Abstract
Cardiomyopathies are a group of heterogeneous diseases that affect the muscles of the heart, leading to early morbidity and mortality in young and adults. Genetic forms of cardiomyopathy are caused predominantly by mutations in structural components of the cardiomyocyte sarcomeres, the contractile units of the heart, which includes cardiac Troponin T (TnT). Here, we generated mutations with CRISPR/Cas9 technology in the zebrafish tnnt2a gene, encoding cardiac TnT, at a mutational “hotspot” site to establish a zebrafish model for genetic cardiomyopathies. We found that a heterozygous tnnt2a mutation deleting Arginine at position 94 and Lysine at position 95 of TnT causes progressive cardiac structural changes resulting in heart failure. The cardiac remodeling is presented by an enlarged atrium, decreased ventricle size, increased myocardial stress as well as increased fibrosis. As early as five days post fertilization, larvae carrying the TnT RK94del mutation display diastolic dysfunction and impaired calcium dynamics related to increased Ca2+ sensitivity. In conclusion, adult zebrafish with a heterozygous TnT-RK94del mutation develop cardiomyopathy as seen in patients with TnT mutations and therefore represent a promising model to study disease mechanisms and to screen for putative therapeutic compounds.
1. Introduction
Cardiomyopathies are a heterogeneous group of diseases that affect the myocardium, causing sudden cardiac death and early mortality in children and adults [1]. Patients within the same family can range from being asymptomatic to having mild-severe symptoms [2]. There are several types of cardiomyopathies that are categorized based on their morphological and pathophysiological differences. These include hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), and restrictive cardiomyopathy (RCM) [3]. Initially, it was thought that these individual diseases have distinctive features where HCM has a thickened left ventricle wall and septum, while DCM causes dilation and thinning of the left ventricle wall, and RCM promotes stiffening of the ventricle walls and restrictive filling. However, in recent years, it is becoming more prominent that these cardiomyopathy types can come as a spectrum of disease with overlapping features [4,5]. These features can include the progression of fibrosis, changes in the ventricle wall structure, disruption in the contractility and intracellular calcium (Ca2+), and dysregulation in the electrical functions of the heart. Although these diseases can have various underlying factors, many progress as a result of mutations in proteins of the sarcomere, the contractile unit of the striated muscles. Hence, they are referred to as sarcomeric cardiomyopathies.
Sarcomeres are composed of thick and thin filaments that slide along each other during muscle contraction. In the thin filaments, cardiac Troponin T (TnT) forms a troponin complex with Troponin I (TnI) and Troponin C (TnC) to interact with Tropomyosin (Tm) coiled-coil dimers. These components closely interact with actin molecules to regulate muscle contraction [2]. The thick filaments of the sarcomeres consist of myosin heavy chains and four myosin light chain molecules. These myosin light chains provide mechanical support to the heavy chain and regulate the interaction of the myosin heads with actin, which eventually affects the force and velocity of contraction [2,6]. The principle of muscle contraction relies on the sliding of actin past myosin to create muscle tension, which is controlled by intracellular [Ca2+]. When an action potential is triggered, voltage-sensitive L-type Ca2+ channels (LTCCs) are activated, leading to Ca2+ ion flow into the cytosol of the cardiomyocytes. Ca2+ binds the contractile elements of the cell at TnC to induce allosteric conformational changes in TnI and TnT, which are transmitted to Tm. This conformational change results in force development by actin and myosin, and contraction occurs due to shortening of the sarcomeres [6]. During relaxation, Ca2+ is taken up by the sarcoplasmic reticulum (SR) and shuttled out of the cell through Na+/Ca2+ exchanger (NCX) causing relaxation of the cardiomyocyte [7]. Ca2+ signaling and handling are affected in cardiomyopathies due to mutations in the genes encoding the myofilament proteins [8].
The TNNT2 gene encodes the thin filament contractile protein TnT [6]. The principle isoform of TNNT2 in the adult human heart encodes 288 amino acids and consists of three structural regions (from N to C terminus): (1) the hypervariable region (residues 1–79) that does not interact with any proteins, (2) the TNT1 region (residues 80–180) which interacts and overlaps with Tm dimer, and (3) the TNT2 region (residues 181–288) that interacts with Tm and the globular end of the troponin complex (TnI and TnC) [8]. Clinical studies on families with mutations in TNNT2 show missense mutations that mostly occur within the TNT1 region (residues 92–144), leading to the progression of cardiomyopathies [9]. This region, in fact, is known as the mutational “hotspot” as mutations that occur here cause changes in the binding affinity of TnT to Tm. Changes in biding affinity lead to alterations in Ca2+ levels, contractility, and the progression of cardiomyopathies [9]. These mutations result in variable symptoms that depend on mutation penetrance, coinciding with the previously mentioned common features of H/D/RCM [10,11,12,13,14,15].
During the past decades, the zebrafish (Danio rerio) has emerged as a favorable model to study cardiovascular development and disease. Embryos that lack blood circulation can still survive by relying on passive diffusion of oxygen to the tissues; this allows the embryos to survive the initial stages of embryonic development to study any cardiovascular defects that might arise [16,17,18,19]. A mutant named silent heart (sih) and affecting the tnnt2a gene was one of the first embryonic cardiomyopathy models established. Homozygous sih/tnnt2a mutant embryos lack cardiac contraction, develop pericardial edema, and display defects in thin filament and sarcomere assembly [20].
Thus far, only embryonic studies of homozygous loss-of-function mutants of tnnt2a have been conducted. As cardiomyopathies arise in young adults with heterozygous mutations in sarcomeric genes including TNNT2, we were intrigued to find out whether heterozygous mutations in the zebrafish tnnt2a gene would result in cardiomyopathies in post-embryonic stages, especially if they are targeting the mutational “hotspot” of TNNT2. Here, we used CRISPR/Cas9 technology to generate an out-of-frame and an in-frame deletion of two amino acids in exon 9 of tnnt2a. While homozygous tnnt2a out-of-frame deletions are embryonically lethal and result in the sih phenotype, fish heterozygous for this mutation display no cardiac phenotypes. Homozygous tnnt2a in-frame deletions are also embryonically lethal; however, our findings indicate that heterozygous zebrafish develop a severe form of cardiomyopathy at the juvenile-adult stages of development. We show that these fish undergo cardiac remodeling, which is accompanied by an increase in myocardial stress and fibrosis. Furthermore, this structural remodeling is preceded by functional cardiac defects that are typical for human familial cardiomyopathies.
2. Materials and Methods
2.1. Zebrafish Husbandry
Fish used in this study were housed under standard conditions as described previously [21]. All experiments were conducted in accordance with the ethical guidelines and approved by the local ethics committee of the Royal Dutch Academy of Sciences (KNAW).
2.2. Generation of Mutant Lines
Tnnt2a lines were generated using CRISPR/Cas9 genome editing technology. Open reading frame of tnnt2a was chosen using CHOPCHOP (http://chopchop.cbu.uib.no/, accessed on 1 May 2014 [22]). sgRNA template was purchased from Integrated DNA Technologies (IDT) as standard desalted same-day oligo, and synthesis was carried out using MEGAshortscript T7 kit (Ambion). Purification of the in vitro synthesized mRNA was performed using RNeasy Mini Kit (Qiagen). sgRNA and Cas9-encoding mRNA were co-injected into one-cell stage wild-type (TL) zebrafish embryos at a concentration of 12.5 ng/µL sgRNA and 150 ng/µL Cas9-encoding mRNA. Injected embryos were grown to adulthood to identify F0 founder fish with indel mutations and screen for germline transmission. Each putative founder adult zebrafish was crossed with a wild-type adult fish (F1). Details of sequencing primers and sgRNA sequences can be found in Figure S1. Sequencing analysis of the targeted region revealed mutation carrier fish with either 5 or 6 nucleotide deletion (referred to as tnnt2a+/hu11260 and tnnt2a+/RK94del).
2.3. Adult Zebrafish Heart Isolations and Preparation
For paraffin sections, adult zebrafish hearts were dissected and fixed in 4% paraformaldehyde (dissolved in phosphate buffer containing 4% sucrose) at 4 °C overnight, washed twice in PBS, dehydrated in EtOH, and embedded in paraffin. Serial sections were made using a microtome (Leica RM2035, Leica, Wetzlar, Germany). Different section thicknesses were used, depending on the size/age of the sample: 10 μm for adult hearts, 8 μm for juvenile hearts, and 6 μm for embryos. For cryosections, zebrafish hearts were extracted and fixed in 4% paraformaldehyde (in phosphate buffer) for 4 h at room temperature (RT). Three washes of 30 min were performed using 4% sucrose (in phosphate buffer) followed by an overnight incubation at 4 °C in 30% sucrose (in phosphate buffer). Hearts were embedded in tissue-freezing medium (Leica, Lot# 03811456), frozen on dry ice, and kept at −80 °C. Cryo-sectioning using Cryostar NK70 (Thermo Scientific, Breda, The Netherlands) was performed to obtain 10 μm thin sections. Images of extracted whole hearts were acquired using a Leica M165 FC stereomicroscope.
2.4. Adult Zebrafish Heart Staining
In situ hybridization (ISH) was performed on paraffin sections as described previously [23], with the exception that the hybridization buffer did not contain heparin and yeast total RNA. vmhcl, amhc, nppb, tnnt2a, and col6a1 probes were previously generated [24]. Primers for the postnb probe generation were forward primer AGAGGTTCTGGACAGGCTCA and reverse primer AAGGCACCATTTTTCACCAG. Haematoxylin and eosin (H&E) staining and acid fuchsin-orange staining (AFOG) were performed on paraffin and cryosections, respectively, in accordance with standard laboratory protocols. Imaging of stained sections was performed using Leica DM4000 B LED upright automated microscope and Olympus Slideview VS200 digital slide scanner.
2.5. High-Speed Brightfield Imaging
Embryos were placed in 200 µM 1-phenyl-2-thiourea (PTU) 20–24 hpf to prevent pigmentation. At 5 dpf, embryos were embedded in 0.3% agarose prepared in E3 medium containing 16 mg/mL MS-222. Recordings were performed at 150 frames per second (fps) using a high-speed inverted light microscope at 28 °C. Heart rate measurements and contractility parameters were analyzed using ImageJ (USA National Institutes of Health, Bethesda, MD, USA). Contraction time, relaxation time, and systolic duration were analyzed by going through the recording frame by frame manually and observing the moments when (1) the ventricular wall moved inward, and the ventricle started expelling blood (start of contraction), (2) the ventricle wall moved back outward (start of relaxation), and (3) the ventricle wall was back at its most dilated position (maximum relaxation). After repeating the process 3 times for each heart, values were averaged, and contractile parameters were calculated. The contraction and relaxation time were corrected for heart rate (HR) by using the formula: time/√(beat to beat interval). The systolic fraction was calculated from the systolic time by using: (systolic time × R)/60.000. The hemodynamic parameters such as surface area and volumes were analyzed using ImageJ by drawing an ellipse on top of the ventricle at end-diastole and end-systole. Six ellipses were analyzed per heart: 3 at diastole and 3 at systole. Values were averaged. ImageJ provided the values for the minor and major axis of each ellipse. End-diastolic and end-systolic volume (EDV/ESV) were calculated by: (1/6) × (π) × (major axis) × (minor axis2). Stroke volume (SV) by: EDV-ESV. Ejection fraction (EF) by: SV/EDV. Cardiac output (CO) by: SV × Heart rate. Surface area was calculated using the following formula: (0.5 × major axis) × (0.5 × minor axis) × π. Fractional area change (FAC) was calculated by: (area diastole − area systole)/(area diastole).
2.6. High-Speed Fluorescence Imaging
tnnt2a+/RK94del fish were crossed to tg (myl7:Gal4FF; UAS:GCaMP6f) fish [25] to obtain tnnt2a fish with a genetically encoded cardiac Ca2+ sensor. Embryos were placed in 200 µM PTU after 20–24 hpf to keep them transparent. At 5 dpf, embryos’ heart contraction was stopped by incubation with E3-MS222-PAB-BDM mixture (PAB: Para-amino blebbistatin from Optopharma, Budapest, Hungary, Cat# DR-Am-89 at 5 µM; BDM: 2,3-Butanedione monoxime from Sigma-Aldrich, St Louis, MO, USA, Cat# B0753 at 100 µM) for 1 h at 28 °C. Embryos were embedded in 0.3% agarose prepared in E3 medium containing 16 mg/mL MS222-100 µM BDM and placed in a heated (28 °C) recording chamber bath containing 100 µM BDM in E3. Recordings were performed using a custom-built upright widefield microscope (Cairn Research, Faversham, UK) equipped with a 20 × 1.0 NA objective (Olympus XLUMPLFLN20X W, Olympus, Tokyo, Japan). Blue LED excitation light (470 nm) was filtered using a 470/40 nm filter (Chroma ET470/40x, Chroma, Bellows Falls, VT, USA) and reflected toward the objective using a 515 nm dichroic mirror (Chroma T515lp). Emitted fluorescence was filtered by a 514 long-pass filter (Semrock LP02-514RU, Semrock, New York, NY, USA), and images were projected on a high-speed camera (Andor Zyla 4.2 plus sCMOS, Andor, Belfast, Northern Ireland ). Recordings were performed at 100 fps, for 1000 frames. Parameters such as heart rate, Ca2+ transient amplitude, peak Ca2+ level, diastolic Ca2+ level, upstroke time, and recovery time were recorded. Recordings were analyzed using ImageJ and Matlab (Version R2015a, Mathworks, Natick, MA, USA).
2.7. Statistical Analysis
Statistical analysis and drawing of graphs and plots were carried out in GraphPad Prism (version 7 for Windows, GraphPad Software, San Diego, CA, USA). Differences between two groups were analyzed using the unpaired Student’s t-test, with the exception of the Kaplan–Meier curve, where Log-rank (Mantel–Cox) test was used. All data are presented as mean ± SEM, and p < 0.05 was considered significant. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, n.s. p > 0.05. N denotes the number of fish used per dataset.
3. Results
3.1. Targeting Exon 9 of tnnt2a via CRISPR/Cas9 Promotes Changes in Heart Morphology of Adult Zebrafish
Zebrafish tnnt2a is located on chromosome 23 and consists of 15 exons. Exon 9 encodes for a highly conserved region within the Tm binding region which is often mutated in patients with familial cardiomyopathy, including the Arginine 92 site (corresponding to Arginine 94 in zebrafish) [9] (Figure 1A). We therefore designed a guide RNA to target this region for mutagenesis with CRISPR/Cas9. From this, we recovered two F1 lines with different mutations. In one of the lines (hu11260), we identified a deletion of five nucleotides causing a frameshift followed by a stop codon resulting in a truncated protein lacking most of the Tm binding region and the entire TnC-TnI binding region. In the second line (hu11320), we identified an in-frame deletion of six nucleotides resulting in the deletion of an Arginine at position 94 and a Lysine at position 95, which hereafter is referred to as tnnt2a+/RK94del (Figure 1A,B).
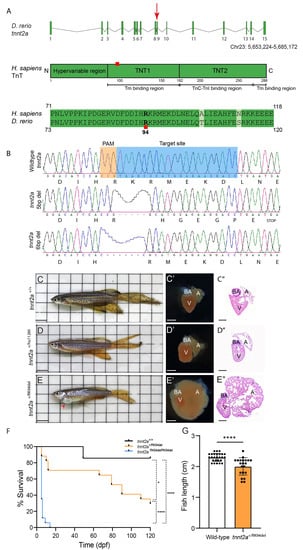
Figure 1.
CRISPR/Cas9-induced mutation in tnnt2a promotes the formation of cardiomyopathy in adult zebrafish. (A) Illustration of tnnt2a exons, introns, and chromosome location. Red arrowhead indicates the CRISPR/Cas9 mutation location. The cardiac Troponin T (TnT) human protein is schematically depicted, showing the different regions (hypervariable region, TNT1, and TNT2) that interact with tropomyosin I binding region and Troponin C and I (TnC-TnI) binding region, spanning 288 amino acids. The Arginine mutation site is highlighted with a red square, located on position 94 in zebrafish (D. rerio), which corresponds to the Arginine amino acid 92 in humans (H. sapiens). (B) Two different mutations targeting exon 9 of tnnt2a were identified, either causing a 5 bp frameshift deletion and a truncated form of the protein or a 6 bp in-frame deletion causing a mutated form of the protein. The target sequence for CRISPR/Cas9 is shown in blue and the PAM sequence in orange. The amino acid sequences show the effect of the mutations on the protein. (C–E) Images of 4-month-old adult zebrafish with the genotypes tnnt2a+/+, tnnt2a+/hu11260, or tnnt2a+/RK94del, respectively. Scale bar of 0.5 cm. (C’–E’) Extracted hearts of the respective fish. Scale bar of 100 µm. (C”–E”) Haematoxylin and Eosin (H&E) stainings of heart sections from tnnt2a+/+, tnnt2a+/hu11260, or tnnt2a+/RK94del, respectively. Images were taken at a magnification of 20×, and scale bars are 100 µm. A: atrium, V: ventricle, BA: bulbus arteriosus. (F) Kaplan–Meier curve, displaying the survival of tnnt2a+/+, tnnt2a+/RK94del, and tnnt2aRK94del/RK94del fish during the first 120 days of development (n = 7, 17, and 17, respectively). Statistics: Log-rank (Mantel–Cox) test, mean ± SEM, * p ≤ 0.05, **** p ≤ 0.0001. (G) Measurement of the length of adult fish from tnnt2a+/+ and tnnt2a +/RK94del. Statistics: mean ± SEM, **** p ≤ 0.0001, tnnt2a+/+ n = 26, tnnt2a +/RK94del n = 21, unpaired Students t-test.
Homozygous tnnt2ahu11260/hu11260 embryos at two days post fertilization (dpf) showed normal embryo morphology but lacking heart contraction, which resembles the previously reported tnnt2a loss-of-function mutation [20]. Heterozygous tnnt2a+/hu11260 zebrafish were viable with no apparent phenotypic changes, including normal heart rate at 5 dpf, and looked similar to their wild-type siblings in embryonic and adult stages (Figure 1C,D and Figure S2). Moreover, extracted hearts of adult tnnt2a+/hu11260 zebrafish appeared normal, with no visible morphological changes (Figure 1C′,C″,D′,D″). Homozygous tnnt2aRK94del/RK94del embryos were suffering from defects after heart looping, evident by the lack of heart chamber ballooning, pericardial edema, and weak heart contraction. Interestingly, also heterozygous tnnt2a+/RK94del embryos showed heart defects with variable penetrance. Survival count revealed that tnnt2aRK94del/RK94del embryos die within the first two weeks (Figure 1F, Figure S3). Strikingly, 70% of tnnt2a+/RK94del zebrafish survive the first two weeks but suffer from heart failure at ages ranging from 10 to 20 weeks post-fertilization (wpf) and have a higher susceptibility for bacterial infection. Only around 30% of tnnt2a+/RK94del survive the first four months of life (120 dpf) (Figure 1F). Females are noticed to have a decline in their egg production as early as 13 wpf. From 10 wpf onward, tnnt2a+/RK94del mutants develop protruding hearts combined with reduced body length (Figure 1G). Hearts extracted from 12-weeks-old tnnt2a+/RK94del zebrafish were enlarged, and it was often hard to distinguish the atrium and the ventricle (Figure 1E–E″). This heart remodeling was observed in 100% of the tnnt2a+/RK94del fish (n = 78) with variable degrees of severity, compared to no remodeling in their wild-type siblings (n = 109). In addition, the phenotype was consistent in more than 15 generations in which the tnnt2a+/RK94del zebrafish were outcrossed with wild-type TL fish, indicating a strong genotype/phenotype correlation. Fish with severely remodeled hearts suffered from breathing difficulties, evident by swimming at the surface gasping for air. These fish also showed a significant decrease in size and length, compared to their wild-type siblings (Figure 1E,G). In conclusion, by targeting exon9 in tnnt2a, we generated different alleles that display very different phenotypes. While the 5 bp out-of-frame deletion in tnnt2a acts as a recessive mutation and does not cause disease in heterozygous fish, the 6 bp deletion that removes Arg94 and Lys95 is dominant and causes phenotypes in heterozygous fish, including heart failure in adults.
3.2. Heart Morphological Changes in tnnt2a+/RK94del Can Be Observed at Early Larval Stages of Development
As tnnt2a+/RK94del fish developed phenotypes such as a protruding heart and altered swimming behavior at 10 weeks of age, we wanted to investigate whether morphological differences in the heart occurred and when these started to develop. To achieve this, live imaging on 5 dpf larvae was performed, as well as histological analysis at three different time points being 2, 4, and 7 wpf. Surprisingly, changes were observed at 5 dpf in the atrium. The atrium appeared larger in 20% of tnnt2a+/RK94del larvae, while the ventricle seemed to be unaffected (n = 24) (Figure 2A,B). Histological analysis confirmed this finding and showed that by 2 wpf, the size of the atrium was slightly increased in all tnnt2a+/RK94del fish, while the size of the ventricle seemed unaffected (n = 6) (Figure 2C,D). Remodeling becomes more apparent at 4 wpf, when the atrium of tnnt2a+/RK94del fish has enlarged further, and the ventricle appears slightly smaller in comparison to the wild-type siblings (n= 6) (Figure 2E,F). By 7 wpf, the size of the atrium in tnnt2a+/RK94del fish is grossly enlarged, and a clear decrease in the size of the ventricle is now apparent (n = 6) (Figure 2G,H). To better distinguish the atrium from the ventricle histologically, we analyzed the expression of atrial myosin heavy chain (amhc) and ventricle myosin heavy chain-like (vmhcl) via mRNA in situ hybridization. Both amhc and vmhcl are expressed at comparable levels in hearts of adult tnnt2a+/+ and tnnt2a+/RK94del zebrafish, but the atrium of tnnt2a+/RK94del zebrafish is much larger and contains a thicker outer layer compared to wild-type controls (n = 6) (Figure 3I,J). The nuclei in the atria of the tnnt2a+/RK94del appear abundant but similarly spaced as in wild-types, suggesting that hyperplasia (an increase in cell number) rather than hypertrophy (an increase in cell size) causes the enlargement of the atrium. The ventricle of tnnt2a+/RK94del fish appears to be morphologically similar to wild-type but is strongly reduced in size (n = 6) (Figure 3K,L). Together, these results demonstrate that cardiac remodeling in tnnt2a+/RK94del fish starts as early as the larval stage and is characterized by a progressive enlargement of the atrium and a reduction of the ventricle.
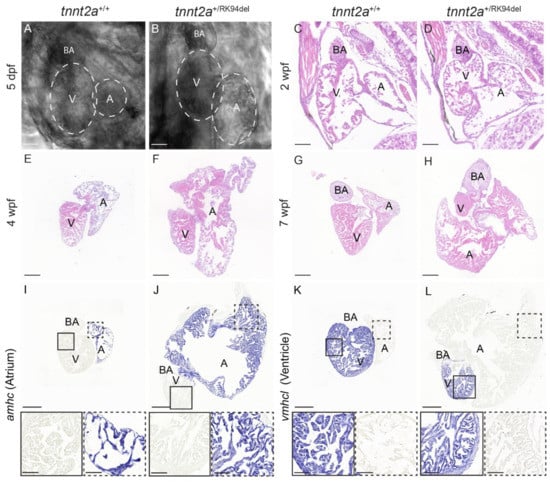
Figure 2.
Morphological changes in tnnt2a+/RK94del observed during different stages of development. (A,B) Images of 5 dpf embryonic hearts for tnnt2a+/+ and tnnt2a+/RK94del. (C–H) H&E staining of heart sections of tnnt2a+/+ and tnnt2a+/RK94del at (C,D) 2 weeks post-fertilization, (E,F) 4 weeks post-fertilization, and (G,H) 7 weeks post-fertilization. (I–L) In situ hybridization on adult heart sections of tnnt2a+/+ and tnnt2a+/RK94del stained for (I,J) amhc, a marker of atrial cells, and (K,L) vmhcl, a marker for adult zebrafish ventricular cells. Images were taken at a magnification of 20×. Scale bars are 100 µm (A–F), 50 µm (G–I), and 200 µm for whole-heart tile scans (I–L) with 50 µm for zoom-in regions. A: atrium, V: ventricle, BA: bulbus arteriosus.
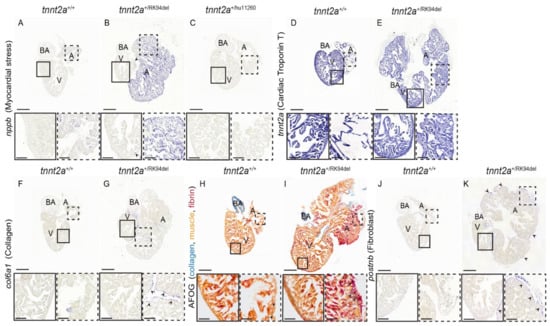
Figure 3.
Hearts of adult tnnt2a+/RK94del display increased myocardial stress and fibrosis. (A–C) In situ hybridization on 4-month-old adult heart sections of tnnt2a+/+, tnnt2a+/RK94del, and tnnt2a+/hu11260 stained for nppb, a marker of myocardial stress. In situ hybridization on adult heart sections of tnnt2a+/+ and tnnt2a+/RK94del stained for (D,E) tnnt2a, to mark cardiac Troponin T expression, and (F,G) col6a1, which marks collagen. (H,I) AFOG staining, which marks collagen in blue, muscle in orange, and fibrin in red of tnnt2a+/+ and tnnt2a+/RK94del. (J,K) In situ hybridization on adult heart sections of tnnt2a+/+ and tnnt2a+/RK94del stained for postnb, to mark fibroblasts. Images were taken at a magnification of 20×, except for (H,I) where magnification was at 60×. Scale bars are 200 µm for whole-heart tile scans, 50 µm for zoom-in regions, and 20 µm for zoom-in regions of H, I. A: atrium, V: ventricle, BA: bulbus arteriosus.
3.3. Heart of Adult tnnt2a+/RK94del Displays Increased Myocardial Stress
As we observed a strong remodeling in tnnt2a+/RK94del hearts, we wondered whether genes that are upregulated in failing mammalian hearts, such as natriuretic peptides [26], are also upregulated in tnnt2a+/RK94del hearts. Indeed, we detected a higher expression of Natriuretic peptide type B (nppb) in the atrium of the tnnt2a+/RK94del hearts and some sparse expression in the outer edges of the ventricle (n = 6) (Figure 3A,B, arrowheads). As previous results indicate that the atrium is more severely affected than the ventricle, we investigated whether tnnt2a is differentially expressed between the two heart chambers. In situ hybridization revealed no difference in tnnt2a expression between the atrium and ventricle of wild-type and tnnt2a+/RK94del hearts (n = 6) (Figure 3D,E). As tnnt2a+/RK94del hearts express only one wild-type copy of the tnnt2a gene, we wondered whether the loss of one tnnt2a wild-type copy is sufficient to induce the observed cardiac remodeling. However, as the hearts of tnnt2a+/hu11260 fish, which also have only one wild-type tnnt2a copy, show normal morphology (n = 6) and lack upregulation of nppb (Figure 3C), remodeling has to be due to the RK94del mutation. Based on these findings, we concluded that tnnt2a+/RK94del mutants develop evident morphological changes of the heart, characterized by an enlarged atrium and a smaller ventricle. These changes are accompanied by myocardial stress throughout the mutant heart.
3.4. Detection of Increased Collagen and Fibrin Deposition in Atrium of Adult tnnt2a+/RK94del Hearts
Myocardial stress and remodeling are often accompanied by increased fibrosis. To investigate whether this also occurred in tnnt2a+/RK94del hearts, col6a1 expression was analyzed. col6a1 is a commonly used fibrosis marker and is implicated in ECM remodeling. It is elevated during fibrotic conditions, such as cardiac fibrosis, via its role in myofibroblast differentiation [27]. While minimal col6a1 expression was detected in wild-type siblings (Figure 3F), we observed patches of col6a1 expression around the outer layer of both the atrium and ventricle of adult tnnt2a+/RK94del zebrafish (n = 6) (Figure 3G, arrowheads). Next, we stained for collagen and fibrin using acid fuchsin orange G (AFOG) staining, where we observed increased collagen levels (in blue) and fibrin deposition (in red) within and around the atrium of tnnt2a+/RK94del, unlike their wild-type siblings (n = 6) (Figure 3H,I, arrowhead). We then stained for fibroblast marker, periostin (postnb), and we observed an increased expression in tnnt2a+/RK94del fish, which overlapped with the areas of elevated col6a1 expression (n = 6) (Figure 3J,K, arrowheads). Taken together, these results indicate a moderate increase in fibrosis, mostly in the outer layer of the atrium in tnnt2a+/RK94del fish.
3.5. Embryonic tnnt2a+/RK94del fish Show Decreased Heart Rate Accompanied by Changes in Contractile and Hemodynamic Parameters
Structural changes were detected early on in the developmental stages of the tnnt2a+/RK94del hearts, which suggests that very early functional defects may underlie the cardiac remodeling in these mutants. We demonstrated a consistent enlargement of the atrium and a reduction of the ventricle in tnnt2a+/RK94del hearts, which appears similar to ventricular dysfunction with atrial compensation [28]. To investigate the role of ventricular dysfunction, we measured early heart rhythm and ventricular contractility in 5 dpf larvae, using brightfield high-speed video recording combined with image analysis. The high-speed video recording was performed on tnnt2a+/RK94del embryos without an apparent phenotype and on their wild-type siblings (Figure 4A,B). Image analysis revealed that the heart rate of tnnt2a+/RK94del embryos was significantly decreased compared to wild-type siblings (Figure 4C). Next, we measured different contractile parameters to observe the ability of the ventricle to contract and relax. Contraction time (time from maximum relaxation to maximum contraction), relaxation time (time from maximum contraction to maximum relaxation), and systolic fraction (the fraction of time the heart is in systole) were measured and corrected for heart rate. We observed a prolonged relaxation time in the ventricle of tnnt2a+/RK94del embryos, indicating that it takes longer before cardiomyocytes return to their uncontracted state (Figure 4E). Contraction time and systolic fraction were not affected (Figure 4D,F). Next, we measured hemodynamic parameters to identify the effect of RK94del mutation on cardiac pump function. An ellipse shape was fitted on the ventricle of brightfield high-speed recordings of embryonic zebrafish hearts (Figure 4G). Using established mathematical formulas, we measured the volume and surface area of each ventricle during diastole and systole. A decrease in end-diastolic volume (EDV) and end-systolic volume (ESV) was observed in tnnt2a+/RK94del embryos (Figure 4H,I). Ejection fraction (EF), stroke volume (SV), and cardiac output (CO) remained unaffected (Figure 4J–L). Surface areas were also affected, where there was a significant decrease in systolic and diastolic surface areas, while fractional area change (FAC) remained unaffected (Figure 4M–O). The results shown here demonstrate that seemingly healthy tnnt2a+/RK94del embryos already display minor changes in heart rhythm and contractility. These changes likely give rise to the remodeling at adult stages.
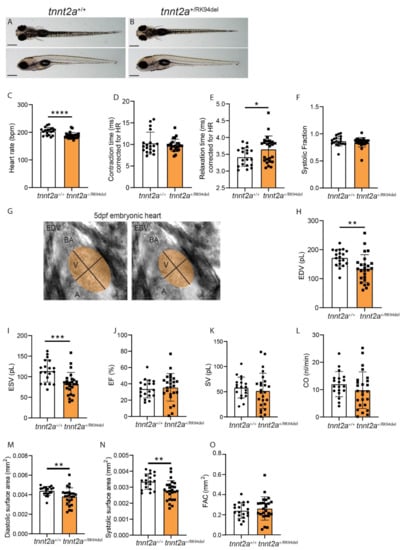
Figure 4.
Embryos of tnnt2a+/RK94del show decreased heart rate accompanied by decreased ventricular diastolic and systolic volumes, as well as increased relaxation time. Side view and top view of 5 dpf (A) tnnt2a+/+ and (B) tnnt2a+/RK94del embryos. (C–F) Bar graph of contractility parameters, including heart rate, contraction time, relaxation time, and systolic fraction for tnnt2a+/+ and tnnt2a+/RK94del. (G) For hemodynamic parameters, we used ImageJ to fit an ellipse over the ventricle in every recording, both at end-diastole (left) and end-systole (right). (H–L) Bar graphs of hemodynamic parameters, including end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction (EF), stroke volume (SV), and cardiac output (CO). (M–O) Bar graphs of surface area parameters: diastolic surface area, systolic diastolic surface area, and fractional area change (FAC). Scale bar: 100 µm. Statistics: mean ± SEM, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, tnnt2a+/+ n = 19, tnnt2a+/RK94del n = 24, unpaired Students t-test.
3.6. The tnnt2a+/RK94del Mutation Causes Impaired Calcium Dynamics in Embryos
While isolating ventricular cardiomyocytes to observe potential structural and functional changes, we observed that cells from tnnt2a+/RK94del fish were highly sensitive and always died during the process of isolation, where wild-type cells remained intact. Since cardiomyopathies progress over a longer time span due to a spectrum of defects, including impaired intracellular Ca2+ handling, we investigated whether we could observe Ca2+ dysfunction well before the end-stages of RK94del remodeling. tnnt2a+/RK94del fish were crossed with a tg (myl7:Gal4FF UAS:GCaMP6f) line, expressing a cytosolic cardiac Ca2+ sensor [25]. Analysis of GCaMP6f (cpEGFP) signal intensity over time allows the examination of Ca2+ transient amplitudes, the speed of intracellular Ca2+ release, and the speed of Ca2+ reuptake/clearance, as shown earlier [25] (Figure 5A,B). We observed changes in Ca2+ parameters in both the ventricle and atrium of 5 dpf tnnt2a+/RK94del embryos. No significant differences were observed in Ca2+ transient upstroke time between wild-type siblings and tnnt2a+/RK94del in both heart chambers (Figure 5C), indicating that the speed of cytosolic Ca2+ influx is unaffected in both heart chambers. Ca2+ recovery time was significantly prolonged, indicating slower clearance of Ca2+ during diastole, and signal amplitudes were significantly increased in the atrium of tnnt2a+/RK94del embryos (Figure 5D,E). Atrial diastolic Ca2+ levels and peak Ca2+ levels were not significantly affected in tnnt2a+/RK94del compared to wild-type siblings (Figure 5F,G); however, a significant decrease in both parameters was detected in the ventricle of the tnnt2a+/RK94del embryos. This suggests that the ventricle of tnnt2a+/RK94del embryos has a reduced amount of cytosolic Ca2+ during contraction and relaxation compared to wild-type siblings. Ca2+ transient frequency was significantly decreased in both the atrium and ventricle of tnnt2a+/RK94del, which is likely linked to the decrease in heart rate that we have shown earlier (Figure 5H and Figure 4C). Overall, the atrium of tnnt2a+/RK94del embryos shows an increase in Ca2+ amplitude and prolonged recovery time, while the ventricle has lower cytosolic Ca2+ levels during contraction and relaxation. Therefore, these findings imply that the Ca2+ dynamics within the hearts of tnnt2a+/RK94del fish are affected as early as 5 dpf.
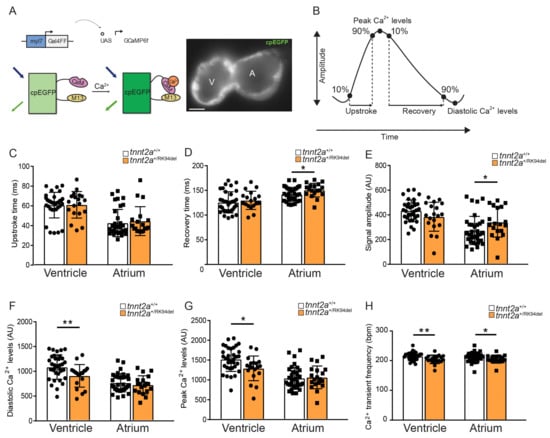
Figure 5.
tnnt2a+/RK94del mutation causes intracellular calcium dysregulation in zebrafish embryos. (A) DNA construct and sensor dynamics of GCaMP6f (left panel). GCaMP6f was placed under the control of the myl7 promoter to restrict its expression to the heart, where the Gal4FF-UAS system amplifies its expression. GCaMP6f consists of a circularly permutated enhanced green fluorescence protein (cpEGFP) fused to calmodulin (CaM) and the M13 peptide. Upon the increase of intracellular calcium (Ca2+), CaM binds to M13, causing increased brightness of cpEGFP. This is visualized using a high-speed epifluorescence microscope, where movies of 5 dpf non-contracting GCaMP6f embryonic hearts were recorded (left panel). (B) Schematic representation of Ca2+ transient parameters examined from the recorded GCaMP6f signals. (C–G) Bar graphs of the different Ca2+ transient parameters for tnnt2a+/+ and tnnt2a+/RK94del, including upstroke time, recovery time, signal amplitude, diastolic Ca2+ levels, and peak Ca2+ levels. (H) Frequency of Ca2+ transients tnnt2a+/+ and tnnt2a+/RK94del. AU: arbitrary unit. Statistics: mean ± SEM, * p ≤ 0.05, ** p ≤ 0.01, tnnt2a+/+ n = 34, tnnt2a+/RK94del n = 19, unpaired Students t-test. A: atrium, V: ventricle.
4. Discussion and Conclusions
Previously characterized sih/tnnt2 mutant alleles in zebrafish result in either no protein or a severely truncated protein only 11-amino-acids-long [20]. In this study, we targeted a location within the tnnt2a gene that is well known in human patients as the mutational “hotspot” for cardiomyopathies [9]. This location is within the TNT1 region of the protein, which is involved in the binding of cardiac TnT to Tm and is essential for the function of the TnT protein. The TNT1 region is a highly helical domain that stabilizes the TNT1-Tm interaction and increases the affinity of Tm to actin [29,30,31]. Earlier work showed that mutations in the region between amino acids 92–110 of TNNT2 impairs Tm binding or the ability of TnT to enhance tropomyosin-actin binding, leading to improper muscle contraction [31,32]. Flexibility of the TNT1 domain is important in allowing the efficient binding to Tm. Mutations in TNT1 that alter its flexibility lead to changes in Ca2+ activation in the thin filaments and propagation of the Ca2+ signal [33]. The regulatory element of Ca2+ is partially retained in the TNT2 domain of TnT; however, for regulation of contractility, Ca2+ needs Tm-TNT1 binding to propagate its signal [29]. The changes in TNT1 flexibility promote Ca2+ alterations, which have been shown to correspond to Ca2+ sensitivity, where increased flexibility leads to increased Ca2+ sensitivity [33,34,35]. Indeed, force measurements on detergent-skinned fiber bundles from transgenic mouse cardiomyocytes with mutations in TNT1 found in human cardiomyopathy patients (e.g., R92Q, I79N, and F110I) display increased Ca2+ sensitivity [36,37,38,39,40,41]. The observed early defects in Ca2+ dynamics and contractile dynamics presented in our study may very well be linked to changes in flexibility of TNT1 due to the deletion of Arginine and Lysine in the mutational hotspot of TnT, leading to inefficient propagation of Ca2+ signal to Tm.
The troponin complex is an important regulator of Ca2+ dynamics as TnC typically binds 50% of the Ca2+ that is released from the SR [42]. An increase in Ca2+ sensitivity of the troponin complex, by an increase in Ca2+ affinity of TnC, is predicted to result in a decrease in peak [Ca2+] [40,43]. This is consistent with our observation of decreased peak Ca2+ levels in the ventricles of tnnt2a+/RK94del embryos measured with the GCaMP6 sensor. A limitation of the GCaMP sensor is that it does not allow measurement of exact [Ca2+]. This would require measurements on isolated cells, which is hampered by the difficulty to isolate intact cardiomyocytes from embryos and from adult tnnt2a+/RK94del hearts. A higher affinity of the troponin complex for Ca2+ would also result in a slower release of Ca2+ into the cytoplasm and a slower clearance from the cell, which translates to a slower Ca2+ recovery and prolonged relaxation time. Indeed, we observed prolonged relaxation times in tnnt2a+/RK94del embryos. In addition, a decrease in diastolic Ca2+ levels in the ventricle of tnnt2a+/RK94del embryos was detected, suggesting that more Ca2+ was bound to troponin when TnT-RK94del is present in the complex. More Ca2+ bound to troponin at diastole is also consistent with the observed reduced EDV of the ventricles in tnnt2a+/RK94del embryos. Taken together, the reduced EDV and ESV combined with the reduction in cytoplasmic Ca2+ levels at diastole and systole are consistent with an increase in Ca2+ sensitivity of the troponin complex due to the TnT-RK94del mutation.
Structural remodeling observed in tnnt2a+/RK94del adult zebrafish is characterized by a reduced ventricular size together with a severely enlarged atrium. The model presented here appears similar to heart failure with preserved EF (HFpEF), as we observed diastolic dysfunction with atrial enlargement while maintaining EF level, a classic appearance of HFpEF [44,45]. The atrium, however, demonstrates increased myocardial stress, collagen deposition, and fibrosis during later stages, causing loss of elasticity and eventual heart failure. Interestingly, transgenic mice expressing a truncated form of Troponin T or mutated form of Troponin T (R92Q mutation) show a similar diastolic dysfunction combined with a decrease in ventricle size and a larger hypertrophic atrium [46,47]. A similar diastolic dysfunction combined with enlargement of the atria has been documented in patients with HCM, including familial cases with mutations in TNNT2 affecting the TNT1 domain [11,12,28,48]. Left atrium dilation is associated with ventricular diastolic dysfunction, which comes as a result of abnormal left ventricular relaxation and changes in actin-myosin interaction [49]. The diastolic dysfunction causes the left atrium pressure to continually increase in order to maintain a proper filling, leading to atrial wall tension and causing dilation and stretching of the atrial myocardium.
In summary, we suggest that the RK94del mutation in TnT affects the ability of Tm to bind TnT, leading to changes in Ca2+ dynamics, increased Ca2+ sensitivity, and contractility defects. We showed that the ventricle displays a form of HFpEF, where diastolic function is dysregulated while EF is maintained. The atrium, in this case, is enlarged to relieve the pressure and promote the proper ventricular filling. At a point where the atrium is unable to efficiently maintain the heart function, remodeling, involving increased collagen deposits and fibrosis, progresses ultimately leading to heart failure. The tnnt2a+/RK94del fish model we described here may help identify potential therapeutics in the future, which have the potential to reduce Ca2+ sensitivity and prevent cardiac remodeling in patients. As zebrafish have a highly conserved drug metabolism, physiology, and pharmacology, and the larvae are small enough to grow in a 96-well plate, they present an ideal model for drug discovery [50,51].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcdd8040046/s1, Figure S1: Sequences for primers and oligonucleotides used in this study, Figure S2: Heart rate from a brightfield high-speed video imaging of tnnt2a+/hu11260 at 5 dpf, Figure S3: Representative embryonic pictures of the different genotypes at 3 dpf and 5 dpf embryos.
Author Contributions
S.M.K.: study design, experiments, data analysis and interpretation, manuscript preparation, expansion, and care of the animal colony. C.D.K.: assistance in high-speed fluorescent imaging recordings, interpretation of calcium experiment findings, manuscript reviewing and editing. F.K.: care of animal colony and initial heart isolations, consultation for techniques, and experimental advice. S.W. and S.C.: design, injections, and generation of the zebrafish F0 generation. J.B.: study conceptualization, main funding, and design, data interpretation, study coordination, and manuscript reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by ZonMW E-rare grant 2016,coHeart project (Project number: 9003037607).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Hubrecht Institute animal caretakers for fish care, Anko de Graaff at the imaging facility, and Jeroen Korving and Harry Begthel at the histology department for their expertise.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Towbin, J.A. Inherited Cardiomyopathies. Circ. J. 2014, 78, 2347–2356. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Watkins, H.; Ashrafian, H.; Redwood, C. Mechanisms of Disease Inherited Cardiomyopathies. N. Engl. J. Med. 2011, 364, 1643–1656. [Google Scholar]
- Braunwald, E. Cardiomyopathies: An overview. Circ. Res. 2017, 121, 711–721. [Google Scholar] [CrossRef]
- McCartan, C.; Mason, R.; Jayasinghe, S.R.; Griffiths, L.R. Cardiomyopathy classification: Ongoing debate in the genomics era. Biochem. Res. Int. 2012, 2012, 796926. [Google Scholar] [CrossRef][Green Version]
- Thiene, G.; Corrado, D.; Basso, C. Revisiting definition and classification of cardiomyopathies in the era of molecular medicine. Eur. Heart J. 2008, 29, 144–146. [Google Scholar] [CrossRef]
- Lopes, L.R.; Elliott, P.M. A straightforward guide to the sarcomeric basis of cardiomyopathies. Heart 2014, 100, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Redwood, C.S.; Moolman-Smook, J.C.; Watkins, H. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc. Res. 1999, 44, 20–36. [Google Scholar] [CrossRef]
- Gangadharan, B.; Sunitha, M.S.; Mukherjee, S.; Chowdhury, R.R.; Hague, F.; Seker, N.; Sowdhamini, R.; Spudich, J.A.; Mercer, J.A. Molecular mechanisms and structural features of cardiomyopathy-causing troponin T mutants in the tropomyosin overlap region. Proc. Natl. Acad. Sci. USA 2017, 114, 11115–11120. [Google Scholar] [CrossRef]
- Pinto, J.R.; Parvatiyar, M.S.; Jones, M.A.; Liang, J.; Potter, J.D. A troponin T mutation that causes infantile restrictive cardiomyopathy increases Ca2+ sensitivity of force development and impairs the inhibitory properties of troponin. J. Biol. Chem. 2008, 283, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Ezekian, J.E.; Clippinger, S.R.; Garcia, J.M.; Yang, Q.; Denfield, S.; Jeewa, A.; Dreyer, W.J.; Zou, W.; Fan, Y.; Allen, H.D.; et al. Variant R94C in TNNT2-Encoded Troponin T Predisposes to Pediatric Restrictive Cardiomyopathy and Sudden Death Through Impaired Thin Filament Relaxation Resulting in Myocardial Diastolic Dysfunction. J. Am. Heart Assoc. 2020, 9, e015111. [Google Scholar] [CrossRef]
- Menon, S.C.; Michels, V.V.; Pellikka, P.A.; Ballew, J.D.; Karst, M.L.; Herron, K.J.; Nelson, S.M.; Rodeheffer, R.J.; Olsen, T.M. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin. Genet. 2008, 74, 445–454. [Google Scholar] [CrossRef]
- Li, D.; Czernuszewicz, G.Z.; Gonzalez, O.; Tapscott, T.; Karibe, A.; Durand, J.-B.; Brugad, R.; Hill, R.; Gregoritch, J.M.; Anderson, J.L.; et al. Novel Cardiac Troponin T Mutation as a Cause of Familial Dilated Cardiomyopathy. Circulation 2001, 104, 2188–2193. [Google Scholar] [CrossRef]
- Thierfelder, L.; Watkins, H.; MacRae, C.; Lamas, R.; McKenna, W.; Vosberg, H.-P.; Seidman, J.G.; Seidman, C.E. Tropomyosin and Cardiac Troponin T Mutations Cause Familial Hypertrophic Cardiomyopathy: A Disease of the Sarcomere. Cell 1994, 77, 701–712. [Google Scholar] [CrossRef]
- Moolman, J.C.; Corfield, V.A.; Posen, B.; Ngumbela, K.; Seidman, C.; Brink, P.; Watkins, H. Sudden Death due to Troponin T Mutations. J. Am. Coll. Cardiol. 1997, 29, 549–555. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Bournele, D.; Beis, D. Zebrafish models of cardiovascular disease. Heart Fail. Rev. 2016, 21, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.J.; Bakkers, J.; Brundel, B.J.; Robbins, J.; Tardiff, J.C.; Carrier, L. Animal and in silico models for the study of sarcomeric cardiomyopathies. Cardiovasc. Res. 2015, 105, 439–448. [Google Scholar] [CrossRef]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y.R. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angeo, L.; Midtlyng, P.J.; Schoderet, D.F.; Shulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Labun, K.; Guo, X.; Chaves, A.; Church, G.; Gagnon, J.A.; Valen, E. Accurate analysis of genuine CRISPR editing events with ampliCan. Genome Res. 2019, 29, 843–847. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Houweling, A.C.; De Boer, P.A.J.; Christoffels, V.M. Sensitive Nonradioactive Detection of mRNA in Tissue Sections: Novel Application of the Whole-mount In Situ Hybridization Protocol. J. Histochem. Cytochem. 2001, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Kruse, F.; Vasudevarao, M.D.; Junker, J.P.; Zebrowski, D.C.; Fischer, K.; Noel, E.S.; Grun, D.; Berezikov, E.; Engel, F.B.; et al. Spatially Resolved Genome-wide Transcriptional Profiling Identifies BMP Signaling as Essential Regulator of Zebrafish Cardiomyocyte Regeneration. Dev. Cell 2016, 36, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Van Opbergen, C.J.M.; Kooman, C.D.; Kok, B.J.; Knopfel, T.; Renninger, S.L.; Orger, M.B.; Vos, M.A.; van Veen, T.A.B.; Bakkers, J.; de Boer, T.P. Optogenetic sensors in the zebrafish heart: A novel in vivo electrophysiological tool to study cardiac arrhythmogenesis. Theranostics 2018, 8, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, I.A.; Hooijkaas, I.B.; van der Made, I.; Jong, W.M.C.; Creemers, E.E.; Christoffels, V.M. A transgenic mouse model for the simultaneous monitoring of ANF and BNP gene activity during heart development and disease. Cardiovasc. Res. 2014, 101, 78–86. [Google Scholar] [CrossRef]
- Sabatelli, P.; Gualandi, F.; Gara, S.K.; Grumati, P.; Zamparelli, A.; Martoni, E.; Pellegrini, C.; Merlini, L.; Ferlini, A.; Bonaldo, P.; et al. Expression of collagen VI α5 and α6 chains in human muscle and in Duchenne muscular dystrophy-related muscle fibrosis. Matrix Biol. 2012, 31, 187–196. [Google Scholar] [CrossRef]
- Tsang, T.S.M.; Barnes, M.E.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Left Atrial Volume as a Morphophysiologic Expression of Left Ventricular Diastolic Dysfunction and Relation to Cardiovascular Risk Burden. Am. J. Cardiol. 2002, 90, 1284–1289. [Google Scholar] [CrossRef]
- Tanokura, M.; Tawada, Y.; Ono, A.; Ohtsuki, L. Chymotryptic Subfragments of Troponin T from Rabbit Skeletal Muscle. Interaction with Tropomyosin, Troponin I and Troponin C. J. Biochem. 1983, 93, 331–337. [Google Scholar] [CrossRef]
- Marston, S.; Zamora, J.E. Troponin structure and function: A view of recent progress. J. Muscle Res. Cell Motil. 2020, 41, 71–89. [Google Scholar] [CrossRef]
- Palm, T.; Graboski, S.; Hitchcock-DeGregori, S.E.; Greenfield, N.J. Disease-causing mutations in cardiac troponin T: Identification of a critical tropomyosin-binding region. Biophys. J. 2001, 81, 2827–2837. [Google Scholar] [CrossRef][Green Version]
- Harada, K.; Potter, J.D. Familial Hypertrophic Cardiomyopathy Mutations from Different Functional Regions of Troponin T Result in Different Effects on the pH and Ca2+ Sensitivity of Cardiac Muscle Contraction. J. Biol. Chem. 2004, 279, 14488–14495. [Google Scholar] [CrossRef]
- Manning, E.P.; Tardiff, J.C.; Schwartz, S.D. Molecular effects of familial hypertrophic cardiomyopathy-related mutations in the TNT1 domain of cTnT. J. Mol. Biol. 2012, 421, 54–66. [Google Scholar] [CrossRef][Green Version]
- Sirenko, S.G.; Potter, J.D.; Knollmann, B.C. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts—Role of myofilament Ca2+ sensitivity increase. J. Physiol. 2006, 575, 201–213. [Google Scholar] [CrossRef]
- Knollmann, B.C.; Krchhof, P.; Sirenko, S.G.; Degen, H.; Greene, A.E.; Scober, T.; Mackow, J.C.; Fabritz, L.; Potter, J.D.; Morad, M. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ. Res. 2003, 92, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.; Rundel, V.L.M.; Tardiff, J.C.; Leinwand, L.A.; de Trombe, P.P.; Solaro, R.J. Ca(2+) activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H705–H713. [Google Scholar] [CrossRef]
- Morimoto, S.; Yanaga, F.; Minakami, R.; Ohtsuki, I. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am. J. Physiol. 1998, 275, C200–C207. [Google Scholar] [CrossRef]
- Szczesna, D.; Zhang, R.; Zhao, J.; Jones, M.; Guzman, G.; Potter, J.D. Altered Regulation of Cardiac Muscle Contraction by Troponin T Mutations That Cause Familial Hypertrophic Cardiomyopathy. J. Biol. Chem. 2000, 275, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Yanaga, F.; Morimoto, S.; Ohtsuki, I. Ca2+ Sensitization and Potentiation of the Maximum Level of Myofibrillar ATPase Activity Caused by Mutations of Troponin T Found in Familial Hypertrophic Cardiomyopathy. J. Biol. Chem. 1999, 274, 8806–8812. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Szczesna, D.; Housmans, P.R.; Zhao, J.; de Freitas, F.; Gomes, A.V.; Culbreath, L.; McCue, J.; Wang, Y.; Xu, Y.; et al. Abnormal Contractile Function in Transgenic Mice Expressing a Familial Hypertrophic Cardiomyopathy linked Troponin T (I79N) Mutation. J. Biol. Chem. 2001, 276, 3743–3755. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.M.; Szczesna-Cordary, D.; Knollmann, B.C.; Miller, T.; Bell, M.; Zhao, J.; Sirenko, S.G.; Diaz, Z.; Guzman, G.; Xu, Y.; et al. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J. Biol. Chem. 2005, 280, 37183–37194. [Google Scholar] [CrossRef] [PubMed]
- Shannon, T.R.; Ginsburg, K.S.; Bers, D.M. Reverse Mode of the Sarcoplasmic Reticulum Calcium Pump and Load-DependentCytosolic Calcium Decline in Voltage-Clamped Cardiac Ventricular Myocytes. Biophys. J. 2000, 78, 322–333. [Google Scholar] [CrossRef]
- Kataoka, A.; Hemmer, C.; Chase, P.B. Computational simulation of hypertrophic cardiomyopathy mutations in Troponin I: Influence of increased myofilament calcium sensitivity on isometric force, ATPase and [Ca2+]i. J. Biomech. 2007, 40, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Lekavich, C.L.; Barksdale, D.J.; Neelon, V.; Wu, J.R. Heart failure preserved ejection fraction (HFpEF): An integrated and strategic review. Heart Fail. Rev. 2015, 20, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, J.C.; Factor, S.M.; Tompkins, B.D.; Hewett, T.E.l.; Palmer, B.M.; Moore, R.L.; Schwartz, S.; Robbins, J.; Leinwand, L.A. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J. Clin. Investig. 1998, 101, 2800–2811. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, J.C.; Hewett, T.E.; Palmer, B.M.; Olsson, C.; Factor, S.M.; Moore, R.L.; Robbins, J.; Leinwand, L.A. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J. Clin. Investig. 1999, 104, 469–481. [Google Scholar] [CrossRef]
- Losi, M.A.; Betocchi, S.; Barbati, G.; Parisi, V.; Tocchetti, C.-G.; Pastore, F.; Migliore, T.; Contaldi, C.; Caput, A.; Romano, R.; et al. Prognostic Significance of Left Atrial Volume Dilatation in Patients with Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2009, 22, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Park, S.W.; Casaclang-Verzosa, G.; Ommen, S.R.; Pelkka, P.A.; Miller, F.A., Jr.; Sarano, M.E.; Kubo, S.H.; Oh, J.K. Diastolic dysfunction and left atrial enlargement as contributing factors to functional mitral regurgitation in dilated cardiomyopathy: Data from the Acorn trial. Am. Heart J. 2009, 157, 762.e3–762.e10. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Tardiff, J.C.; Carrier, L.; Bers, D.M.; Poggesi, C.; Ferrantini, C.; Copini, R.; Maier, L.S.; Ashrafian, H.; Huke, S.; van der Velden, J. Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc. Res. 2015, 105, 457–470. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).