Myocardial TGFβ2 Is Required for Atrioventricular Cushion Remodeling and Myocardial Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains and Breeding Scheme
2.2. Embryo Collection, Processing, Genotyping, Histology and Cell Lineage Tracing
2.3. TUNEL Assay
2.4. Cell Proliferation
2.5. Immunohistochemistry
2.6. RNAscope In Situ Hybridization (ISH)
2.7. Statistics
3. Results
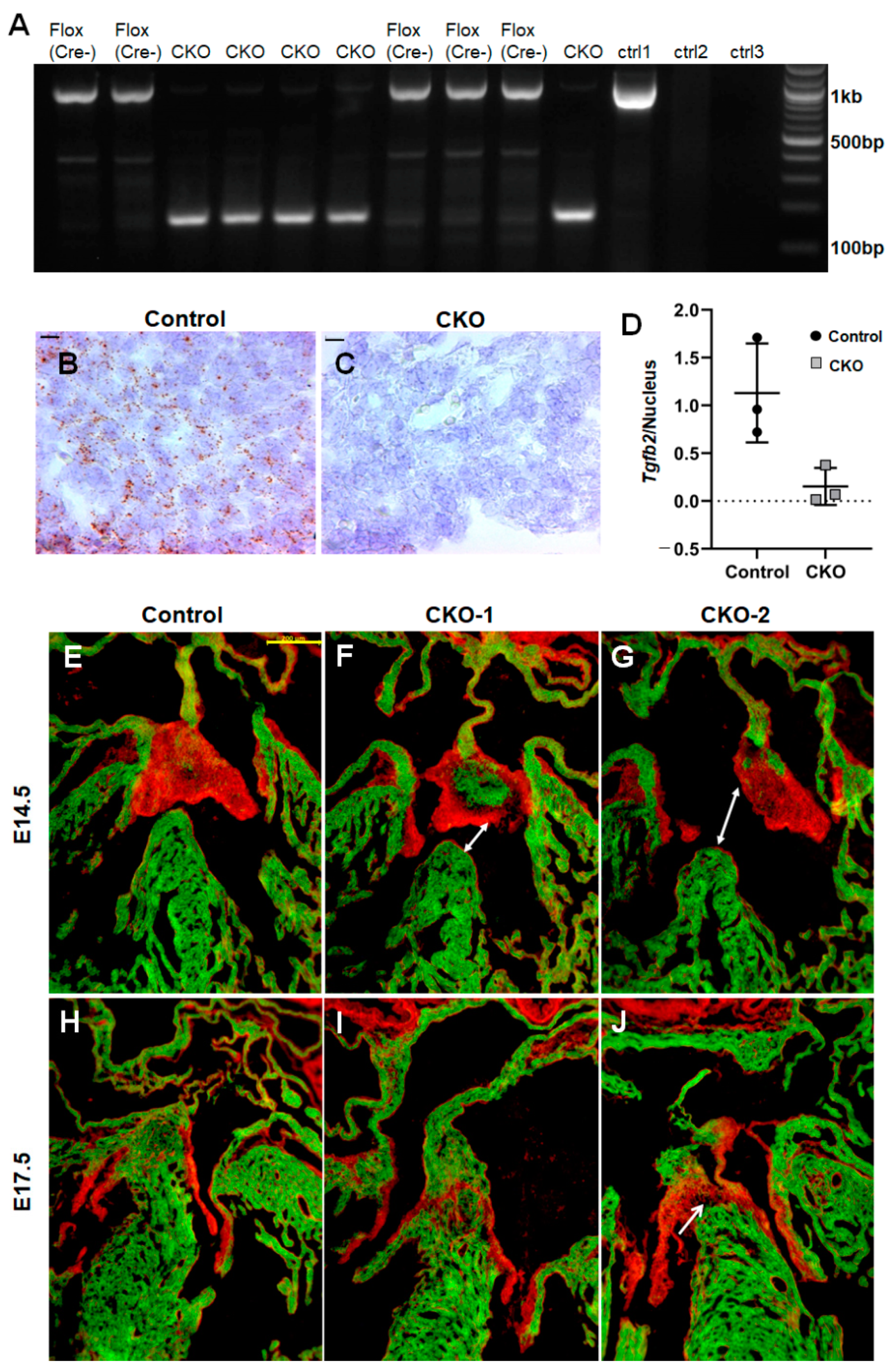
3.1. cTntCre Efficiently Deletes Tgfb2 in Early Cardiomyocytes
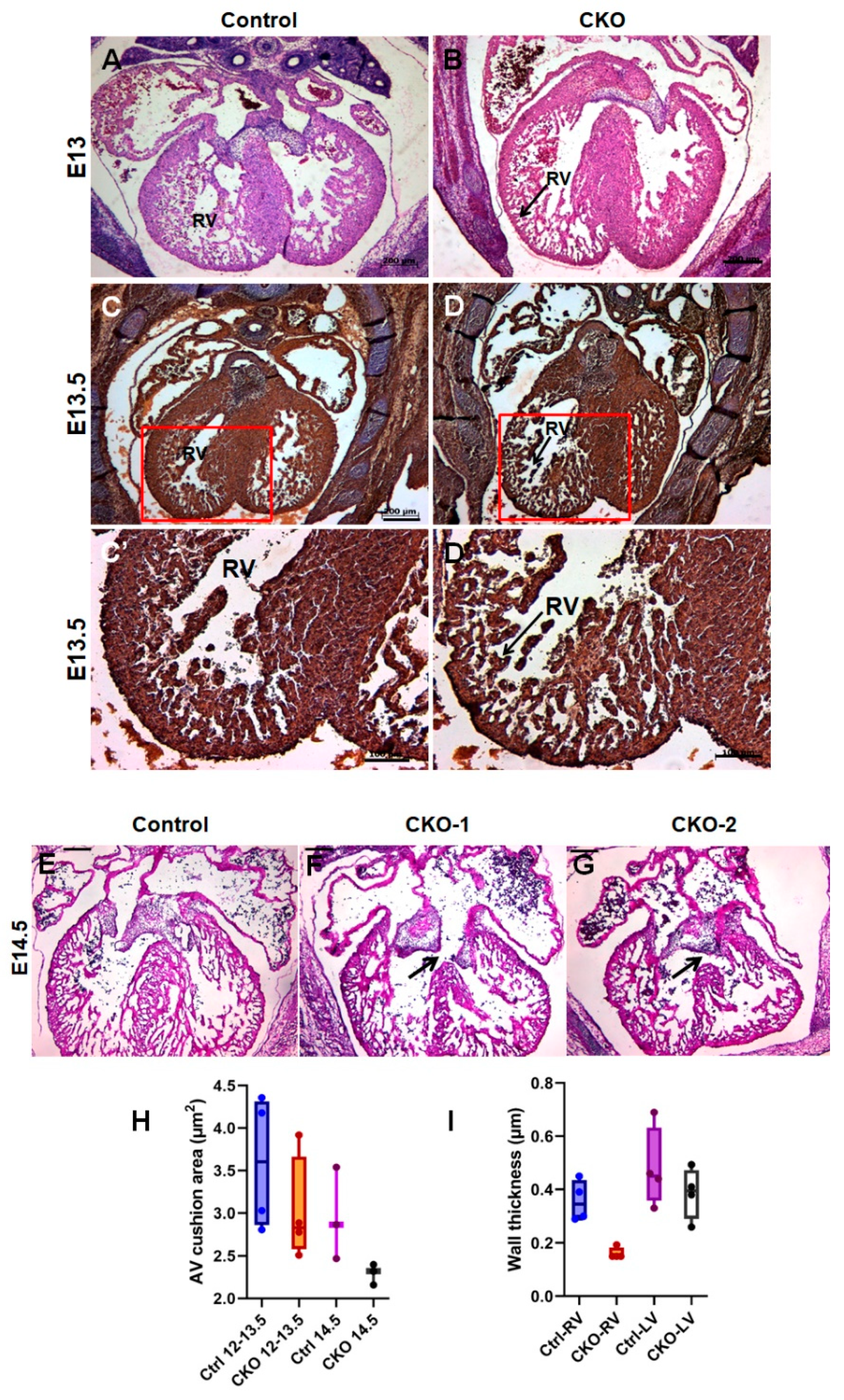
3.2. Conditional Deletion of Tgfb2 in Early Cardiomyocytes Leads to Cushion Remodeling Defect, Severe Thinning of Right Ventricle, and Muscular Type VSD
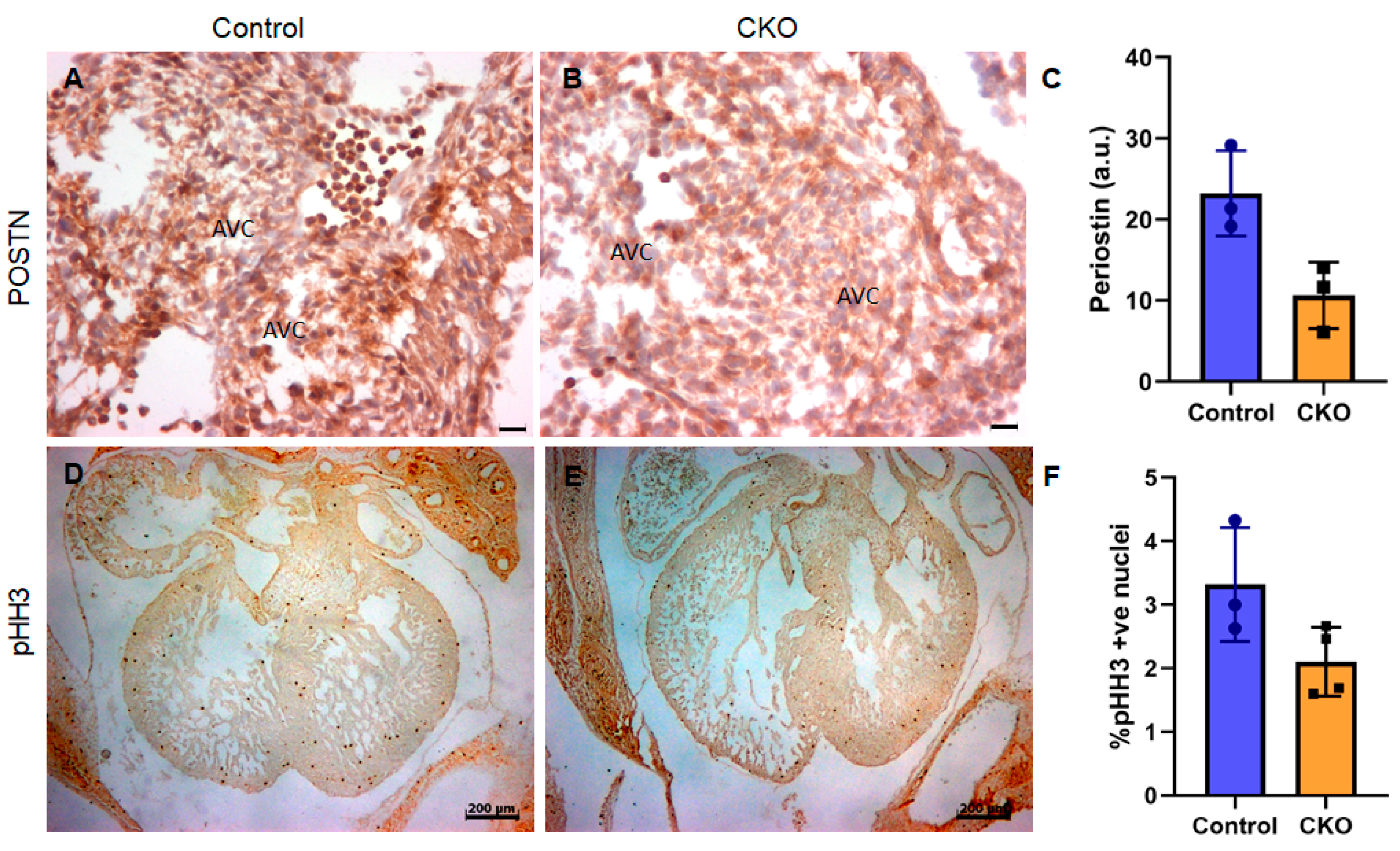
3.3. Cardiomyocyte-Derived TGFβ2 Is Required for Cushion Remodeling during Heart Development
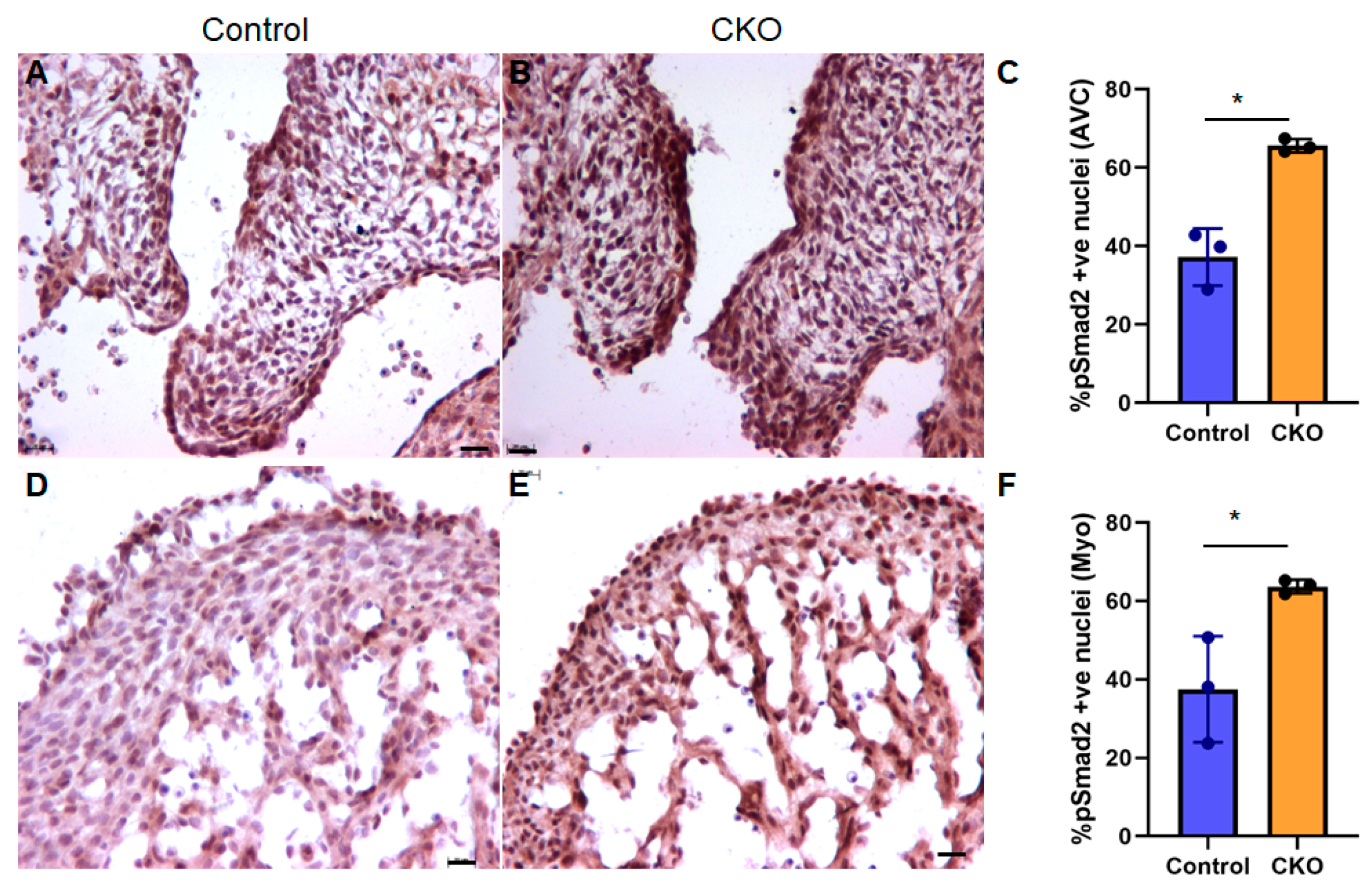
3.4. TGFβ Signaling Is “Paradoxically” Increased upon Myocardial Tgfb2 Deletion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derynck, R.; Budi, E.H. Specificity, versatility, and control of TGF-beta family signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamagishi, T.; Narematsu, M.; Kamimura, T.; Kai, M.; Nakajima, Y. Epicardium is required for sarcomeric maturation and cardiomyocyte growth in the ventricular compact layer mediated by transforming growth factor beta and fibroblast growth factor before the onset of coronary circulation. Congenit. Anom. Kyoto 2014, 54, 162–171. [Google Scholar] [CrossRef]
- Russo, I.; Cavalera, M.; Huang, S.; Su, Y.; Hanna, A.; Chen, B.; Shinde, A.V.; Conway, S.J.; Graff, J.; Frangogiannis, N.G. Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program. Circ. Res. 2019, 124, 1214–1227. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The role of transforming growth factor (TGF)-beta in the infarcted myocardium. J. Thorac. Dis. 2017, 9 (Suppl. 1), S52–S63. [Google Scholar] [CrossRef] [PubMed]
- Doetschman, T.; Barnett, J.V.; Runyan, R.B.; Camenisch, T.D.; Heimark, R.L.; Granzier, H.L.; Conway, S.J.; Azhar, M. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res. 2012, 347, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Molin, D.G.; Bartram, U.; Van der Heiden, K.; Van Iperen, L.; Speer, C.P.; Hierck, B.P.; Beerend, P.; Poelmann, R.E.; Gittenberger-de-Groot, A.C. Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev. Dyn. 2003, 227, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Boileau, C.; Guo, D.-C.; Hanna, N.; Regalado, E.S.; Detaint, D.; Gong, L.; Varret, M.; Prakash, S.K.; Li, A.H.; D’Indy, H.; et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet. 2012, 44, 916–921. [Google Scholar] [CrossRef]
- Lindsay, M.E.; Schepers, D.; Bolar, N.A.; Doyle, J.J.; Gallo, E.; Fert-Bober, J.; Kempers, M.J.; Fishman, E.K.; Chen, Y.; Myers, L.; et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 2012, 44, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Markwald, R.R.; Norris, R.A.; Moreno-Rodriguez, R.; Levine, R.A. Developmental basis of adult cardiovascular diseases: Valvular heart diseases. Ann. N. Y. Acad. Sci. 2010, 1188, 177–183. [Google Scholar] [CrossRef]
- Durst, R.; Sauls, K.; Peal, D.S.; DeVlaming, A.; Toomer, K.; Leyne, M.; Salani, M.; Talkowski, M.E.; Brand, H.; Perrocheau, M.; et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015, 525, 109–113. [Google Scholar] [CrossRef]
- Azhar, M.; Ware, S.M. Genetic and Developmental Basis of Cardiovascular Malformations. Clin. Perinatol. 2016, 43, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Runyan, R.B.; Gard, C.; Sanford, L.P.; Miller, M.L.; Andringa, A.; Pawlowski, S.; Rajan, S.; Doetschman, T. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev. Dyn. 2009, 238, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Azhar, M.; Brown, K.; Gard, C.; Chen, H.; Rajan, S.; Elliott, D.A.; Stevens, M.V.; Camenisch, T.D.; Conway, S.J.; Doetschman, T. Transforming growth factor Beta2 is required for valve remodeling during heart development. Dev. Dyn. 2011, 240, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Azhar, M.; Molin, D.G. Transforming growth factor beta-SMAD2 signaling and aortic arch development. Trends Cardiovasc. Med. 2006, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.W.; Liu, J.; Thorn, K.S.; Stuurman, N.; Liebling, M.; Stainier, D.Y. High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 2014, 141, 585–593. [Google Scholar] [CrossRef]
- Icardo, J.M.; Fernandez-Teran, A. Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat. (Basel) 1987, 130, 264–274. [Google Scholar] [CrossRef]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; de la Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, B.P.; Kruithof-De-Julio, M.; Poelmann, R.E.; Gittenberger-De-Groot, A.C.; Gaussin, V.; Goumans, M.J. Remodeling of the myocardium in early trabeculation and cardiac valve formation; a role for TGFbeta2. Int. J. Dev. Biol. 2013, 57, 853–863. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.N.; van der Loo, B. Isolated ventricular non-compaction of the myocardium in adults. Heart 2007, 93, 11–15. [Google Scholar] [CrossRef]
- Ishtiaq Ahmed, A.S.; Bose, G.C.; Huang, L.; Azhar, M. Generation of mice carrying a knockout-first and conditional-ready allele of transforming growth factor beta2 gene. Genesis 2014, 52, 817–826. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, W.R.; Brand, T.; Schneider, M.D. Transforming growth factor-beta in cardiac ontogeny and adaptation. Circ. Res. 1993, 73, 783–791. [Google Scholar] [CrossRef]
- Bartram, U.; Molin, D.G.M.; Wisse, L.J.; Mohamad, A.; Sanford, L.P.; Doetschman, T.; Speer, C.P.; Poelmann, R.E.; Groot, A.C.G.-D. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 2001, 103, 2745–2752. [Google Scholar] [CrossRef]
- van den Hoff, M.J.B.; Wessels, A. Muscularization of the Mesenchymal Outlet Septum during Cardiac Development. J. Cardiovasc. Dev. Dis. 2020, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Kulessa, H.; Tompkins, K.; Zhou, Y.; Batts, L.; Baldwin, H.S.; Hogan, B.L. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003, 17, 2362–2367. [Google Scholar] [CrossRef]
- Sanford, L.P.; Ormsby, I.; Groot, A.C.G.-D.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 1997, 124, 2659–2670. [Google Scholar]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.A.; Moreno-Rodriguez, R.A.; Sugi, Y.; Hoffman, S.; Amos, J.; Hart, M.M.; Potts, J.D.; Goodwin, R.L.; Markwald, R.R. Periostin regulates atrioventricular valve maturation. Dev. Biol. 2008, 316, 200–213. [Google Scholar] [CrossRef]
- Massague, J. TGFbeta signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Koinuma, D.; Miyazono, K.; Heldin, C.H. Genome-wide mechanisms of Smad binding. Oncogene 2013, 32, 1609–1615. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Xie, J.; Li, Y.; Shi, S.; Qian, H.; Yan, Z.; Rao, L. Genetic variance of transforming growth factor beta2 gene in conotruncal heart defects. Biomarkers 2017, 22, 287–290. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Li, H.; Zhou, B.; Rao, L. Association between rs6658835 polymorphism of transforming growth factor beta 2 gene and congenital heart diseases in Chinese Han population. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2012, 29, 210–213. [Google Scholar]
- Scalise, M.; Torella, M.; Marino, F.; Ravo, M.; Giurato, G.; Vicinanza, C.; Cianflone, E.; Mancuso, T.; Aquila, I.; Salerno, L.; et al. Atrial myxomas arise from multipotent cardiac stem cells. Eur. Heart J. 2020, 41, 4332–4345. [Google Scholar] [CrossRef]
- Azhar, M.; Schultz Jel, J.; Grupp, I.; Dorn, G.W., II; Meneton, P.; Molin, D.G.; Gittenberger-de Groot, A.C.; Doetschman, T. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003, 14, 391–407. [Google Scholar] [CrossRef]
- Macfarlane, E.G.; Parker, S.J.; Shin, J.Y.; Ziegler, S.G.; Creamer, T.J.; Bagirzadeh, R.; Bedja, D.; Chen, Y.; Calderon, J.F.; Weissler, K.; et al. Lineage-specific events underlie aortic root aneurysm pathogenesis in Loeys-Dietz syndrome. J. Clin. Investig. 2019, 129, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Gaussin, V.; Van De Putte, T.; Mishina, Y.; Hanks, M.C.; Zwijsen, A.; Huylebroeck, D.; Behringer, R.R.; Schneider, M.D. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc. Natl. Acad. Sci. USA 2002, 99, 2878–2883. [Google Scholar] [CrossRef]
- Burns, T.; Yang, Y.; Hiriart, E.; Wessels, A. The Dorsal Mesenchymal Protrusion and the Pathogenesis of Atrioventricular Septal Defects. J. Cardiovasc. Dev. Dis. 2016, 3, 29. [Google Scholar] [CrossRef]
- Cianflone, E.; Aquila, I.; Scalise, M.; Marotta, P.; Torella, M.; Nadal-Ginard, B.; Torella, D. Molecular basis of functional myogenic specification of Bona Fide multipotent adult cardiac stem cells. Cell Cycle. 2018, 17, 927–946. [Google Scholar] [CrossRef] [PubMed]
- Verzi, M.P.; McCulley, D.J.; De Val, S.; Dodou, E.; Black, B.L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005, 287, 134–145. [Google Scholar] [CrossRef]
- Sridurongrit, S.; Larsson, J.; Schwartz, R.; Ruiz-Lozano, P.; Kaartinen, V. Signaling via the Tgf-beta type I receptor Alk5 in heart development. Dev. Biol. 2008, 322, 208–218. [Google Scholar] [CrossRef]
- Jiao, K.; Langworthy, M.; Batts, L.; Brown, C.B.; Moses, H.L.; Baldwin, H.S. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 2006, 133, 4585–4593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eley, L.; Alqahtani, A.M.; MacGrogan, D.; Richardson, R.V.; Murphy, L.; Salguero-Jimenez, A.; Pedro, M.S.R.S.; Tiurma, S.; McCutcheon, L.; Gilmore, A.; et al. A novel source of arterial valve cells linked to bicuspid aortic valve without raphe in mice. eLife 2018, 7, e34110. [Google Scholar] [CrossRef] [PubMed]
- Mifflin, J.J.; Dupuis, L.E.; Alcala, N.E.; Russell, L.G.; Kern, C.B. Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev. Dyn. 2018, 247, 1005–1017. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Al-Sammarraie, N.; Gebere, M.G.; Bhattacharya, A.; Chopra, S.; Johnson, J.; Peña, E.A.; Eberth, J.F.; Poelmann, R.E.; Groot, A.C.G.-D.; et al. Transforming Growth Factor Beta3 is Required for Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Cheng, A.; A Myers, L.; Martinez-Murillo, F.; Jie, C.; Bedja, D.; Gabrielson, K.L.; Hausladen, J.M.W.; Mecham, R.P.; Judge, D.P.; et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Investig. 2004, 114, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Dietz, H.C. Loeys-Dietz Syndrome. In GeneReviews®; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Ghayda Mirzaa, G., Anne Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
| ID | Age | Cardiac Defects | |||
|---|---|---|---|---|---|
| OFT Cushion Thickening | VSD | AV Cushion and Septation Defects | Myocardial Defects | ||
| CKO-1 | E12.5–13 | ND | Yes | Slightly smaller but not dysmorphic, incomplete AVSD | Yes; RV more affected than LV, |
| CKO-2 | E12.5–13 | ND | Yes | Smaller, dysmorphic, incomplete AVSD | Yes; RV more affected than LV, |
| CKO-3 | E12.5–13 | ND | Yes | Dysmorphic, incomplete AVSD | yes |
| CKO-4 | E12.5 | ND | ND | ND | |
| CKO-5 | E13.5 | Yes | ND | ND | Yes; RV more affected than LV |
| CKO-6 | E14.5 | yes | Muscular | Smaller but not dysmorphic | Yes |
| CKO-7 | E14.5 | ND | Yes | Dysmorphic, incomplete AVSD | Yes |
| CKO-8 | E14.5 | ND | Yes | Dysmorphic, incomplete AVSD | Yes |
| CKO-9 | E16.5 | No | No | AV valves normal | Yes |
| CKO-10 | E16.5 | No | Muscular | AV valves normal | Yes; RV more affected than LV |
| CKO-11 | E17.5 | No | Yes | ND | Yes |
| CKO-12 | E17.5 | ND | Perimembranous | ND | Yes |
| CKO-13 | E18.5 | Yes | No | ND | Yes |
| Total CKO | 13 | ||||
| Controls | 19 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, A.; Al-Sammarraie, N.; Gebere, M.G.; Johnson, J.; Eberth, J.F.; Azhar, M. Myocardial TGFβ2 Is Required for Atrioventricular Cushion Remodeling and Myocardial Development. J. Cardiovasc. Dev. Dis. 2021, 8, 26. https://doi.org/10.3390/jcdd8030026
Bhattacharya A, Al-Sammarraie N, Gebere MG, Johnson J, Eberth JF, Azhar M. Myocardial TGFβ2 Is Required for Atrioventricular Cushion Remodeling and Myocardial Development. Journal of Cardiovascular Development and Disease. 2021; 8(3):26. https://doi.org/10.3390/jcdd8030026
Chicago/Turabian StyleBhattacharya, Aniket, Nadia Al-Sammarraie, Mengistu G. Gebere, John Johnson, John F. Eberth, and Mohamad Azhar. 2021. "Myocardial TGFβ2 Is Required for Atrioventricular Cushion Remodeling and Myocardial Development" Journal of Cardiovascular Development and Disease 8, no. 3: 26. https://doi.org/10.3390/jcdd8030026
APA StyleBhattacharya, A., Al-Sammarraie, N., Gebere, M. G., Johnson, J., Eberth, J. F., & Azhar, M. (2021). Myocardial TGFβ2 Is Required for Atrioventricular Cushion Remodeling and Myocardial Development. Journal of Cardiovascular Development and Disease, 8(3), 26. https://doi.org/10.3390/jcdd8030026









