. 2022, 13, 816865. [Google Scholar] [CrossRef] [PubMed]
| SP Patients | Non-SP Patients | Tipdisp Measurement Patients | |
|---|---|---|---|
| Number of cases | 11 | 33 | 11 |
| Age (years) | 61.9 ± 12.6 | 63.0 ± 11.6 | 62.3 ± 10.8 |
| Male, n (%) | 7 (63.6) | 21 (63.6) | 7 (63.6) |
| Duration of AF (months) | 4 (1, 24) | 6 (1, 60) | 11 (3, 12) |
| Comorbidities | |||
| Hypertension, n (%) | 7 (63.6) | 17 (51.5) | 7 (63.6) |
| Diabetes Mellitus, n (%) | 0 (0) | 6 (18.2) | 4 (36.4) |
| Coronary artery disease, n (%) | 1 (9.1) | 6 (18.2) | 0(0) |
| Heart failure, n (%) | 5 (45.4) * | 2 (6.1) * | 1 (9.1) |
| History of stroke, n (%) | 0 (0) | 2 (6.1) | 1 (9.1) |
| History of LAAC, n (%) | 2 (18.2) | 0 (0) | 0 (0) |
| TTE measurement | |||
| LAD (mm) | 46.6 ± 4.9 | 46.2 ± 4.4 | 44.6 ± 6.2 |
| LVEDD (mm) | 51.6 ± 4.6 | 49.5 ± 5.8 | 47.1 ± 5.8 |
| LVESD (mm) | 36.6 ± 6.7 | 33.7 ± 6.1 | 30.1 ± 5.1 |
| LVEF (%) | 55.8 ± 13.0 | 60.2 ± 8.6 | 62.6 ± 8.7 |
| SP Patients (n = 11) | Non-SP Patients (n = 33) | p Value | |
|---|---|---|---|
| Number of lesions | 11 | 99 | |
| RF power (Watts) | 40.4 ± 3.5 | 40.3 ± 2.8 | 0.87 |
| RF energy delivery duration (s) | 13.9 ± 6.3 | 23.3 ± 6.0 | <0.001 |
| Average temperature (°C) | 22.2 ± 1.3 | 22.1 ± 2.0 | 0.98 |
| Maximum temperature (°C) | 24.9 ± 3.5 | 26.0 ± 3.0 | 0.25 |
| Average contact force (g) | 6.9 ± 1.8 | 6.4 ± 1.7 | 0.34 |
| Maximum contact force (g) | 12.8 ± 3.4 | 15.0 ± 5.3 | 0.19 |
| Δimpedance (Ω) | 17.6 ± 6.7 | 6.7 ± 4.1 | <0.001 |
| Impedance at the beginning of ablation (Ω) | 112.7 ± 10.4 | 127.1 ± 15.7 | 0.004 |
| Impedance at the end of ablation | 119.1 ± 21.5 | 122.2 ± 14.0 | 0.5 |
| AI value | 357.7 ± 68.8 | 430.2 ± 30.7 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuang, T.; Kong, L.; Cheng, F.; Wang, X. Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2022, 9, 441. https://doi.org/10.3390/jcdd9120441
Shuang T, Kong L, Cheng F, Wang X. Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease. 2022; 9(12):441. https://doi.org/10.3390/jcdd9120441
Chicago/Turabian StyleShuang, Tian, Lingcong Kong, Fuyu Cheng, and Xinhua Wang. 2022. "Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation" Journal of Cardiovascular Development and Disease 9, no. 12: 441. https://doi.org/10.3390/jcdd9120441
APA StyleShuang, T., Kong, L., Cheng, F., & Wang, X. (2022). Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease, 9(12), 441. https://doi.org/10.3390/jcdd9120441






