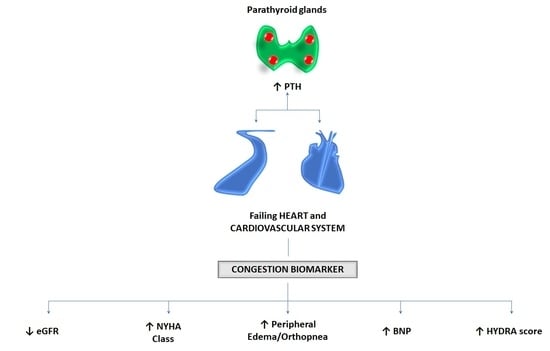
Plasma Levels of Intact Parathyroid Hormone and Congestion Burden in Heart Failure: Clinical Correlations and Prognostic Role
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Corrected Serum Calcium
2.3. Creatinine-Based Estimated Glomerular Filtration Rate (eGFR) Formula
2.4. BUN to Creatinine Ratio
2.5. Estimated Plasma Volume Status
2.6. Hydration Status Estimated by Bioimpedance Vector Analysis
2.7. Hydra Score
2.8. Statistical Analysis
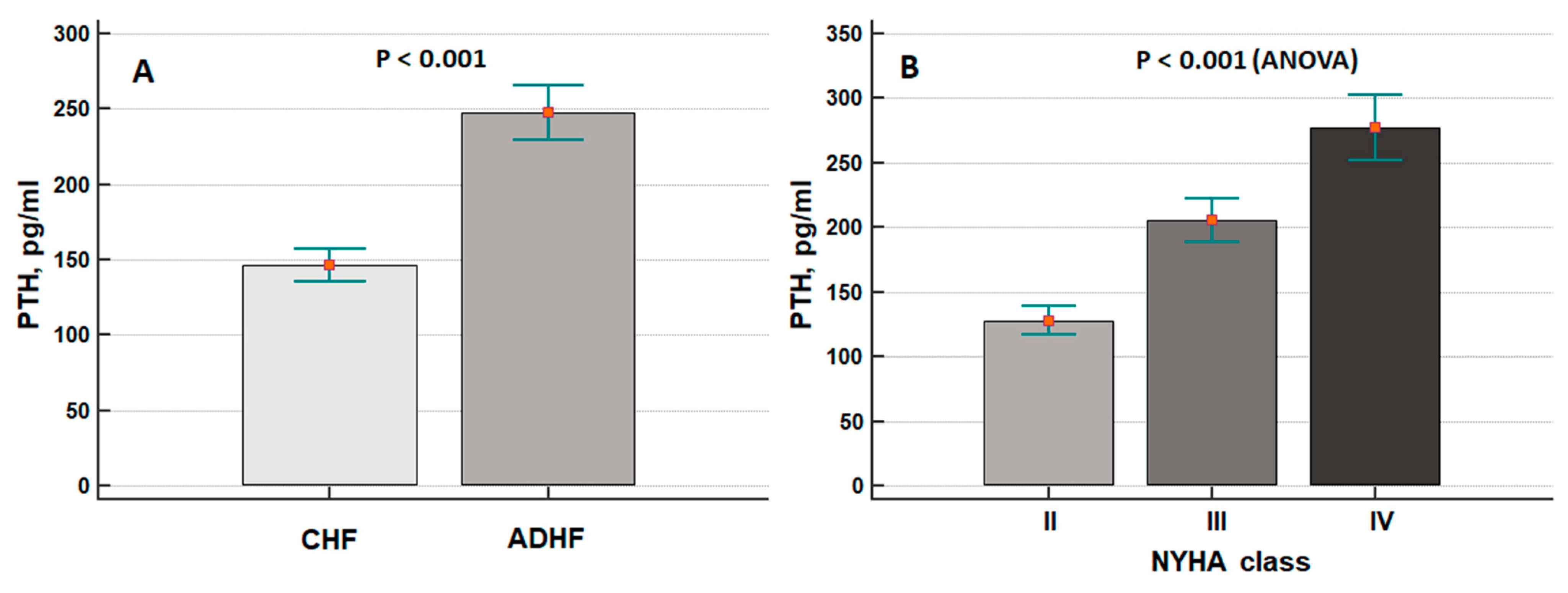
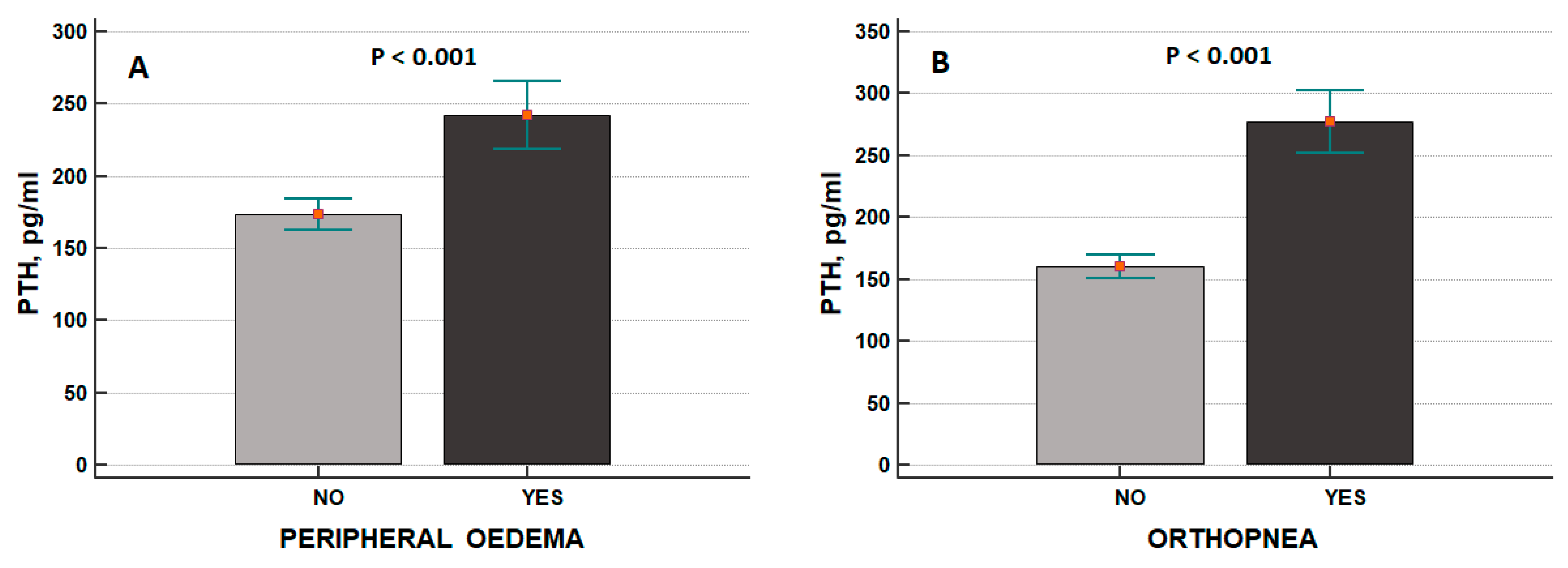
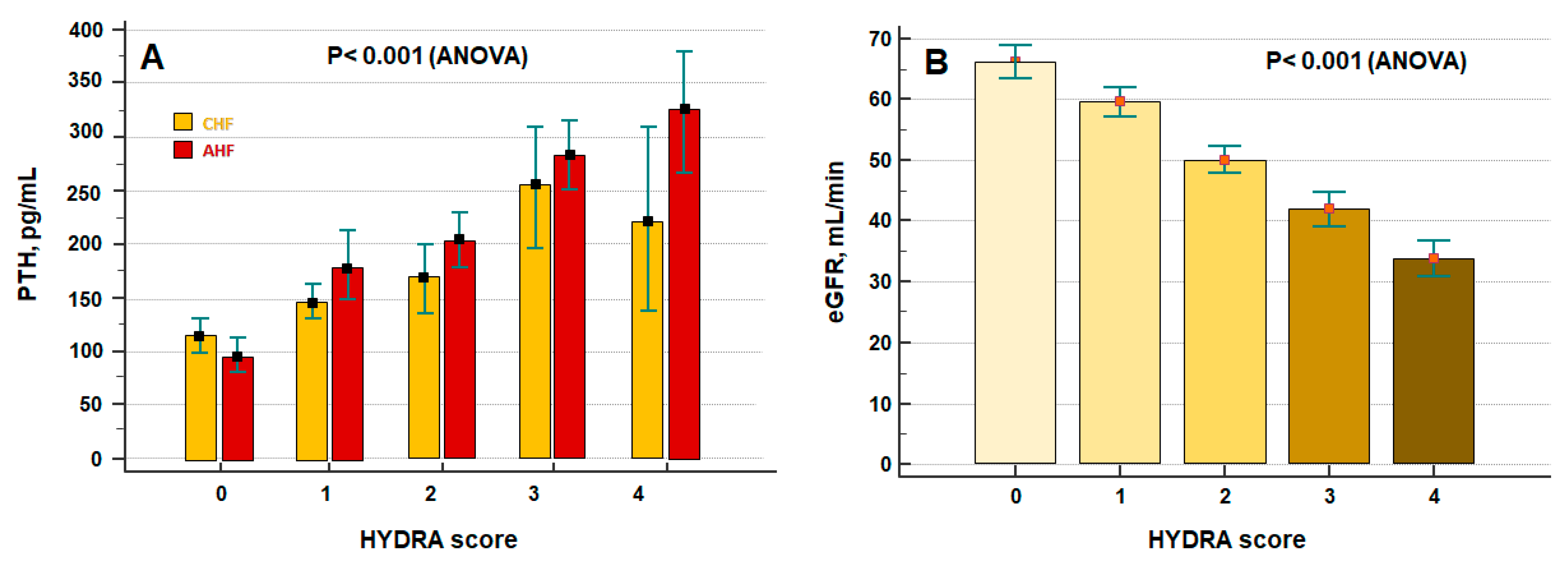
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruson, D.; Buglioni, A.; Burnett, J.C., Jr. PTH: Potential role in management of heart failure. Clin Chim Acta. 2014, 433, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tomaschitz, A.; Ritz, E.; Pieske, B.; Rus-Machan, J.; Kienreich, K.; Verheyen, N.; Gaksch, M.; Grübler, M.; Fahrleitner-Pammer, A.; Mrak, P.; et al. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism 2014, 63, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Wolzt, M.; Schmetterer, L.; Dorner, G.; Zelger, G.; Entlicher, J.; Kapiotis, S.; Eichler, H.G. Hemodynamic effects of parathyroid hormone-related peptide-(1-34) in humans. J. Clin. Endocrinol. Metab. 1997, 82, 2548–2551. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; Sachs, M.C.; Duprez, D.; Hoofnagle, A.N.; Ix, J.H.; Jacobs, D.R., Jr.; Peralta, C.A.; Siscovick, D.S.; Kestenbaum, B.; de Boer, I.H. Parathyroid hormone and arterial dysfunction in the multi-ethnic study of atherosclerosis. Clin. Endocrinol. 2013, 79, 429–436. [Google Scholar] [CrossRef]
- Sugimoto, T.; Tanigawa, T.; Onishi, K.; Fujimoto, N.; Matsuda, A.; Nakamori, S.; Matsuoka, K.; Nakamura, T.; Koji, T.; Ito, M. Serum intact parathyroid hormone levels predict hospitalisation for heart failure. Heart 2009, 95, 395–398. [Google Scholar] [CrossRef]
- Hagström, E.; Ingelsson, E.; Sundström, J.; Hellman, P.; Larsson, T.E.; Berglund, L.; Melhus, H.; Held, C.; Michaëlsson, K.; Lind, L.; et al. Plasma parathyroid hormone and risk of congestive heart failure in the community. Eur. J. Heart Fail. 2010, 12, 1186–1192. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Welsh, P.; Papacosta, O.; Lennon, L.; Whincup, P.H.; Sattar, N. Elevated parathyroid hormone, but not vitamin D deficiency, is associated with increased risk of heart failure in older men with and without cardiovascular disease. Circ. Heart Fail. 2014, 7, 732–739. [Google Scholar] [CrossRef]
- Meng, F.; Wang, W.; Ma, J.; Lin, B. Parathyroid hormone and risk of heart failure in the general population: A meta-analysis of prospective studies. Medicine 2016, 95, e4810. [Google Scholar] [CrossRef]
- Schierbeck, L.L.; Jensen, T.S.; Bang, U.; Jensen, G.; Køber, L.; Jensen, J.E. Parathyroid hormone and vitamin D--markers for cardiovascular and all cause mortality in heart failure. Eur. J. Heart Fail. 2011, 13, 626–632. [Google Scholar] [CrossRef]
- Bansal, N.; Zelnick, L.; Robinson-Cohen, C.; Hoofnagle, A.N.; Ix, J.H.; Lima, J.A.; Shoben, A.B.; Peralta, C.A.; Siscovick, D.S.; Kestenbaum, B.; et al. Serum parathyroid hormone and 25-hydroxyvitamin D concentrations and risk of incident heart failure: The Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2014, 3, e001278. [Google Scholar] [CrossRef]
- Hassan, M.; Qureshi, W.; Sroujieh, L.S.; Albashaireh, D.; BouMalham, S.; Liroff, M.; Amjad, W.; Khalid, F.; Hadid, H.; Alirhayim, Z. Interplay of parathyroid hormone and aldosterone antagonist in prevention of heart failure hospitalizations in chronic kidney disease. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dohi, K.; Onishi, K.; Yamada, T.; Horiguchi, M.; Takamura, T.; Kawamura, A.; Seko, T.; Nakamura, M.; Kasai, A.; et al. Prognostic value of serum parathyroid hormone level in acute decompensated heart failure. Circ. J. 2014, 78, 2704–2710. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Vanwoerkom, R.C.; Horne, B.D.; Bair, T.L.; May, H.T.; Lappé, D.L.; Muhlestein, J.B. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: Dependent or independent risk factors? Am. Heart J. 2011, 162, 331–339.e2. [Google Scholar] [CrossRef] [PubMed]
- Meems, L.M.; Brouwers, F.P.; Joosten, M.M.; Lambers Heerspink, H.J.; de Zeeuw, D.; Bakker, S.J.; Gansevoort, R.T.; van Gilst, W.H.; van der Harst, P.; de Boer, R.A. Plasma calcidiol, calcitriol, and parathyroid hormone and risk of new onset heart failure in a population-based cohort study. ESC Heart Fail. 2016, 3, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Gruson, D.; Ferracin, B.; Ahn, S.A.; Zierold, C.; Blocki, F.; Hawkins, D.M.; Bonelli, F.; Rousseau, M.F. 1,25-Dihydroxyvitamin D to PTH(1-84) Ratios Strongly Predict Cardiovascular Death in Heart Failure. PLoS ONE 2015, 10, e0135427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, Y.; Zhou, L.; Chen, X.; Wang, Y.; Wu, J.; He, H.; Gao, Y. Correlations between serum intact parathyroid hormone (PTH) and N-terminal-probrain natriuretic peptide levels in elderly patients with chronic heart failure (CHF). Arch. Gerontol. Geriatr. 2015, 60, 359–365. [Google Scholar] [CrossRef]
- Kolaszko, A.; Nowalany-Kozielska, E.; Ceranowicz, P.; Morawiec, B.; Kubiak, G. The Role of Parathyroid Hormone and Vitamin D Serum Concentrations in Patients with Cardiovascular Diseases. Dis. Markers 2018, 2018, 5287573. [Google Scholar]
- Wu, G.; Wang, X.; Wang, X.; Jiang, H.; Wang, L.; Wang, T.; Liu, J.; An, D.; Cao, L.; Xia, Y.; et al. Serum Parathyroid Hormone Levels Predict Discharge and Readmission for Heart Failure. Genet. Test Mol. Biomarkers 2016, 20, 328–334. [Google Scholar] [CrossRef]
- Gruson, D.; Lepoutre, T.; Ahn, S.A.; Ketelslegers, J.M.; Rousseau, M.F. Increased circulating concentrations of bioactive PTH 1-84 in patients with heart failure. J. Endocrinol. Investig. 2012, 35, 987–991. [Google Scholar]
- Loncar, G.; Bozic, B.; Dimkovic, S.; Prodanovic, N.; Radojicic, Z.; Cvorovic, V.; Putnikovic, B.; Popovic, V. Association of increased parathyroid hormone with neuroendocrine activation and endothelial dysfunction in elderly men with heart failure. J. Endocrinol. Investig. 2011, 34, e78–e85. [Google Scholar] [CrossRef]
- Gruson, D.; Ahn, S.A.; Rousseau, M.F. Multiple biomarker strategy based on parathyroid hormone and natriuretic peptides testing for improved prognosis of chronic heart failure. Peptides 2015, 64, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Altay, H.; Zorlu, A.; Binici, S.; Bilgi, M.; Yilmaz, M.B.; Colkesen, Y.; Erol, T.; Muderrisoglu, H. Relation of serum parathyroid hormone level to severity of heart failure. Am. J. Cardiol. 2012, 109, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.Y.; Shen, Q.; Wu, T.; Shi, Y.C.; Wang, T.X.; Zong, G.J.; Yang, X.J. Serum parathyroid hormone levels in patients with chronic right heart failure. Biomed. Rep. 2020, 12, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Dohi, K.; Onishi, K.; Watanabe, K.; Sato, Y.; Sugiura, E.; Nakamori, S.; Nakajima, H.; Nakamura, M.; Ito, M. Interrelatiosseonship between haemodynamic state and serum intact parathyroid hormone levels in patients with chronic heart failure. Heart 2013, 99, 111–115. [Google Scholar] [CrossRef]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar]
- Dickerson, R.N.; Alexander, K.H.; Minard, G.; Croce, M.A.; Brown, R.O. Accuracy of methods to estimate ionized and “corrected” serum calcium concentrations in critically ill multiple trauma patients receiving specialized nutrition support. JPEN J. Parenter. Enter. Nutr. 2004, 28, 133–141. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Matsue, Y.; van der Meer, P.; Damman, K.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Blood urea nitrogen-to-creatinine ratio in the general opulationandin patients with acute heart failure. Heart 2017, 103, 407–413. [Google Scholar] [CrossRef]
- Parrinello, G.; Torres, D.; Testani, J.M.; Almasio, P.L.; Bellanca, M.; Pizzo, G.; Cuttitta, F.; Pinto, A.; Butler, J.; Paterna, S. Blood urea nitrogen to creatinine ratio is associated with congestion and mortality in heart failure patients with renal dysfunction. Intern. Emerg. Med. 2015, 10, 965–972. [Google Scholar] [CrossRef]
- Duarte, K.; Monnez, J.M.; Albuisson, E.; Pitt, B.; Zannad, F.; Rossignol, P. Prognostic Value of Estimated Plasma Volume in Heart Failure. JACC Heart Fail. 2015, 3, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Iacoviello, M.; Scicchitano, P.; Mastropasqua, F.; Guida, P.; Riccioni, G.; Speziale, G.; Caldarola, P.; Ciccone, M.M.; Di Somma, S. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung 2016, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Mastropasqua, F.; Guida, P.; De Tommasi, E.; Rizzon, B.; Pontraldolfo, G.; Pitzalis, M.V.; Rizzon, P. Whole-body bioelectrical impedance analysis in patients with chronic heart failure: Reproducibility of the method and effects of body side. Ital. Heart J. 2001, 2, 594–598. [Google Scholar] [PubMed]
- Bosselmann, H.; Tonder, N.; Sölétormos, G.; Gaborit, F.; Rossing, K.; Iversen, K.; Goetze, J.P.; Gustafsson, F.; Schou, M. Influence of renal impairment on aldosterone status, calcium metabolism, and vasopressin activity in outpatients with systolic heart failure. ESC Heart Fail. 2017, 4, 554–562. [Google Scholar] [CrossRef]
- Scagliola, R.; Brunelli, C. Venous Congestion and Systemic Hypoperfusion in Cardiorenal Syndrome: Two Sides of the Same Coin. Rev. Cardiovasc. Med. 2022, 23, 111. [Google Scholar] [CrossRef]
- Evenepoel, P.; Bover, J.; Ureña Torres, P. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016, 90, 1184–1190. [Google Scholar] [CrossRef]
- Dahlen, B.; Müller, F.; Tröbs, S.O.; Heidorn, M.W.; Schulz, A.; Arnold, N.; Hermanns, M.I.; Schwuchow-Thonke, S.; Prochaska, J.H.; Gori, T.; et al. Sex-Specific Relationship Between Parathyroid Hormone and Platelet Indices in Phenotypes of Heart Failure-Results From the MyoVasc Study. Front. Cardiovasc. Med. 2021, 8, 682521. [Google Scholar] [CrossRef]
- Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; De Palo, M.; Piscopo, A.; Gesualdo, M.; Caldarola, P.; Massari, F. The Prognostic Impact of Estimated Creatinine Clearance by Bioelectrical Impedance Analysis in Heart Failure: Comparison of Different eGFR Formulas. Biomedicines 2021, 9, 1307. [Google Scholar] [CrossRef]
- Okuhara, Y.; Asakura, M.; Orihara, Y.; Morisawa, D.; Matsumoto, Y.; Naito, Y.; Tsujino, T.; Ishihara, M.; Masuyama, T. Reduction in Left Ventricular Ejection Fraction is Associated with Subsequent Cardiac Events in Outpatients with Chronic Heart Failure. Sci. Rep. 2019, 9, 17271. [Google Scholar] [CrossRef]
| n = 228 | |
|---|---|
| Clinical characteristics | |
| Age, yrs | 75 ± 0.7 |
| Male, % | 52 |
| BMI, kg/m2 | 28 ± 0.3 |
| NYHA class II/III/IV, n | 98/69/61 |
| Medical history, % | |
| Coronary artery disease | 28 |
| Diabetes | 21 |
| Atrial fibrillation | 43 |
| PM/ICD | 16 |
| AHF | 44 |
| LVEF, % | 42 ± 0.8 |
| Clinical congestion | |
| Peripheral oedema, % | 26 |
| Orthoponea, % | 27 |
| Laboratory values | |
| PTH, pg/dL | 191 ± 10 |
| Haemoglobin, g/dL | 13 ± 0.1 |
| Uric acid, mg/dL | 6.4 ± 0.1 |
| BUN, mg/dL | 31 ± 2.4 |
| Creatinine, mg/dL | 1.4 ± 0.04 |
| eGFR, mL/min/1.73 m2 | 53 ± 1.8 |
| Sodium, mmol/L | 139 ± 0.2 |
| Potassium, mmol/L | 4.0 ± 0.04 |
| Chloride, mmol/L | 103 ± 0.3 |
| Calcium, mmol/L | 8.7 ± 0.05 |
| Inorganic phosphorus (mg/dL) | 3.6 ± 0.07 |
| Albumin, g/dL | 3.3 ± 0.04 |
| Corrected calcium (mg/dL) | 9.2 ± 0.05 |
| Biomarkers of congestion | |
| BNP, pg/mL | 978 ± 78 |
| ePVS, dL/g | 5.0 ± 0.1 |
| Hydration index, % | 75 ± 0.3 |
| BUN/Cr ratio | 54 ± 1.1 |
| HYDRA score | 1.7 ± 0.08 |
| Therapies, % | |
| Furosemide | 69 |
| Beta-blockers | 50 |
| ACE inhibitors/ARBs | 59 |
| MRAs | 50 |
| Digitalis | 22 |
| Ivabradine | 5 |
| Univariate Cox Regression Analysis | Adjusted Cox Regression Analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | Wald | |
| AHF vs. CHF | 2.85 (1.58–5.12) | =0.005 | 0.67 (0.31–1.47) | =0.3 | |
| Age, year | 1.05 (1.02–1.09) | =0.001 | 0.94 (0.96–1.04.994) | =0.9 | |
| NYHA class | 1.75 (1.25–5.46) | =0.001 | 0.94 (0.59–1.52) | = 0.8 | |
| LVEF, % | 0.97 (0.94–0.99) | =0.005 | 0.97 (0.94–0.99) | =0.009 | 6.9 |
| PTH, pg/mL | 1.003 (1.002–1.005) | <0.0001 | 1.001 (0.999–1.003) | =0.15 | |
| HYDRA score | 2.13 (1.67–2.70) | <0.0001 | 1.96 (1.44–2.67) | <0.0001 | 18 |
| Corrected calcium | 1.15 (0.57–1.50) | =0.47 | |||
| eGFR, mL/min | 0.97 (0.95–0.98) | <0.0001 | 0.98 (0.96–0.99) | =0.04 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Iacoviello, M.; Passantino, A.; Gesualdo, M.; Trotta, F.; Basile, M.; De Palo, M.; Guida, P.; Paolillo, C.; Riccioni, G.; et al. Plasma Levels of Intact Parathyroid Hormone and Congestion Burden in Heart Failure: Clinical Correlations and Prognostic Role. J. Cardiovasc. Dev. Dis. 2022, 9, 334. https://doi.org/10.3390/jcdd9100334
Scicchitano P, Iacoviello M, Passantino A, Gesualdo M, Trotta F, Basile M, De Palo M, Guida P, Paolillo C, Riccioni G, et al. Plasma Levels of Intact Parathyroid Hormone and Congestion Burden in Heart Failure: Clinical Correlations and Prognostic Role. Journal of Cardiovascular Development and Disease. 2022; 9(10):334. https://doi.org/10.3390/jcdd9100334
Chicago/Turabian StyleScicchitano, Pietro, Massimo Iacoviello, Andrea Passantino, Michele Gesualdo, Francesco Trotta, Marco Basile, Micaela De Palo, Piero Guida, Claudio Paolillo, Graziano Riccioni, and et al. 2022. "Plasma Levels of Intact Parathyroid Hormone and Congestion Burden in Heart Failure: Clinical Correlations and Prognostic Role" Journal of Cardiovascular Development and Disease 9, no. 10: 334. https://doi.org/10.3390/jcdd9100334
APA StyleScicchitano, P., Iacoviello, M., Passantino, A., Gesualdo, M., Trotta, F., Basile, M., De Palo, M., Guida, P., Paolillo, C., Riccioni, G., Ciccone, M. M., Caldarola, P., & Massari, F. (2022). Plasma Levels of Intact Parathyroid Hormone and Congestion Burden in Heart Failure: Clinical Correlations and Prognostic Role. Journal of Cardiovascular Development and Disease, 9(10), 334. https://doi.org/10.3390/jcdd9100334