The Role of Multimodality Imaging in Left-Sided Prosthetic Valve Dysfunction
Abstract
:1. Introduction
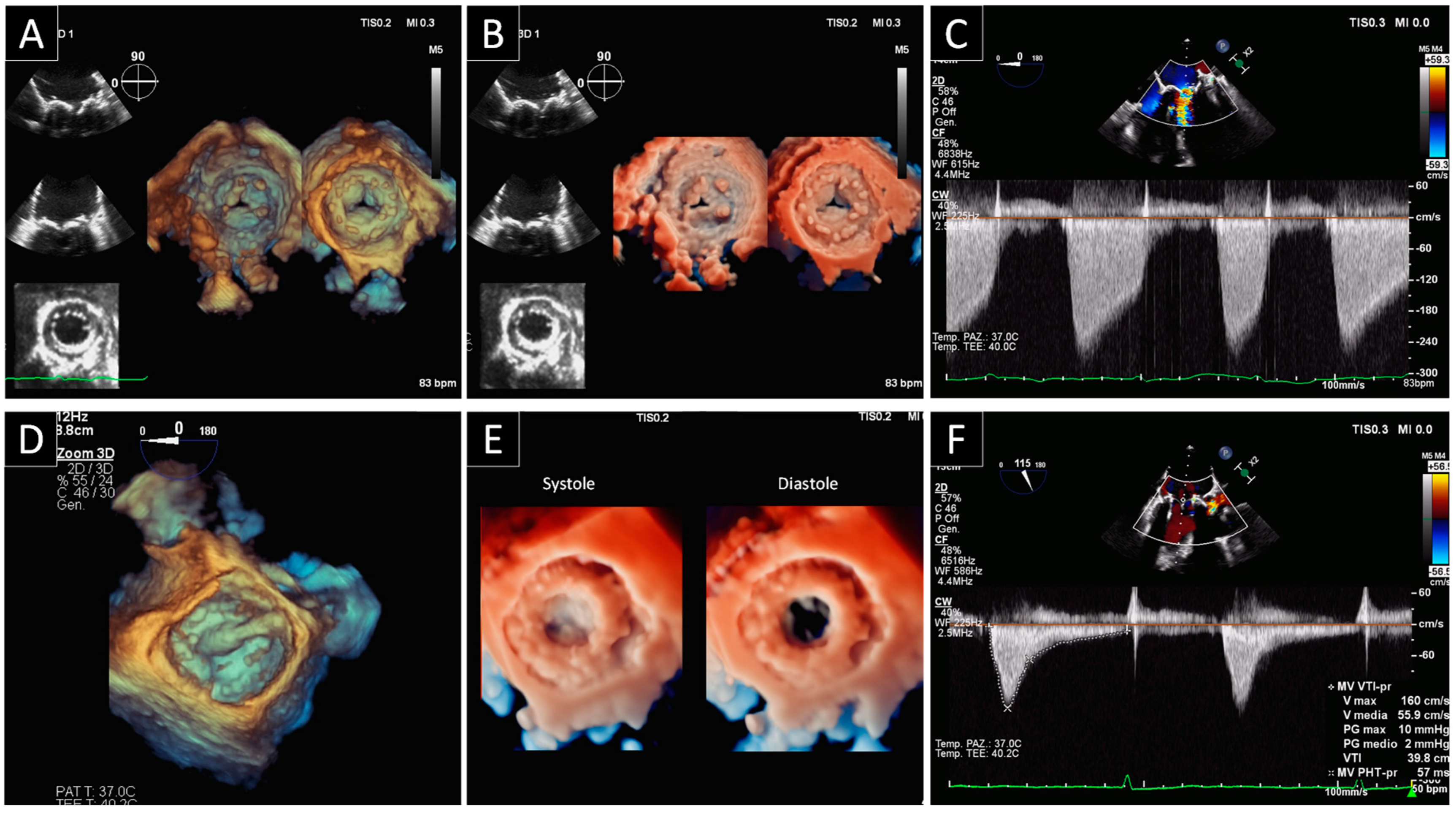
2. Prosthetic Valve Obstruction
2.1. Prosthetic Mitral Valve Obstruction
2.2. Prosthetic Aortic Valve Obstruction
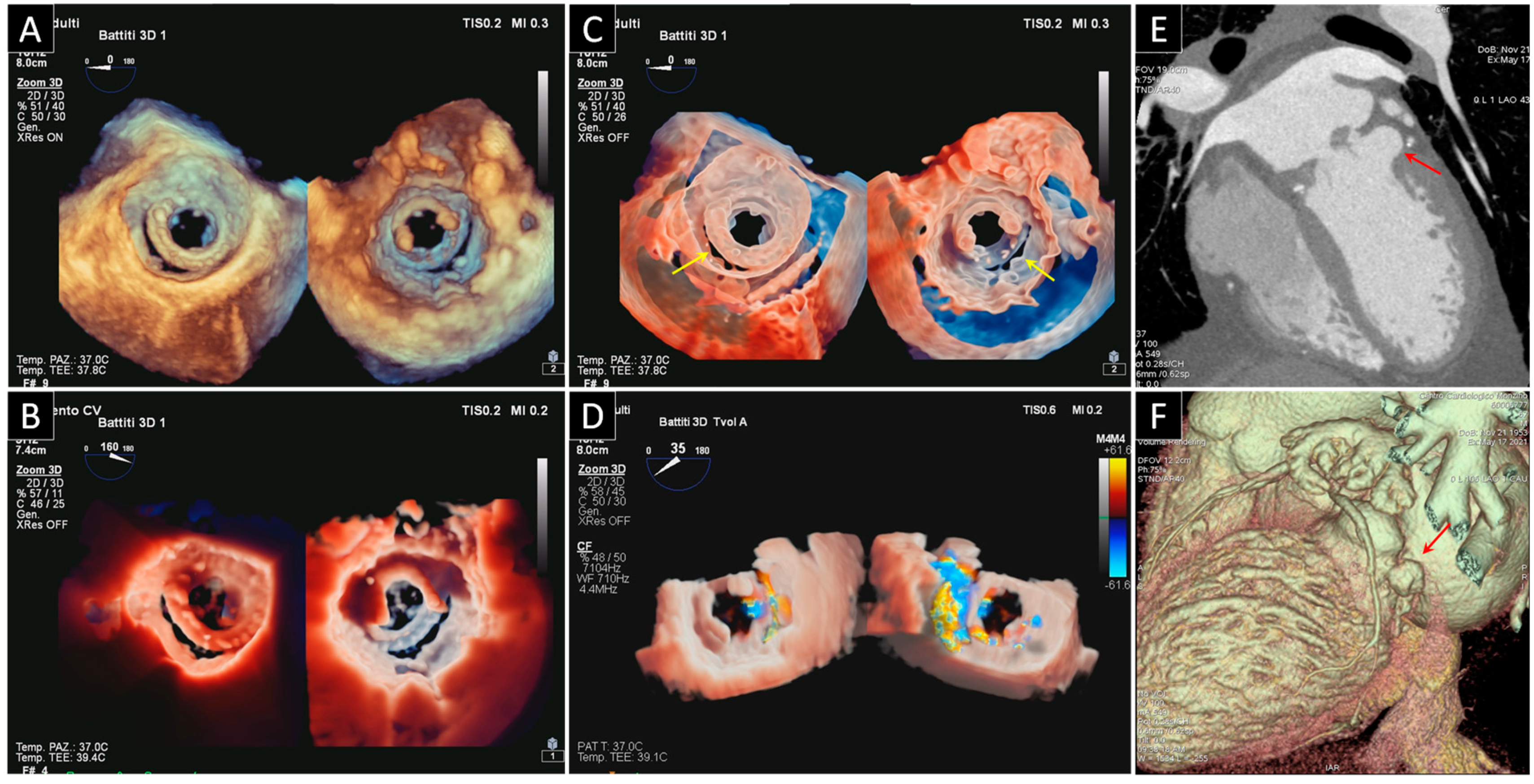
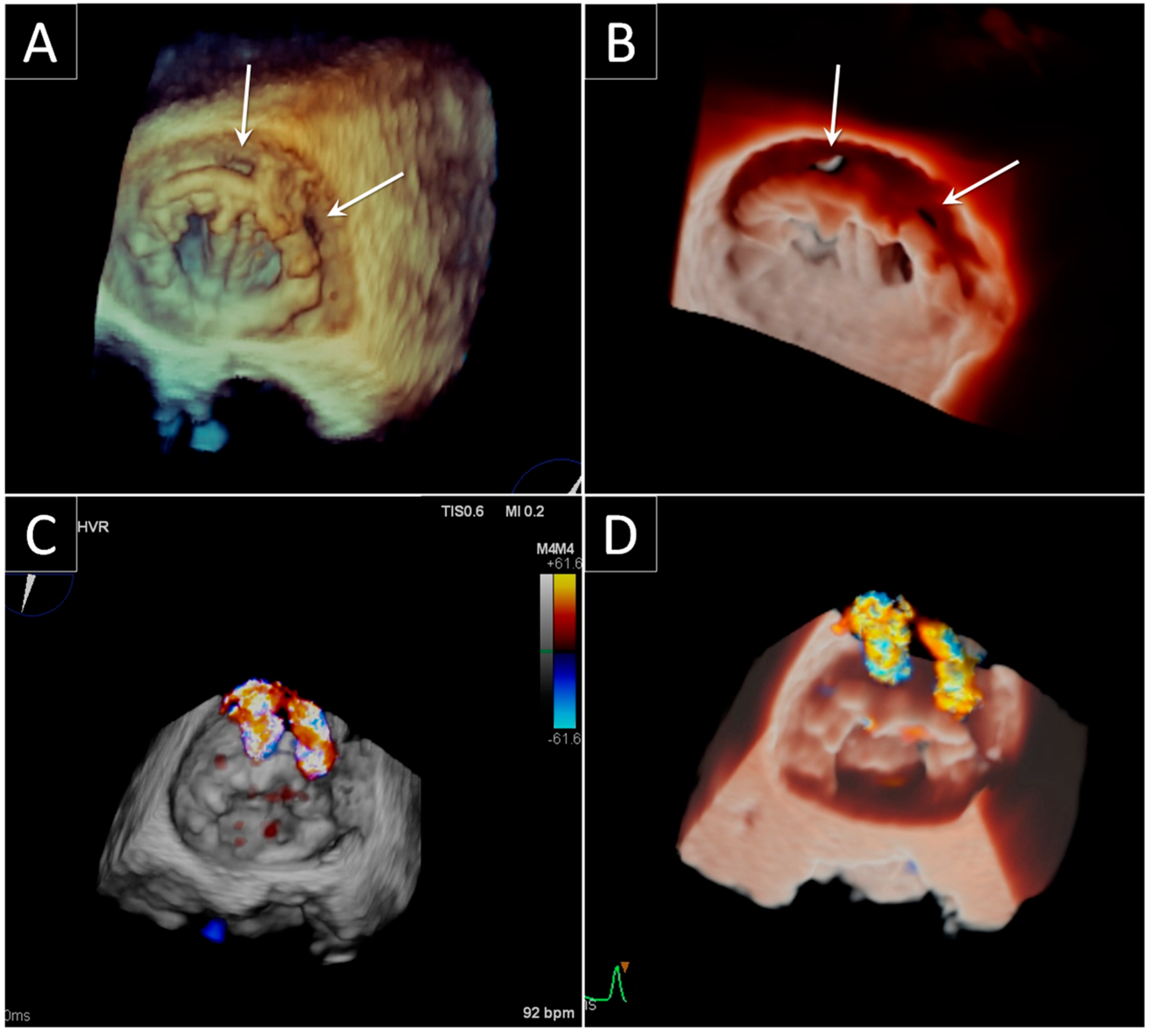
3. Prosthetic Paravalvular Leak
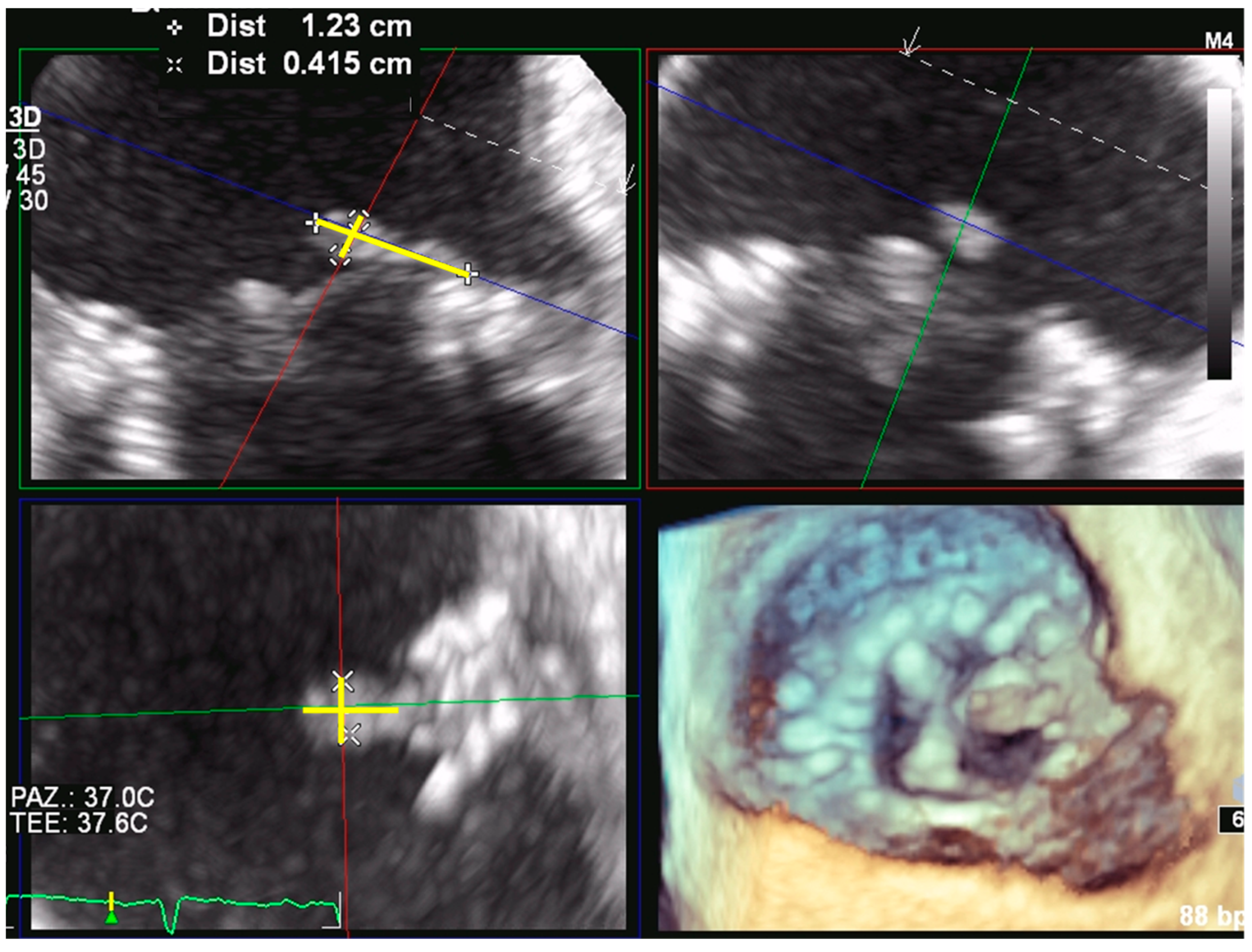
3.1. Mitral Paravalvular Leak
3.2. Aortic Paravalvular Leak
4. Bioprosthetic Structural Valve Degeneration
4.1. Mitral Bioprosthetic SVD
4.2. Aortic Bioprosthetic SVD
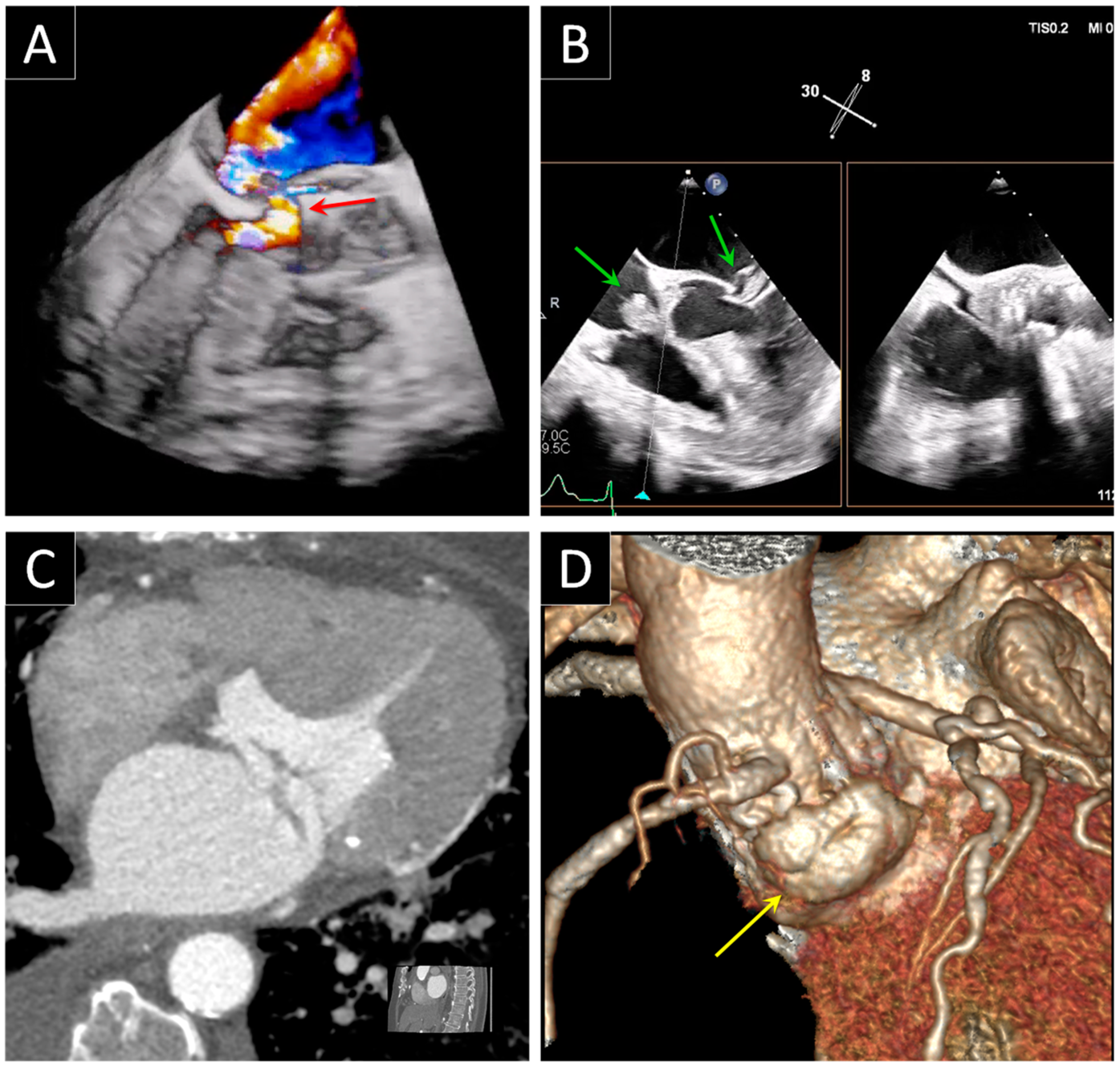
5. Prosthetic Endocarditis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A., Jr.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014. [Google Scholar] [CrossRef]
- Barbetseas, J.; Nagueh, S.F.; Pitsavos, C.; Toutouzas, P.K.; Quinones, M.A.; Zoghbi, W.A. Differentiating thrombus from pannus formation in obstructed mechanical prosthetic valves: An evaluation of clinical, transthoracic and transesophageal echocardiographic parameters. J. Am. Coll. Cardiol. 1998, 32, 1410–1417. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Schaff, H.V.; Sundt, T.M.; Rahimtoola, S.H. Treatment of obstructive thrombosed prosthetic heart valve. J. Am. Coll. Cardiol. 2013, 62, 1731–1736. [Google Scholar] [CrossRef] [Green Version]
- Pislaru, S.V.; Hussain, I.; Pellikka, P.A.; Maleszewski, J.J.; Hanna, R.D.; Schaff, H.V.; Connolly, H.M. Misconceptions, diagnostic challenges and treatment opportunities in bioprosthetic valve thrombosis: Lessons from a case series. Eur. J. Cardiothorac. Surg. 2015, 47, 725–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanis, W.; Habets, J.; van den Brink, R.B.; Symersky, P.; Budde, R.P.; Chamuleau, S.A. Differentiation of thrombus from pannus as the cause of acquired mechanical prosthetic heart valve obstruction by non-invasive imaging: A review of the literature. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Muratori, M.; Montorsi, P.; Teruzzi, G.; Celeste, F.; Doria, E.; Alamanni, F.; Pepi, M. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am. J. Cardiol. 2006, 97, 94–100. [Google Scholar] [CrossRef]
- Montorsi, P.; De Bernardi, F.; Muratori, M.; Cavoretto, D.; Pepi, M. Role of cine-fluoroscopy, transthoracic, and transesophageal echocardiography in patients with suspected prosthetic heart valve thrombosis. Am. J. Cardiol. 2000, 85, 58–64. [Google Scholar] [CrossRef]
- Montorsi, P.; Cavoretto, D.; Alimento, M.; Muratori, M.; Pepi, M. Prosthetic mitral valve thrombosis: Can fluoroscopy predict the efficacy of thrombolytic treatment? Circulation 2003, 108, II79–II84. [Google Scholar] [CrossRef] [Green Version]
- Montorsi, P.; Cavoretto, D.; Parolari, A.; Muratori, M.; Alimento, M.; Pepi, M. Diagnosing prosthetic mitral valve thrombosis and the effect of the type of prosthesis. Am. J. Cardiol. 2002, 90, 73–76. [Google Scholar] [CrossRef]
- Muratori, M.; Fusini, L.; Ghulam Ali, S.; Teruzzi, G.; Corrieri, N.; Gripari, P.; Mapelli, M.; Annoni, A.; Tamborini, G.; Rabbat, M.G.; et al. Detection of Mechanical Prosthetic Valve Dysfunction. Am. J. Cardiol. 2021, 150, 101–109. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, Y.J.; Han, K.; Kim, Y.J.; Choi, B.W. Diagnostic Value of Advanced Imaging Modalities for the Detection and Differentiation of Prosthetic Valve Obstruction: A Systematic Review and Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 2182–2192. [Google Scholar] [CrossRef]
- Habets, J.; Tanis, W.; Mali, W.P.; Chamuleau, S.A.; Budde, R.P. Imaging of prosthetic heart valve dysfunction: Complementary diagnostic value of TEE and MDCT? JACC Cardiovasc. Imaging 2012, 5, 956–961. [Google Scholar] [CrossRef]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Muratori, M.; Montorsi, P.; Maffessanti, F.; Teruzzi, G.; Zoghbi, W.A.; Gripari, P.; Tamborini, G.; Ghulam Ali, S.; Fusini, L.; Fiorentini, C.; et al. Dysfunction of bileaflet aortic prosthesis: Accuracy of echocardiography versus fluoroscopy. JACC Cardiovasc. Imaging 2013, 6, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Ben Zekry, S.; Saad, R.M.; Ozkan, M.; Al Shahid, M.S.; Pepi, M.; Muratori, M.; Xu, J.; Little, S.H.; Zoghbi, W.A. Flow acceleration time and ratio of acceleration time to ejection time for prosthetic aortic valve function. JACC Cardiovasc. Imaging 2011, 4, 1161–1170. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Yang, D.H.; Shin, S.Y.; Kim, N.; Kang, J.W.; Kim, D.H.; Song, J.M.; Kang, D.H.; Song, J.K.; Kim, J.B.; et al. Subprosthetic Pannus after Aortic Valve Replacement Surgery: Cardiac CT Findings and Clinical Features. Radiology 2015, 276, 724–731. [Google Scholar] [CrossRef]
- Ha, H.; Koo, H.J.; Huh, H.K.; Kim, G.B.; Kweon, J.; Kim, N.; Kim, Y.H.; Kang, J.W.; Lim, T.H.; Song, J.K.; et al. Effect of pannus formation on the prosthetic heart valve: In vitro demonstration using particle image velocimetry. PLoS ONE 2018, 13, e0199792. [Google Scholar] [CrossRef]
- Aladmawi, M.A.; Pragliola, C.; Vriz, O.; Galzerano, D. Use of multidetector-row computed tomography scan to detect pannus formation in prosthetic mechanical aortic valves. J. Thorac. Dis. 2017, 9, S343–S348. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.J.; Dweck, M.R.; Dreisbach, J.G.; Williams, M.C.; Mak, S.M.; Cartlidge, T.; Nicol, E.D.; Morgan-Hughes, G.J. Complementary role of cardiac CT in the assessment of aortic valve replacement dysfunction. Open Heart 2016, 3, e000494. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Hahn, R.T.; Weissman, N.J.; Monaghan, M.J. Assessment of paravalvular regurgitation following TAVR: A proposal of unifying grading scheme. JACC Cardiovasc. Imaging 2015, 8, 340–360. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, C.E.; Hahn, R.T.; Berrebi, A.; Borer, J.S.; Cutlip, D.E.; Fontana, G.; Gerosa, G.; Ibrahim, R.; Jelnin, V.; Jilaihawi, H.; et al. Clinical Trial Principles and Endpoint Definitions for Paravalvular Leaks in Surgical Prosthesis. Eur. Heart J. 2018, 39, 1224–1245. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, ehab395, In press. [Google Scholar] [CrossRef]
- Taramasso, M.; Maisano, F.; Denti, P.; Guidotti, A.; Sticchi, A.; Pozzoli, A.; Buzzatti, N.; De Bonis, M.; La Canna, G.; Alfieri, O. Surgical treatment of paravalvular leak: Long-term results in a single-center experience (up to 14 years). J. Thorac. Cardiovasc. Surg. 2015, 149, 1270–1275. [Google Scholar] [CrossRef] [Green Version]
- Alkhouli, M.; Rihal, C.S.; Zack, C.J.; Eleid, M.F.; Maor, E.; Sarraf, M.; Cabalka, A.K.; Reeder, G.S.; Hagler, D.J.; Maalouf, J.F.; et al. Transcatheter and Surgical Management of Mitral Paravalvular Leak: Long-Term Outcomes. JACC Cardiovasc. Interv. 2017, 10, 1946–1956. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef] [Green Version]
- Kronzon, I.; Sugeng, L.; Perk, G.; Hirsh, D.; Weinert, L.; Garcia Fernandez, M.A.; Lang, R.M. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. J. Am. Coll. Cardiol. 2009, 53, 1543–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Fernandez, M.A.; Cortes, M.; Garcia-Robles, J.A.; Gomez de Diego, J.J.; Perez-David, E.; Garcia, E. Utility of real-time three-dimensional transesophageal echocardiography in evaluating the success of percutaneous transcatheter closure of mitral paravalvular leaks. J. Am. Soc. Echocardiogr. 2010, 23, 26–32. [Google Scholar] [CrossRef]
- Ionescu, A.; Fraser, A.G.; Butchart, E.G. Prevalence and clinical significance of incidental paraprosthetic valvar regurgitation: A prospective study using transoesophageal echocardiography. Heart 2003, 89, 1316–1321. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [CrossRef]
- Dvir, D.; Bourguignon, T.; Otto, C.M.; Hahn, R.T.; Rosenhek, R.; Webb, J.G.; Treede, H.; Sarano, M.E.; Feldman, T.; Wijeysundera, H.C.; et al. Standardized Definition of Structural Valve Degeneration for Surgical and Transcatheter Bioprosthetic Aortic Valves. Circulation 2018, 137, 388–399. [Google Scholar] [CrossRef]
- Arsalan, M.; Walther, T. Durability of prostheses for transcatheter aortic valve implantation. Nat. Rev. Cardiol. 2016, 13, 360–367. [Google Scholar] [CrossRef]
- Côté, N.; Pibarot, P.; Clavel, M.A. Incidence, risk factors, clinical impact, and management of bioprosthesis structural valve degeneration. Curr. Opin. Cardiol. 2017, 32, 123–129. [Google Scholar] [CrossRef]
- Rodriguez-Gabella, T.; Voisine, P.; Puri, R.; Pibarot, P.; Rodes-Cabau, J. Aortic Bioprosthetic Valve Durability: Incidence, Mechanisms, Predictors, and Management of Surgical and Transcatheter Valve Degeneration. J. Am. Coll. Cardiol. 2017, 70, 1013–1028. [Google Scholar] [CrossRef]
- Paradis, J.M.; Del Trigo, M.; Puri, R.; Rodes-Cabau, J. Transcatheter Valve-in-Valve and Valve-in-Ring for Treating Aortic and Mitral Surgical Prosthetic Dysfunction. J. Am. Coll. Cardiol. 2015, 66, 2019–2037. [Google Scholar] [CrossRef] [Green Version]
- Little, S.H.; Bapat, V.; Blanke, P.; Guerrero, M.; Rajagopal, V.; Siegel, R. Imaging Guidance for Transcatheter Mitral Valve Intervention on Prosthetic Valves, Rings, and Annular Calcification. JACC Cardiovasc. Imaging 2021, 14, 22–40. [Google Scholar] [CrossRef]
- Reid, A.; Ben Zekry, S.; Turaga, M.; Tarazi, S.; Bax, J.J.; Wang, D.D.; Piazza, N.; Bapat, V.N.; Ihdayhid, A.R.; Cavalcante, J.L.; et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc. Imaging 2021, 14, 854–866. [Google Scholar] [CrossRef]
- Salaun, E.; Clavel, M.A.; Rodes-Cabau, J.; Pibarot, P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart 2018, 104, 1323–1332. [Google Scholar] [CrossRef]
- Egbe, A.C.; Pislaru, S.V.; Pellikka, P.A.; Poterucha, J.T.; Schaff, H.V.; Maleszewski, J.J.; Connolly, H.M. Bioprosthetic Valve Thrombosis Versus Structural Failure: Clinical and Echocardiographic Predictors. J. Am. Coll. Cardiol. 2015, 66, 2285–2294. [Google Scholar] [CrossRef]
- Pache, G.; Blanke, P.; Zeh, W.; Jander, N. Cusp thrombosis after transcatheter aortic valve replacement detected by computed tomography and echocardiography. Eur. Heart J. 2013, 34, 3546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.P.M.; Cartlidge, T.R.; Dweck, M.R.; Moss, A.J. Cardiac CT in prosthetic aortic valve complications. Br. J. Radiol 2019, 92, 20180237. [Google Scholar] [CrossRef]
- Habib, G.; Thuny, F.; Avierinos, J.F. Prosthetic valve endocarditis: Current approach and therapeutic options. Prog. Cardiovasc. Dis. 2008, 50, 274–281. [Google Scholar] [CrossRef]
- Perez-Vazquez, A.; Farinas, M.C.; Garcia-Palomo, J.D.; Bernal, J.M.; Revuelta, J.M.; Gonzalez-Macias, J. Evaluation of the Duke criteria in 93 episodes of prosthetic valve endocarditis: Could sensitivity be improved? Arch. Intern. Med. 2000, 160, 1185–1191. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef] [Green Version]
- Sordelli, C.; Fele, N.; Mocerino, R.; Weisz, S.H.; Ascione, L.; Caso, P.; Carrozza, A.; Tascini, C.; De Vivo, S.; Severino, S. Infective Endocarditis: Echocardiographic Imaging and New Imaging Modalities. J. Cardiovasc. Echogr. 2019, 29, 149–155. [Google Scholar] [CrossRef]
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schafers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344. [Google Scholar] [CrossRef]
- Salaun, E.; Habib, G. Beyond Standard Echocardiography in Infective Endocarditis: Computed Tomography, 3-Dimensional Imaging, and Multi-Imaging. Circ. Cardiovasc. Imaging 2018, 11, e007626. [Google Scholar] [CrossRef]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: Increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef] [Green Version]
- Dilsizian, V.; Budde, R.P.J.; Chen, W.; Mankad, S.V.; Lindner, J.R.; Nieman, K. Best Practices for Imaging Cardiac Device-Related Infections and Endocarditis: A JACC: Cardiovascular Imaging Expert Panel Statement. JACC Cardiovasc. Imaging, 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Mikail, N.; Benali, K.; Iung, B.; Duval, X.; Nataf, P.; Jondeau, G.; Hyafil, F.; Le Guludec, D.; Rouzet, F. Characterization of (18)F-Fluorodeoxyglucose Uptake Pattern in Noninfected Prosthetic Heart Valves. Circ. Cardiovasc. Imaging 2017, 10, e005585. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratori, M.; Fusini, L.; Mancini, M.E.; Tamborini, G.; Ghulam Ali, S.; Gripari, P.; Doldi, M.; Frappampina, A.; Teruzzi, G.; Pontone, G.; et al. The Role of Multimodality Imaging in Left-Sided Prosthetic Valve Dysfunction. J. Cardiovasc. Dev. Dis. 2022, 9, 12. https://doi.org/10.3390/jcdd9010012
Muratori M, Fusini L, Mancini ME, Tamborini G, Ghulam Ali S, Gripari P, Doldi M, Frappampina A, Teruzzi G, Pontone G, et al. The Role of Multimodality Imaging in Left-Sided Prosthetic Valve Dysfunction. Journal of Cardiovascular Development and Disease. 2022; 9(1):12. https://doi.org/10.3390/jcdd9010012
Chicago/Turabian StyleMuratori, Manuela, Laura Fusini, Maria Elisabetta Mancini, Gloria Tamborini, Sarah Ghulam Ali, Paola Gripari, Marco Doldi, Antonio Frappampina, Giovanni Teruzzi, Gianluca Pontone, and et al. 2022. "The Role of Multimodality Imaging in Left-Sided Prosthetic Valve Dysfunction" Journal of Cardiovascular Development and Disease 9, no. 1: 12. https://doi.org/10.3390/jcdd9010012
APA StyleMuratori, M., Fusini, L., Mancini, M. E., Tamborini, G., Ghulam Ali, S., Gripari, P., Doldi, M., Frappampina, A., Teruzzi, G., Pontone, G., Montorsi, P., & Pepi, M. (2022). The Role of Multimodality Imaging in Left-Sided Prosthetic Valve Dysfunction. Journal of Cardiovascular Development and Disease, 9(1), 12. https://doi.org/10.3390/jcdd9010012








