Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Experimental Protocol
2.2.1. Tissue Factor Gene and Protein Levels, Surface Translocation and Functional Activity
2.2.2. Evaluation of ACE2 Receptor-mRNA and Protein Levels
2.2.3. Effects on Adhesion Molecules Expression
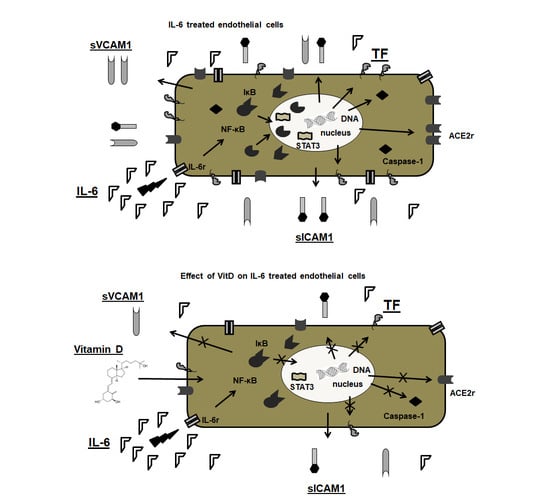
2.2.4. Regulation of Intracellular Signaling: The Role of NF-κB Nuclear Translocation and STAT3
2.2.5. Modulation of NLRP3 Protein
2.3. Statistical Analysis
3. Results
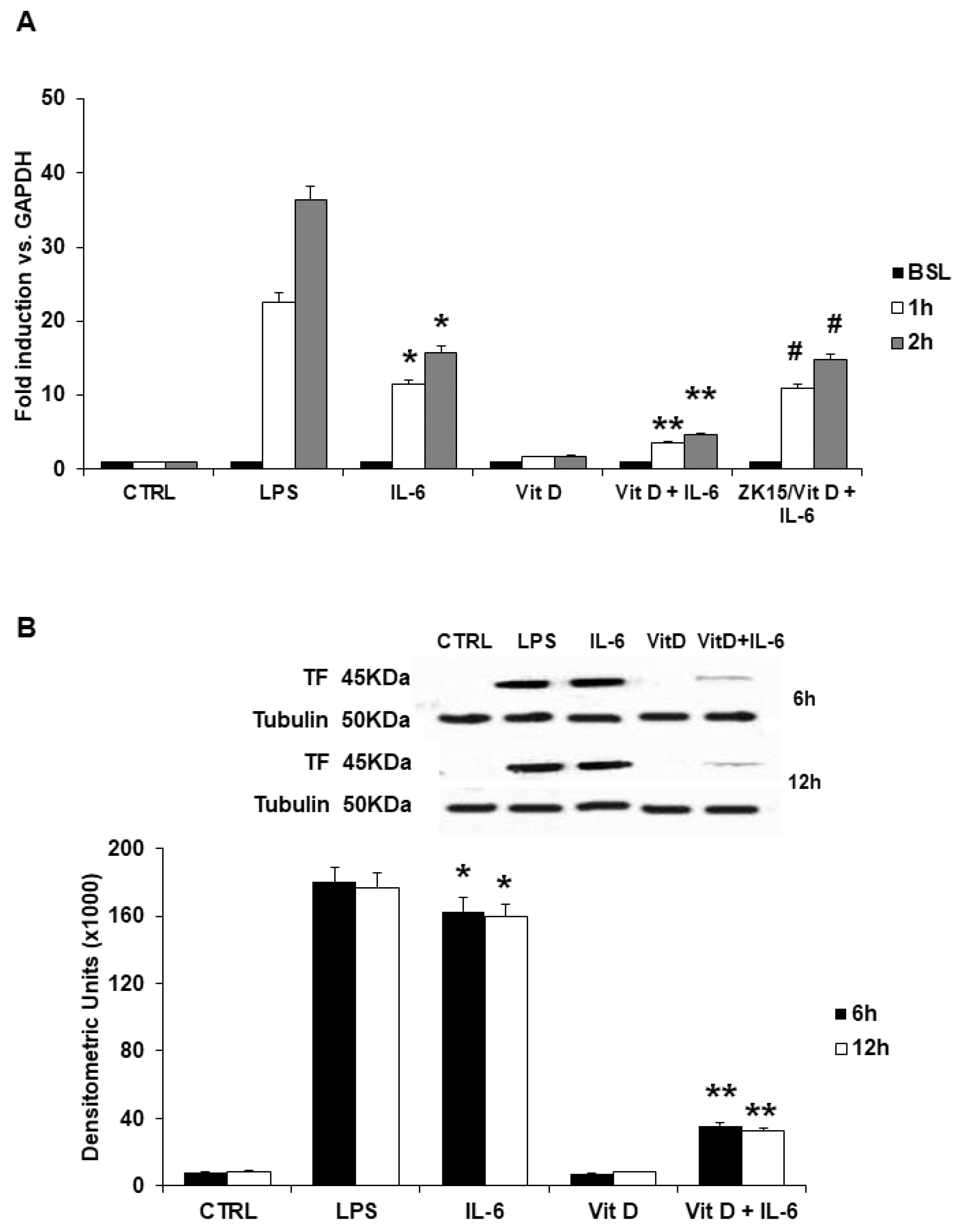
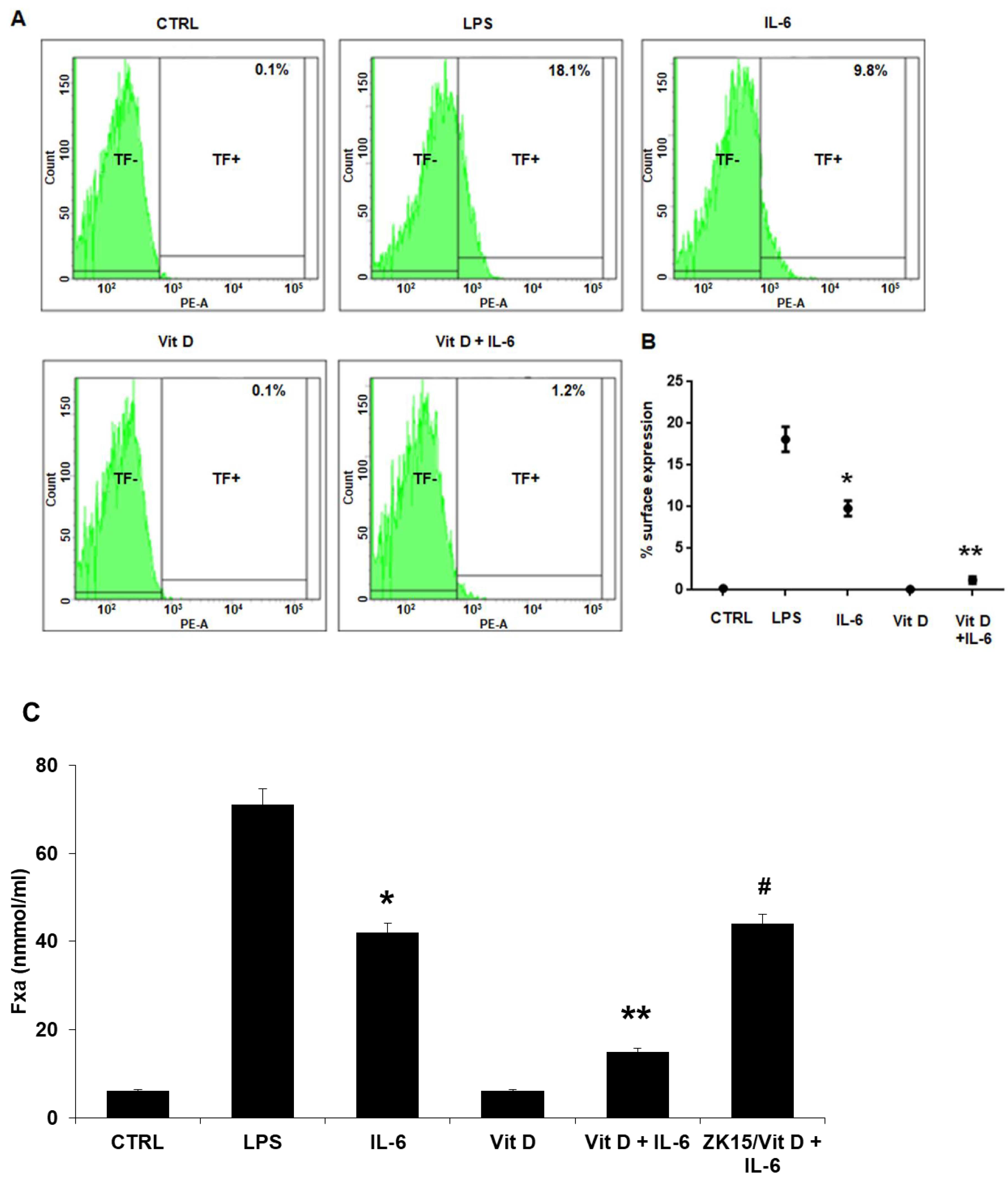
3.1. Tissue Factor Gene and Protein Levels, Surface Translocation and Procoagulant Activity
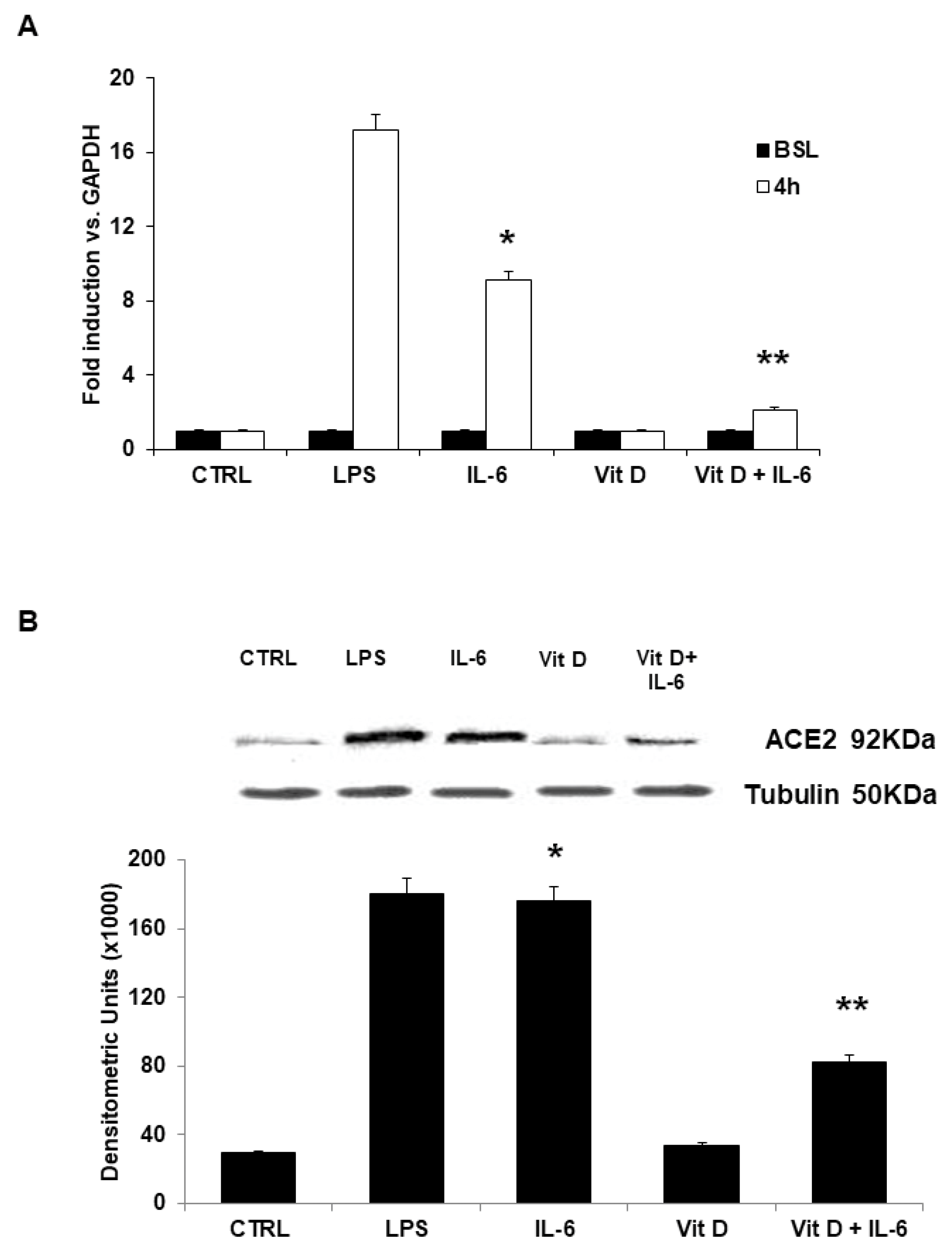
3.2. ACE2 Receptor mRNA and Protein Expression in Vitamin D Pretreated Cells
3.3. Vitamin D Reduces Adhesion Molecules’ Expression
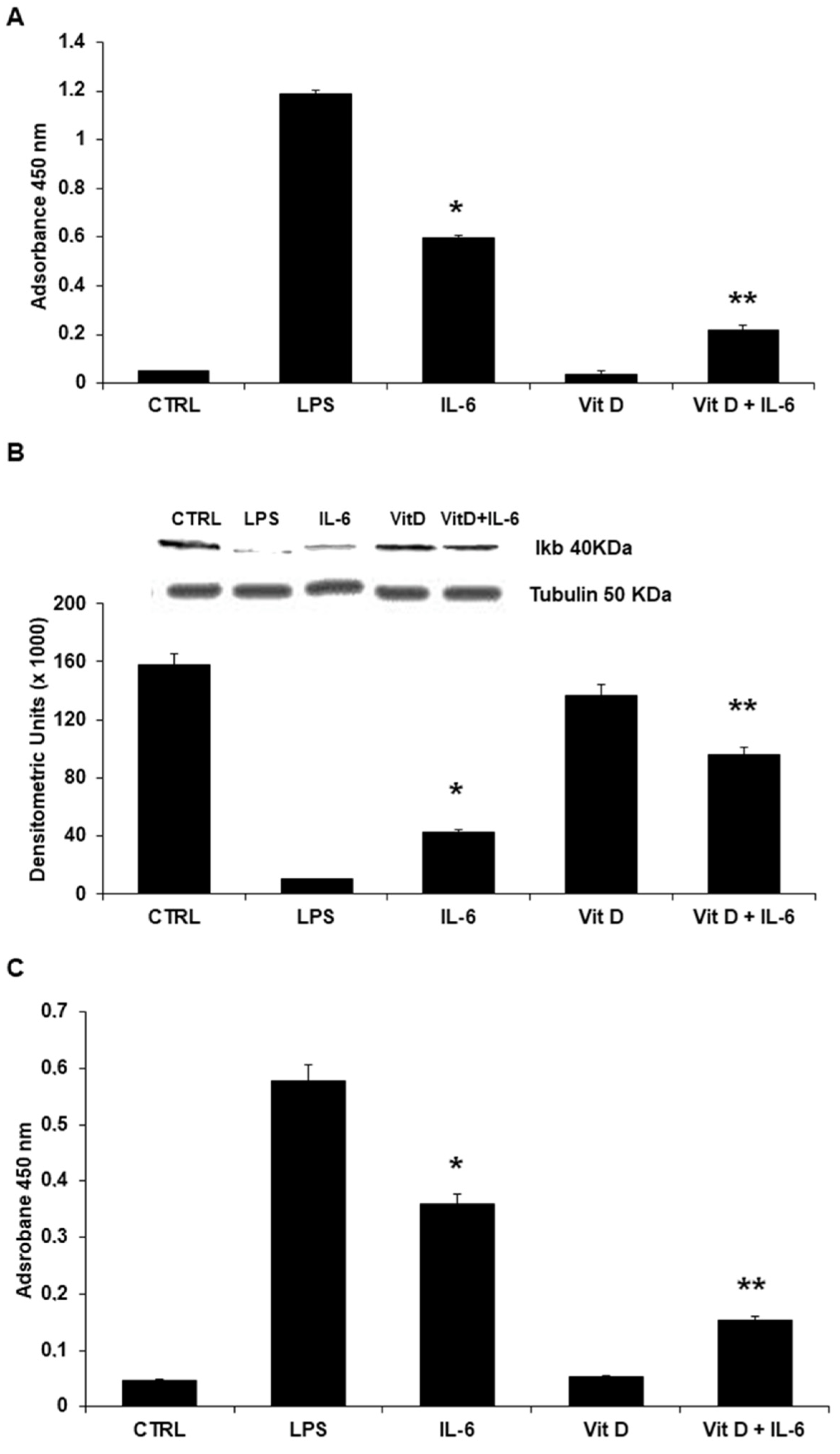
3.4. Regulation of Intracellular Signaling: The Role of NF-κB Nuclear Translocation and STAT3
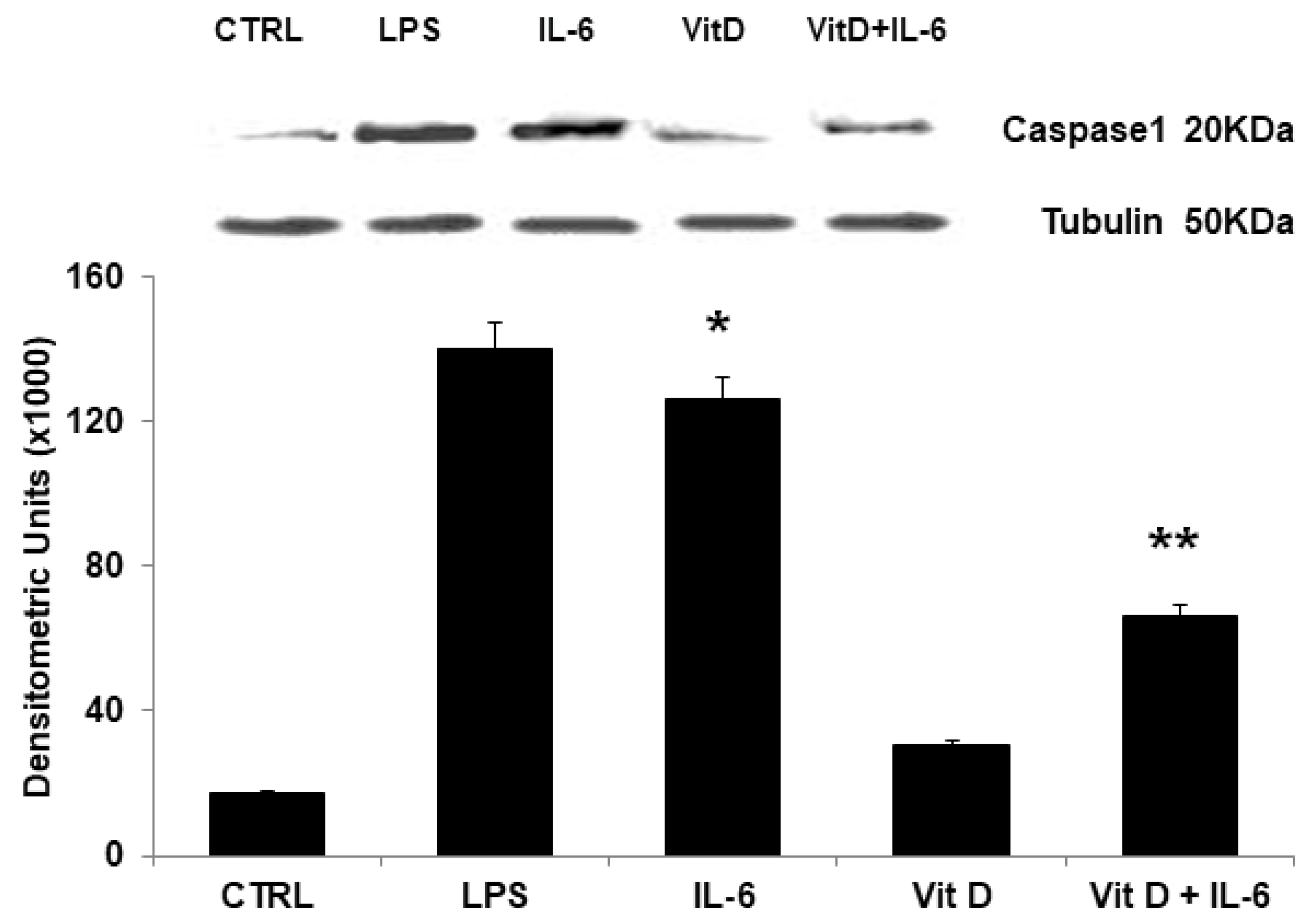
3.5. Modulation of NLRP3 Protein Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.C.J.; Voetsch, B.; Loscalzo, J. Endogenous mechanisms of inhibition of platelet function. Microcirculation 2005, 12, 247–258. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labò, N.; Ohnuki, H.; Tosato, G. Vasculopathy and Coagulopathy Associated with SARS-CoV-2 Infection. Cells 2020, 9, 1583. [Google Scholar] [CrossRef] [PubMed]
- Godino, C.; Scotti, A.; Maugeri, N.; Mancini, N.; Fominskiy, E.; Margonato, A.; Landoni, G. Antithrombotic therapy in patients with COVID-19?—Rationale and Evidence. Int. J. Cardiol. 2021, 324, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Uchida, M.; Tanaka, K.; Hasebe, R.; Tanaka, Y.; Murakami, M.; Hirano, T. How COVID-19 induces cytokine storm with high mortality. Inflamm. Regen. 2020, 40, 37. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Panagiotakos, D.B. Vitamin D status, gender and cardiovascular diseases: A systematic review of prospective epidemiological studies. Expert Rev. Cardiovasc. Ther. 2019, 17, 545–555. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Nicholls, S.J.; Psaltis, P.J. Vitamin D and Cardiovascular Disease. Heart Lung Circ. 2018, 27, 903–906. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef] [Green Version]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and Inflammation: Potential Implications for Severity of COVID-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Martinez-Moreno, J.M.; Herencia, C.; Montes de Oca, A.; Munoz-Castaneda, J.R.; Rodriguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Peralbo-Santaella, E.; Camargo, A.; Canalejo, A.; Rodriguez, M.; et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016, 30, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, G.; Morello, A.; Conte, S.; Pellegrino, G.; Marra, L.; Golino, P.; Cirillo, P. Vitamin D inhibits Tissue Factor and CAMs expression in oxidized low-density lipoproteins-treated human endothelial cells by modulating NF-kappaB pathway. Eur. J. Pharmacol. 2020, 885, 173422. [Google Scholar] [CrossRef]
- Cirillo, P.; Conte, S.; Cimmino, G.; Pellegrino, G.; Ziviello, F.; Barra, G.; Sasso, F.C.; Borgia, F.; De Palma, R.; Trimarco, B. Nobiletin inhibits oxidized-LDL mediated expression of Tissue Factor in human endothelial cells through inhibition of NF-kappaB. Biochem. Pharmacol. 2017, 128, 26–33. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Kucia, M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia 2020, 34, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Loffredo, F.S.; Morello, A.; D’Elia, S.; De Palma, R.; Cirillo, P.; Golino, P. Immune-Inflammatory Activation in Acute Coronary Syndromes: A Look into the Heart of Unstable Coronary Plaque. Curr. Cardiol. Rev. 2017, 13, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Ismail, M.Y.; Diamond, A.; Kapoor, S.; Arafah, Y.; Nayak, L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb. Res. 2020, 194, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Gladka, M.M.; Maack, C. The endothelium as Achilles’ heel in COVID-19 patients. Cardiovasc. Res. 2020, 116, e195–e197. [Google Scholar] [CrossRef]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.; Meijers, J.C.; Brandjes, D.P.; van Gorp, E.C. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [Green Version]
- Giannakodimos, I.; Gkountana, G.-V.; Lykouras, D.; Karkoulias, K.; Tsakas, S. The role of Interleukin-6 in the pathogenesis, prognosis and treatment of severe COVID-19. Curr. Med. Chem. 2021, 28, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; McGary, H.; Razmpour, R.; Galie, P.A.; Potula, R.; Andrews, A.M.; Ramirez, S.H. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in vitro models of the human blood-brain barrier. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Haasbach, E.; Lueftenegger, D.; Heyken, W.T.; Ocker, M.; Planz, O. NF-kappaB Pathway as a Potential Target for Treatment of Critical Stage COVID-19 Patients. Front. Immunol. 2020, 11, 598444. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Ziviello, F.; Pellegrino, G.; Conte, S.; Cimmino, G.; Giaquinto, A.; Pacifico, F.; Leonardi, A.; Golino, P.; Trimarco, B. The adipokine apelin-13 induces expression of prothrombotic tissue factor. Thromb. Haemost. 2015, 113, 363–372. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef]
- Chen, J.; Mei, K.; Xie, L.; Yuan, P.; Ma, J.; Yu, P.; Zhu, W.; Zheng, C.; Liu, X. Low vitamin D levels do not aggravate COVID-19 risk or death, and vitamin D supplementation does not improve outcomes in hospitalized patients with COVID-19: A meta-analysis and GRADE assessment of cohort studies and RCTs. Nutr. J. 2021, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Crafa, A.; Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; Barbagallo, F.; Aversa, A.; La Vignera, S.; Calogero, A.E. Influence of 25-hydroxy-cholecalciferol levels on SARS-CoV-2 infection and COVID-19 severity: A systematic review and meta-analysis. EClinicalMedicine 2021, 37, 100967. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef] [PubMed]
- Telcian, A.G.; Zdrenghea, M.T.; Edwards, M.R.; Laza-Stanca, V.; Mallia, P.; Johnston, S.L.; Stanciu, L.A. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antivir. Res. 2017, 137, 93–101. [Google Scholar] [CrossRef]
- Schögler, A.; Muster, R.J.; Kieninger, E.; Casaulta, C.; Tapparel, C.; Jung, A.; Moeller, A.; Geiser, T.; Regamey, N.; Alves, M.P. Vitamin D represses rhinovirus replication in cystic fibrosis cells by inducing LL-37. Eur. Respir. J. 2016, 47, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [Green Version]
- Tsujino, I.; Ushikoshi-Nakayama, R.; Yamazaki, T.; Matsumoto, N.; Saito, I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J. Clin. Biochem. Nutr. 2019, 65, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, G.; Conte, S.; Morello, M.; Pellegrino, G.; Marra, L.; Morello, A.; Nicoletti, G.; De Rosa, G.; Golino, P.; Cirillo, P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J. Cardiovasc. Dev. Dis. 2022, 9, 27. https://doi.org/10.3390/jcdd9010027
Cimmino G, Conte S, Morello M, Pellegrino G, Marra L, Morello A, Nicoletti G, De Rosa G, Golino P, Cirillo P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? Journal of Cardiovascular Development and Disease. 2022; 9(1):27. https://doi.org/10.3390/jcdd9010027
Chicago/Turabian StyleCimmino, Giovanni, Stefano Conte, Mariarosaria Morello, Grazia Pellegrino, Laura Marra, Andrea Morello, Giuseppe Nicoletti, Gennaro De Rosa, Paolo Golino, and Plinio Cirillo. 2022. "Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection?" Journal of Cardiovascular Development and Disease 9, no. 1: 27. https://doi.org/10.3390/jcdd9010027
APA StyleCimmino, G., Conte, S., Morello, M., Pellegrino, G., Marra, L., Morello, A., Nicoletti, G., De Rosa, G., Golino, P., & Cirillo, P. (2022). Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? Journal of Cardiovascular Development and Disease, 9(1), 27. https://doi.org/10.3390/jcdd9010027