Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Abstract
:1. Introduction
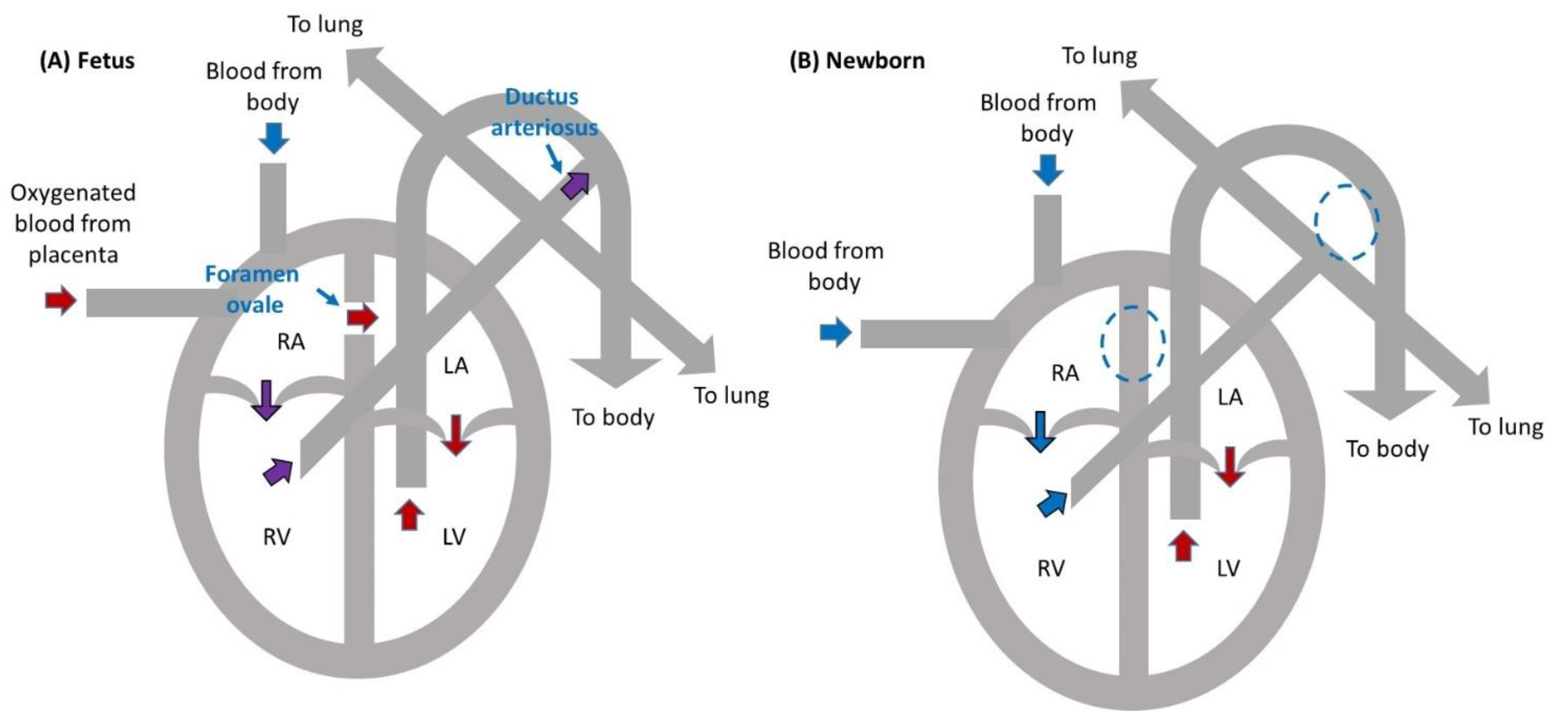
2. Fetal Heart Circulation
3. Abnormal Blood Flow in Congenital Heart Disease
4. Early Perturbed Flow and TOF
5. Genetic Mutations Associated with TOF
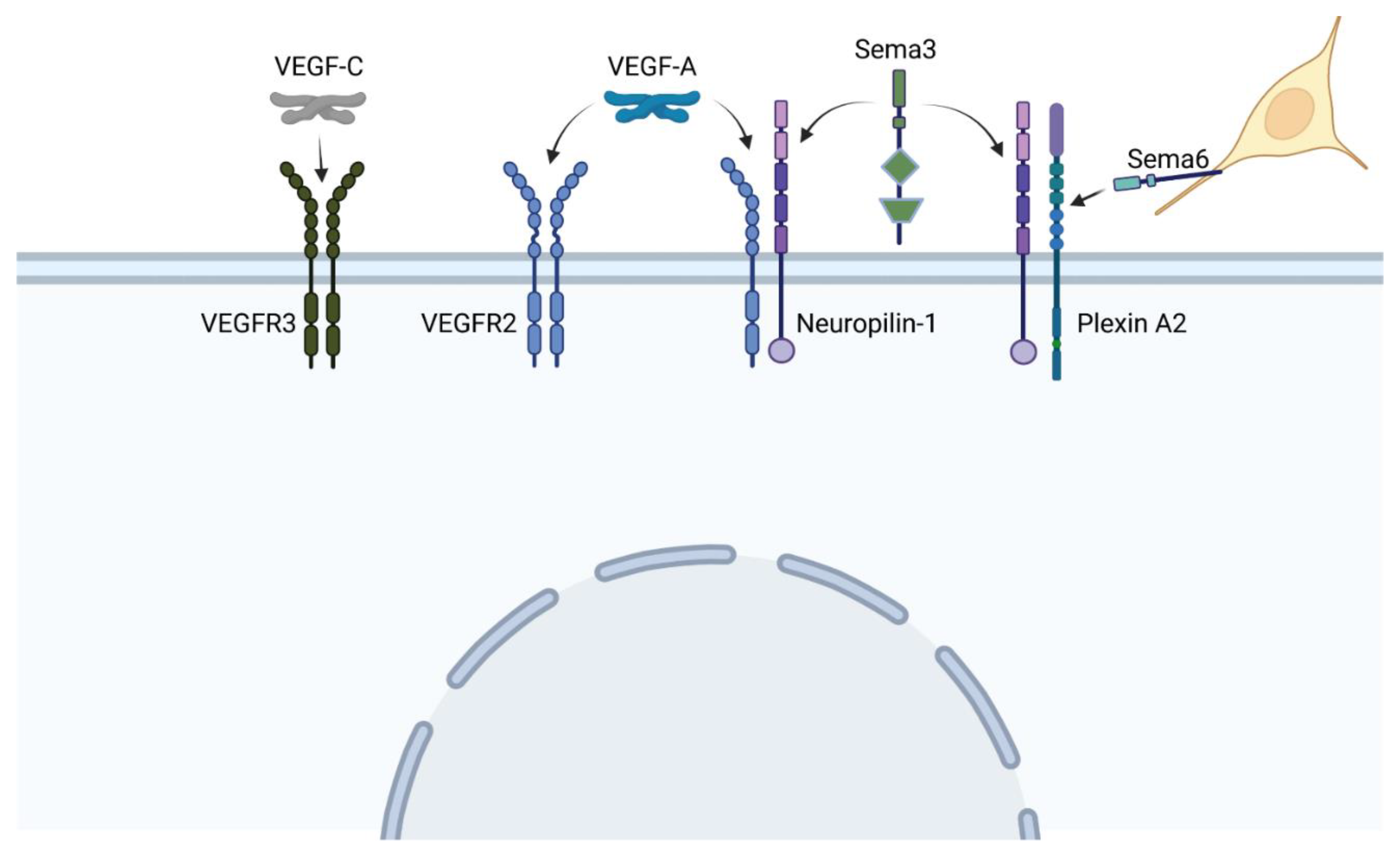
5.1. The Vascular Endothelial Growth Factor (VEGF) Family
5.2. Mutations within the VEGF-A Pathway Are Associated with TOF
5.3. Mutations within the VEGF-Semaphorin Crosstalk Mediator Neuropilin-1 Can Lead to TOF or Persistent Truncus Arteriosus
5.4. Mutations within the Semaphorin Signaling Pathway Lead to TOF or Persistent Truncus Arteriosus
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ward, C.; Stadt, H.; Hutson, M.; Kirby, M.L. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Dev. Biol. 2005, 284, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Scherptong, R.W.; Jongbloed, M.R.; Wisse, L.J.; Vicente-Steijn, R.; Bartelings, M.M.; Poelmann, R.E.; Schalij, M.J.; Groot, A.C.G.-D. Morphogenesis of outflow tract rotation during cardiac development: The pulmonary push concept. Dev. Dyn. 2012, 241, 1413–1422. [Google Scholar] [CrossRef]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef]
- Hiruma, T.; Hirakow, R. Formation of the pharyngeal arch arteries in the chick embryo. Observations of corrosion casts by scanning electron microscopy. Anat. Embryol. 1995, 191, 415–423. [Google Scholar] [CrossRef]
- Bertrand, N.; Roux, M.; Ryckebüsch, L.; Niederreither, K.; Dollé, P.; Moon, A.; Capecchi, M.; Zaffran, S. Hox genes define distinct progenitor sub-domains within the second heart field. Dev. Biol. 2011, 353, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Kirby, M.L. Pulmonary Atresia or Persistent Truncus Arteriosus. Circ. Res. 2008, 103, 337–339. [Google Scholar] [CrossRef]
- Hernandez-Andrade, E.; Patwardhan, M.; Cruz-Lemini, M.; Luewan, S. Early Evaluation of the Fetal Heart. Fetal Diagn. Ther. 2017, 42, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Pervolaraki, E.; Anderson, R.A.; Benson, A.P.; Hayes-Gill, B.; Holden, A.V.; Moore, B.J.R.; Paley, M.N.; Zhang, H. Antenatal architecture and activity of the human heart. Interface Focus 2013, 3, 20120065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.; Glickstein, J.S.; Kleinman, C.S.; Levasseur, S.M.; Chen, J.; Gersony, W.M.; Williams, I.A. The Impact of Prenatal Diagnosis of Complex Congenital Heart Disease on Neonatal Outcomes. Pediatr. Cardiol. 2010, 31, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, B.; Levasseur, S.M.; Woldu, K.; Glickstein, J.S.; Andrews, H.F.; Williams, I.A. Fetal Echocardiographic Measurements and the Need for Neonatal Surgical Intervention in Tetralogy of Fallot. Pediatr. Cardiol. 2013, 35, 810–816. [Google Scholar] [CrossRef]
- Landis, B.J.; Levey, A.; Levasseur, S.M.; Glickstein, J.S.; Kleinman, C.S.; Simpson, L.L.; Williams, I.A. Prenatal Diagnosis of Congenital Heart Disease and Birth Outcomes. Pediatr. Cardiol. 2012, 34, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Holland, B.J.; Myers, J.A.; Woods, C.R. Prenatal diagnosis of critical congenital heart disease reduces risk of death from cardiovascular compromise prior to planned neonatal cardiac surgery: A meta-analysis. Ultrasound Obstet. Gynecol. 2015, 45, 631–638. [Google Scholar] [CrossRef]
- Jatavan, P.; Tongprasert, F.; Srisupundit, K.; Luewan, S.; Traisrisilp, K.; Tongsong, T. Quantitative Cardiac Assessment in Fetal Tetralogy of Fallot. J. Ultrasound Med. 2016, 35, 1481–1488. [Google Scholar] [CrossRef]
- Wiputra, H. Right Ventricular Physiology in Health and Disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, 1649–1659. [Google Scholar] [CrossRef]
- Emidgett, M.; Erugonyi, S. Congenital heart malformations induced by hemodynamic altering surgical interventions. Front. Physiol. 2014, 5, 287. [Google Scholar] [CrossRef] [Green Version]
- Midgett, M.; Thornburg, K.L.; Rugonyi, S. Blood flow patterns underlie developmental heart defects. Am. J. Physiol. Circ. Physiol. 2017, 312, H632–H642. [Google Scholar] [CrossRef]
- Karakaya, C.; Goktas, S.; Celik, M.; Kowalski, W.J.; Keller, B.B.; Pekkan, K. Asymmetry in Mechanosensitive Gene Expression during Aortic Arch Morphogenesis. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Lashkarinia, S.S.; Çoban, G.; Ermek, E.; Çelik, M.; Pekkan, K. Spatiotemporal remodeling of embryonic aortic arch: Stress distribution, microstructure, and vascular growth in silico. Biomech. Model. Mechanobiol. 2020, 19, 1897–1915. [Google Scholar] [CrossRef] [PubMed]
- Celik, M.; Goktas, S.; Karakaya, C.; Cakiroglu, A.I.; Karahuseyinoglu, S.; Lashkarinia, S.S.; Ermek, E.; Pekkan, K. Microstructure of early embryonic aortic arch and its reversibility following mechanically altered hemodynamic load release. Am. J. Physiol. Circ. Physiol. 2020, 318, H1208–H1218. [Google Scholar] [CrossRef]
- Lawson, T.B.; Scott-Drechsel, D.E.; Chivukula, V.K.; Rugonyi, S.; Thornburg, K.L.; Hinds, M.T. Hyperglycemia Alters the Structure and Hemodynamics of the Developing Embryonic Heart. J. Cardiovasc. Dev. Dis. 2018, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Karunamuni, G.; Gu, S.; Doughman, Y.Q.; Peterson, L.M.; Mai, K.; McHale, Q.; Jenkins, M.W.; Linask, K.K.; Rollins, A.M.; Watanabe, M. Ethanol exposure alters early cardiac function in the looping heart: A mechanism for congenital heart defects? Am. J. Physiol. Circ. Physiol. 2014, 306, H414–H421. [Google Scholar] [CrossRef] [Green Version]
- Groenendijk, B.C.; Hierck, B.P.; Groot, A.C.G.-D.; Poelmann, R.E. Development-related changes in the expression of shear stress responsive genesKLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev. Dyn. 2004, 230, 57–68. [Google Scholar] [CrossRef]
- Alser, M.; Shurbaji, S.; Yalcin, H. Mechanosensitive Pathways in Heart Development: Findings from Chick Embryo Studies. J. Cardiovasc. Dev. Dis. 2021, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, S.J.; El-Brolosy, M.; Tsedeke, A.T.; Bensimon-Brito, A.; Ghanbari, P.; Maischein, H.-M.; Kuenne, C.; Stainier, D.Y. The flow responsive transcription factor Klf2 is required for myocardial wall integrity by modulating Fgf signaling. Elife 2018, 7, e38889. [Google Scholar] [CrossRef] [PubMed]
- Midgett, M.; López, C.S.; David, L.; Maloyan, A.; Rugonyi, S. Increased Hemodynamic Load in Early Embryonic Stages Alters Endocardial to Mesenchymal Transition. Front. Physiol. 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midgett, M.; López, C.S.; David, L.; Maloyan, A.; Rugonyi, S. Increased Hemodynamic Load in Early Embryonic Stages Alters Myofibril and Mitochondrial Organization in the Myocardium. Front. Physiol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.; Eberth, J.F.; Goodwin, R.L.; Potts, J.D. Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development. J. Cardiovasc. Dev. Dis. 2015, 2, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Steed, E.; Faggianelli, N.; Roth, S.; Ramspacher, C.; Concordet, J.-P.; Vermot, J. klf2a couples mechanotransduction and zebrafish valve morphogenesis through fibronectin synthesis. Nat. Commun. 2016, 7, 11646. [Google Scholar] [CrossRef] [Green Version]
- Dyer, L.A.; Kirby, M.L. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev. Biol. 2009, 330, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanneman, K.; Newman, B.; Chan, F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics 2017, 37, 32–51. [Google Scholar] [CrossRef]
- Nishibatake, M.; Kirby, M.L.; Van Mierop, L.H. Pathogenesis of persistent truncus arteriosus and dextroposed aorta in the chick embryo after neural crest ablation. Circulation 1987, 75, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Tomita, H.; Connuck, D.M.; Leatherbury, L.; Kirby, M.L. Relation of Early Hemodynamic Changes to Final Cardiac Phenotpe and Survival After Neural Crest Ablation in Chick Embryos. Circulation 1991, 84, 1289–1295. [Google Scholar] [CrossRef] [Green Version]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vion, A.-C.; Perovic, T.; Petit, C.; Hollfinger, I.; Bartels-Klein, E.; Frampton, E.; Gordon, E.; Claesson-Welsh, L.; Gerhardt, H. Endothelial Cell Orientation and Polarity Are Controlled by Shear Stress and VEGF Through Distinct Signaling Pathways. Front. Physiol. 2021, 11, 1743. [Google Scholar] [CrossRef]
- Reuter, M.S.; Jobling, R.; Chaturvedi, R.R.; Manshaei, R.; Costain, G.; Heung, T.; Curtis, M.; Hosseini, S.M.; Liston, E.; Lowther, C.; et al. Haploinsufficiency of vascular endothelial growth factor related signaling genes is associated with tetralogy of Fallot. Genet. Med. 2018, 21, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrechts, D.; Devriendt, K.; Driscoll, D.A.; Goldmuntz, E.; Gewillig, M.; Vlietinck, R.; Collen, D.; Carmeliet, P. Low expression VEGF haplotype increases the risk for tetralogy of Fallot: A family based association study. J. Med Genet. 2005, 42, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, C.-L.; Li, X.-X.; Li, Q.-C.; Ma, L.-M.; Liu, G.-L. VEGF Gene Polymorphisms are Associated with Risk of Tetralogy of Fallot. Med. Sci. Monit. 2015, 21, 3474–3482. [Google Scholar] [CrossRef] [Green Version]
- Doi, K.; Noiri, E.; Nakao, A.; Fujita, T.; Kobayashi, S.; Tokunaga, K. Functional Polymorphisms in the Vascular Endothelial Growth Factor Gene Are Associated with Development of End-Stage Renal Disease in Males. J. Am. Soc. Nephrol. 2006, 17, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Ferreira, V.; Breier, G.; Pollefeyt, S.; Kieckens, L.; Gertsenstein, M.; Fahrig, M.; Vandenhoeck, A.; Harpal, K.; Eberhardt, C.; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nat. Cell Biol. 1996, 380, 435–439. [Google Scholar] [CrossRef]
- Ferrara, N.; Carver-Moore, K.; Chen, H.; Dowd, M.; Lu, L.; O’Shea, K.S.; Powell-Braxton, L.; Hillan, K.J.; Moore, M.W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nat. Cell Biol. 1996, 380, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Damert, A.; Miquerol, L.; Gertsenstein, M.; Risau, W.; Nagy, A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development 2002, 129, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, F.; Rossant, J.; Yamaguchi, T.P.; Gertsenstein, M.; Wu, X.-F.; Breitman, M.L.; Schuh, A.C. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nat. Cell Biol. 1995, 376, 62–66. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Chen, K.; Jubran, A.; Ramirez, A.; Astrof, S. Endothelium in the pharyngeal arches 3, 4 and 6 is derived from the second heart field. Dev. Biol. 2017, 421, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, K.; Shiratori, H.; Hamada, H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nat. Cell Biol. 2007, 450, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Azhar, M.; Molin, D.G.M. Transforming Growth Factor [beta] -SMAD2 Signaling and Aortic Arch Development. Trends Cardiovasc. Med. 2006, 16, 1–6. [Google Scholar] [CrossRef]
- Maruyama, K.; Miyagawa-Tomita, S.; Mizukami, K.; Matsuzaki, F.; Kurihara, H. Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev. Biol. 2019, 452, 134–143. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 2003, 5, 74–80. [Google Scholar] [CrossRef]
- Page, D.J.; Miossec, M.J.; Williams, S.; Monaghan, R.; Fotiou, E.; Cordell, H.J.; Sutcliffe, L.; Topf, A.; Bourgey, M.; Bourque, G.; et al. Whole Exome Sequencing Reveals the Major Genetic Contributors to Nonsyndromic Tetralogy of Fallot. Circ. Res. 2019, 124, 553–563. [Google Scholar] [CrossRef]
- Dumont, D.J.; Jussila, L.; Taipale, J.; Lymboussaki, A.; Mustonen, T.; Pajusola, K.; Breitman, M.; Alitalo, K. Cardiovascular Failure in Mouse Embryos Deficient in VEGF Receptor-3. Science 1998, 282, 946–949. [Google Scholar] [CrossRef]
- Groppa, E.; Brkic, S.; Bovo, E.; Reginato, S.; Sacchi, V.; Di Maggio, N.; Muraro, M.G.; Calabrese, D.; Heberer, M.; Gianni-Barrera, R.; et al. VEGF dose regulates vascular stabilization through Semaphorin3A and the Neuropilin-1 + monocyte/ TGF -β1 paracrine axis. EMBO Mol. Med. 2015, 7, 1366–1384. [Google Scholar] [CrossRef]
- Yelland, T.; Djordjevic, S. Crystal Structure of the Neuropilin-1 MAM Domain: Completing the Neuropilin-1 Ectodomain Picture. Structure 2016, 24, 2008–2015. [Google Scholar] [CrossRef] [Green Version]
- Mehta, V.; Fields, L.; Evans, I.M.; Yamaji, M.; Pellet-Many, C.; Jones, T.; Mahmoud, M.; Zachary, I. VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling. Arter. Thromb. Vasc. Biol. 2018, 38, 1845–1858. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Rodriguez, E.R.; Reimert, D.V.; Shu, T.; Fritzsch, B.; Richards, L.J.; Kolodkin, A.L.; Ginty, D.D. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 2003, 5, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Cordell, H.J.; Töpf, A.; Mamasoula, C.; Postma, A.; Bentham, J.; Zelenika, D.; Heath, S.; Blue, G.M.; Cosgrove, C.; Granados-Riveron, J.T.; et al. Genome-wide association study identifies loci on 12q24 and 13q32 associated with Tetralogy of Fallot. Hum. Mol. Genet. 2013, 22, 1473–1481. [Google Scholar] [CrossRef]
- Fan, S.-H.; Shen, Z.-Y.; Xiao, Y.-M. Functional polymorphisms of the neuropilin 1 gene are associated with the risk of tetralogy of Fallot in a Chinese Han population. Gene 2018, 653, 72–79. [Google Scholar] [CrossRef]
- Roy, S.; Bag, A.K.; Singh, R.K.; Talmadge, J.E.; Batra, S.K.; Datta, K. Multifaceted Role of Neuropilins in the Immune System: Potential Targets for Immunotherapy. Front. Immunol. 2017, 8, 1228. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.F.; Fitzgerald, D.J. Vascular endothelial cell growth factor (VEGF) induces cyclooxygenase (COX)-dependent proliferation of endothelial cells (EC) via the VEGF-2 receptor. FASEB J. 2001, 15, 1667–1669. [Google Scholar] [CrossRef]
- Dixelius, J.; Olsson, A.K.; Thulin, Å.; Lee, C.; Johansson, I.; Claesson-Welsh, L. Minimal Active Domain and Mechanism of Action of the Angiogenesis Inhibitor Histidine-Rich Glycoprotein. Cancer Res. 2006, 66, 2089–2097. [Google Scholar] [CrossRef] [Green Version]
- Duran, I.; Tenney, J.; Warren, C.M.; Sarukhanov, A.; Csukasi, F.; Skalansky, M.; Iruela-Arispe, L.; Krakow, D. NRP1 haploinsufficiency predisposes to the development of Tetralogy of Fallot. Am. J. Med Genet. Part A 2018, 176, 649–656. [Google Scholar] [CrossRef]
- Koch, S.; van Meeteren, L.; Morin, E.; Testini, C.; Weström, S.; Björkelund, H.; LE Jan, S.; Adler, J.; Berger, P.; Claesson-Welsh, L. NRP1 Presented in trans to the Endothelium Arrests VEGFR2 Endocytosis, Preventing Angiogenic Signaling and Tumor Initiation. Dev. Cell 2014, 28, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, R.; Alhashem, A.; Alghamdi, M.H.; Seidahmad, M.Z.; Wakil, S.; Dagriri, K.; Keavney, B.; Goodship, J.; Alyousif, S.; Alhabshan, F.; et al. Positional mapping ofPRKD1,NRP1andPRDM1as novel candidate disease genes in truncus arteriosus. J. Med Genet. 2015, 52, 322–329. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kitsukawa, T.; Bekku, Y.; Matsuda, Y.; Sanbo, M.; Yagi, T.; Fujisawa, H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Newbern, J.; Zhong, J.; Wickramasinghe, R.S.; Li, X.; Wu, Y.; Samuels, I.; Cherosky, N.; Karlo, J.C.; O’Loughlin, B.; Wikenheiser, J.; et al. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc. Natl. Acad. Sci. USA 2008, 105, 17115–17120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Taniguchi, M. Congenital diseases and semaphorin signaling: Overview to date of the evidence linking them. Congenit. Anom. 2014, 55, 26–30. [Google Scholar] [CrossRef]
- van Gils, J.; Ramkhelawon, B.; Fernandes, L.; Stewart, M.C.; Guo, L.; Seibert, T.; Menezes, G.B.; Cara, D.C.; Chow, C.; Kinane, T.B.; et al. Endothelial Expression of Guidance Cues in Vessel Wall Homeostasis Dysregulation Under Proatherosclerotic Conditions. Arter. Thromb. Vasc. Biol. 2013, 33, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttmann-Raviv, N.; Shraga-Heled, N.; Varshavsky, A.; Guimaraes-Sternberg, C.; Kessler, O.; Neufeld, G. Semaphorin-3A and Semaphorin-3F Work Together to Repel Endothelial Cells and to Inhibit Their Survival by Induction of Apoptosis. J. Biol. Chem. 2007, 282, 26294–26305. [Google Scholar] [CrossRef] [Green Version]
- Silversides, C.K.; Lionel, A.C.; Costain, G.; Merico, D.; Migita, O.; Liu, B.; Yuen, T.; Rickaby, J.; Thiruvahindrapuram, B.; Marshall, C.R.; et al. Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways. PLoS Genet. 2012, 8, e1002843. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Shibata, S.; Miyagawa-Tomita, S.; Ong, S.-G.; Takahashi, H.; Kume, T.; Okano, H.; Matsuoka, R.; Yamagishi, H. Regulation of Sema3c and the Interaction between Cardiac Neural Crest and Second Heart Field during Outflow Tract Development. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plein, A.; Calmont, A.; Fantin, A.; Denti, L.; Anderson, N.A.; Scambler, P.J.; Ruhrberg, C. Neural crest–derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J. Clin. Investig. 2015, 125, 2661–2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toyofuku, T.; Yoshida, J.; Sugimoto, T.; Yamamoto, M.; Makino, N.; Takamatsu, H.; Takegahara, N.; Suto, F.; Hori, M.; Fujisawa, H.; et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 2008, 321, 251–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.B.; Feiner, L.; Lu, M.M.; Li, J.; Ma, X.; Webber, A.L.; Jia, L.; Raper, J.A.; Epstein, J.A. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 2001, 128, 3071–3080. [Google Scholar] [CrossRef]
- Feiner, L.; Webber, A.L.; Brown, C.B.; Lu, M.M.; Jia, L.; Feinstein, P.; Mombaerts, P.; Epstein, J.A.; Raper, J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001, 128, 3061–3070. [Google Scholar] [CrossRef]
- Kodo, K.; Nishizawa, T.; Furutani, M.; Arai, S.; Yamamura, E.; Joo, K.; Takahashi, T.; Matsuoka, R.; Yamagishi, H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 13933–13938. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Yoshida, Y.; Livet, J.; Reimert, D.V.; Mann, F.; Merte, J.; Henderson, C.E.; Jessell, T.M.; Kolodkin, A.L.; Ginty, D.D. Semaphorin 3E and Plexin-D1 Control Vascular Pattern Independently of Neuropilins. Science 2005, 307, 265–268. [Google Scholar] [CrossRef]
- Aghajanian, H.; Choi, C.; Ho, V.C.; Gupta, M.; Singh, M.; Epstein, J.A. Semaphorin 3d and Semaphorin 3e Direct Endothelial Motility through Distinct Molecular Signaling Pathways. J. Biol. Chem. 2014, 289, 17971–17979. [Google Scholar] [CrossRef] [Green Version]
- Gitler, A.D.; Lu, M.M.; A Epstein, J. PlexinD1 and Semaphorin Signaling Are Required in Endothelial Cells for Cardiovascular Development. Dev. Cell 2004, 7, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Ta-Shma, A.; Pierri, C.L.; Stepensky, P.; Shaag, A.; Zenvirt, S.; Elpeleg, O.; Rein, A.J. Isolated truncus arteriosus associated with a mutation in the plexin-D1 gene. Am. J. Med Genet. Part A 2013, 161, 3115–3120. [Google Scholar] [CrossRef]
- Moriya, J.; Minamino, T.; Tateno, K.; Okada, S.; Uemura, A.; Shimizu, I.; Yokoyama, M.; Nojima, A.; Okada, M.; Koga, H.; et al. Inhibition of Semaphorin As a Novel Strategy for Therapeutic Angiogenesis. Circ. Res. 2010, 106, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Mehta, V.; Pang, K.-L.; Rozbesky, D.; Nather, K.; Keen, A.; Lachowski, D.; Kong, Y.; Karia, D.; Ameismeier, M.; Huang, J.; et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nat. Cell Biol. 2020, 578, 290–295. [Google Scholar] [CrossRef]
- Chen, H.; He, Z.; Bagri, A.; Tessier-Lavigne, M. Semaphorin–Neuropilin Interactions Underlying Sympathetic Axon Responses to Class III Semaphorins. Neuron 1998, 21, 1283–1290. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Fournier, A.; Nakamura, F.; Wang, L.-H.; Murakami, Y.; Kalb, R.G.; Fujisawa, H.; Strittmatter, S.M. Plexin-Neuropilin-1 Complexes Form Functional Semaphorin-3A Receptors. Cell 1999, 99, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Manshaei, R.; Merico, D.; Reuter, M.S.; Engchuan, W.; Mojarad, B.A.; Chaturvedi, R.; Heung, T.; Pellecchia, G.; Zarrei, M.; Nalpathamkalam, T.; et al. Genes and Pathways Implicated in Tetralogy of Fallot Revealed by Ultra-Rare Variant Burden Analysis in 231 Genome Sequences. Front. Genet. 2020, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.J.; Vedula, V.; Baek, K.I.; Chen, C.; Chen, J.; Chou, M.I.; Lam, J.; Subhedar, S.; Wang, J.; Ding, Y.; et al. Contractile and hemodynamic forces coordinate Notch1b-mediated outflow tract valve formation. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Mutation | Mutation Effects, If Known |
|---|---|---|
| VEGF-A | C2578A C634G C936T Haploinsufficiency (mouse) | Reduces VEGF-A expression Reduces VEGF-A expression Reduces VEGF-A expression Abnormal vasculogenesis and hematopoiesis |
| VEGFR2 | Stop-gain Missense Knockout (mouse) | Loss of blood islands |
| VEGFR3 | Loss-of-function Nonsense | |
| Neuropilin-1 | V733I Predicted miRNA binding site mutation | Sequesters VEGF-A Reduces neuropilin-1 expression |
| PlexinA2 | Copy number loss | |
| Semaphorin 3D | Copy number loss | |
| Semaphorin 3E | Copy number loss | Destabilizes actin cytoskeleton |
| Gene | Mutation | Mutation Effects, If Known |
| Erk2 | Haploinsufficiency | |
| Semaphorin 3C | Null (mouse) | Reduces cardiac neural crest migration |
| Plexin A2 | Null (mouse) | |
| Neuropilin-1 | Truncation | No functional protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyer, L.A.; Rugonyi, S. Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 90. https://doi.org/10.3390/jcdd8080090
Dyer LA, Rugonyi S. Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2021; 8(8):90. https://doi.org/10.3390/jcdd8080090
Chicago/Turabian StyleDyer, Laura A., and Sandra Rugonyi. 2021. "Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease" Journal of Cardiovascular Development and Disease 8, no. 8: 90. https://doi.org/10.3390/jcdd8080090
APA StyleDyer, L. A., & Rugonyi, S. (2021). Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease. Journal of Cardiovascular Development and Disease, 8(8), 90. https://doi.org/10.3390/jcdd8080090







