Impact of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Renal Function in Patient with Heart Failure
Abstract
:1. Introduction
2. Case Report
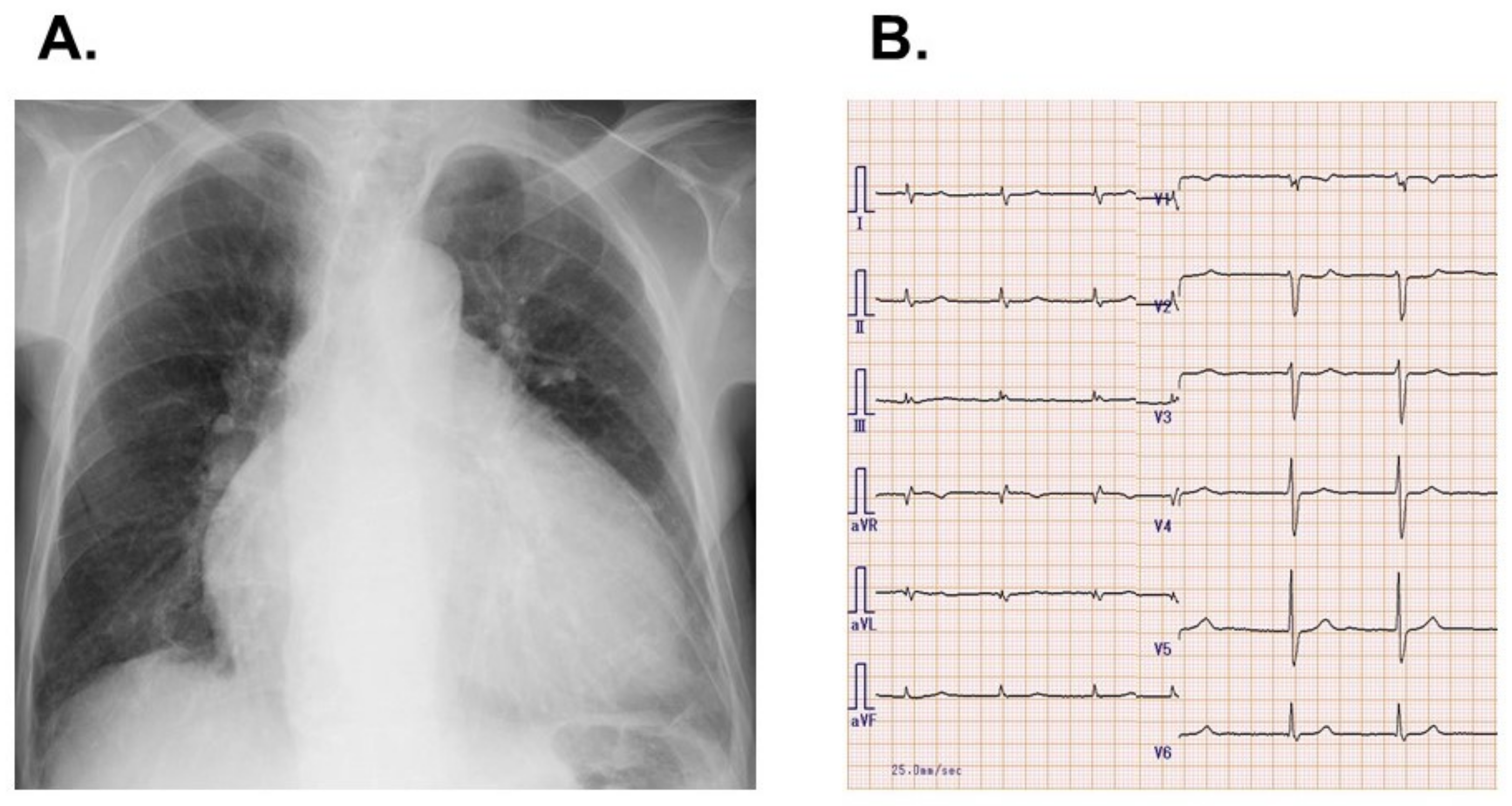
2.1. On Admission
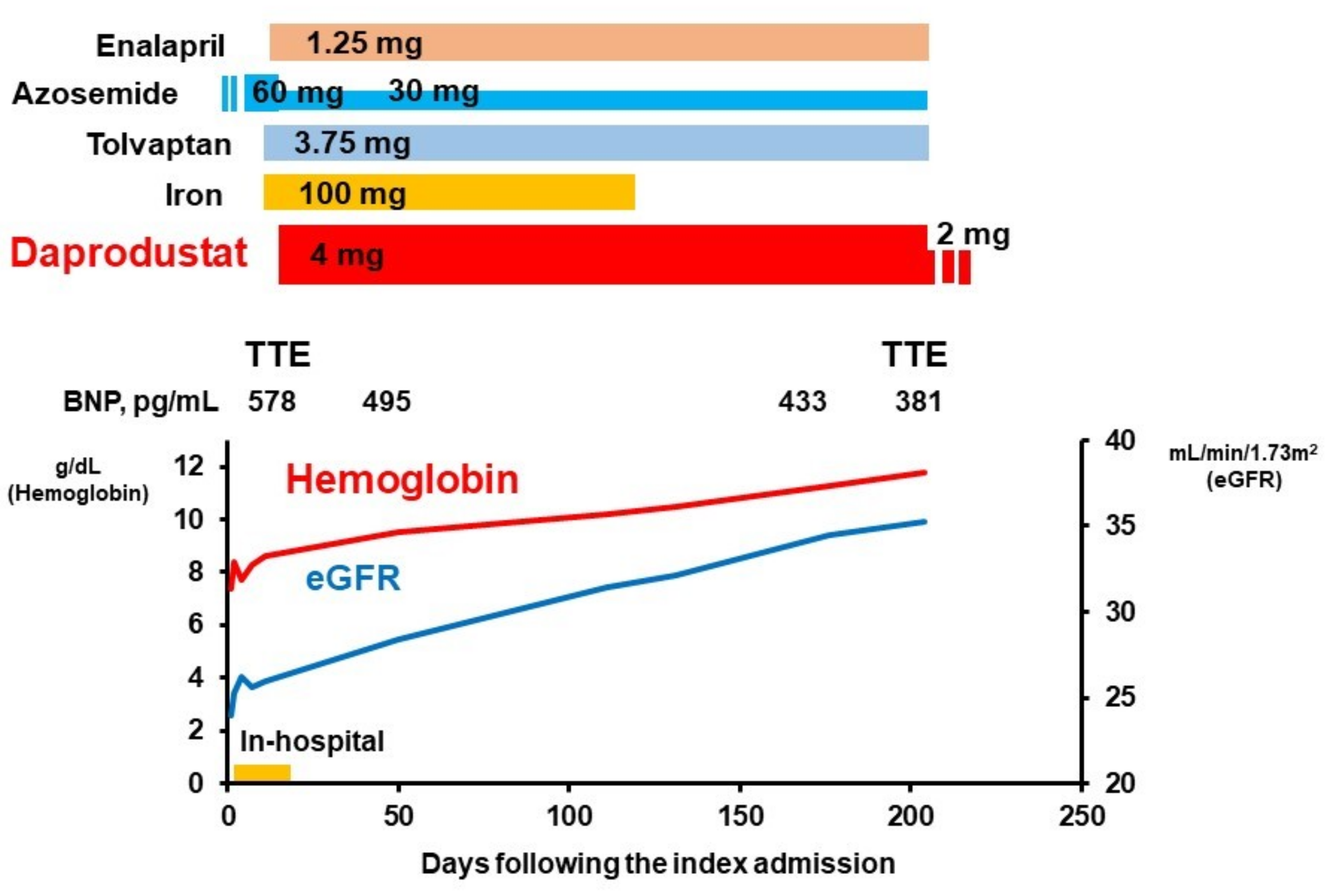
2.2. In-Hospital Course
2.3. Post-Discharge Course
3. Discussion
3.1. Cause of Anemia
3.2. Impact of HIF-PH Inhibitor on Renal Function
3.3. Future Concern
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Righetti, M. Cardio-renal-anemia syndrome: A link between erythropoietin, dimethylarginine and homocysteine. Curr. Med. Chem. 2012, 19, 3502–3507. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Eckardt, K.U. Pathogenesis of renal anemia. Semin. Nephrol. 2006, 26, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Tomonari, H.; Yoshida, H.; Hashimoto, T.; Kawaguchi, Y.; Sakai, O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 1997, 77, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Wish, J.B. Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors: A Potential New Treatment for Anemia in Patients With CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akizawa, T.; Nangaku, M.; Yonekawa, T.; Okuda, N.; Kawamatsu, S.; Onoue, T.; Endo, Y.; Hara, K.; Cobitz, A.R. Efficacy and Safety of Daprodustat Compared with Darbepoetin Alfa in Japanese Hemodialysis Patients with Anemia: A Randomized, Double-Blind, Phase 3 Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, D.S.; Wexler, D.; Iaina, A. The importance of anemia and its correction in the management of severe congestive heart failure. Eur. J. Heart Fail. 2002, 4, 681–686. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Burdmann, E.A.; Chen, C.Y.; Cooper, M.E.; de Zeeuw, D.; Eckardt, K.U.; Feyzi, J.M.; Ivanovich, P.; Kewalramani, R.; Levey, A.S.; et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 2009, 361, 2019–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swedberg, K.; Young, J.B.; Anand, I.S.; Cheng, S.; Desai, A.S.; Diaz, R.; Maggioni, A.P.; McMurray, J.J.; O’Connor, C.; Pfeffer, M.A.; et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N. Engl. J. Med. 2013, 368, 1210–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Ueno, Y.; Kinugawa, K. Impact of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Renal Function in Patient with Heart Failure. J. Cardiovasc. Dev. Dis. 2021, 8, 189. https://doi.org/10.3390/jcdd8120189
Imamura T, Ueno Y, Kinugawa K. Impact of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Renal Function in Patient with Heart Failure. Journal of Cardiovascular Development and Disease. 2021; 8(12):189. https://doi.org/10.3390/jcdd8120189
Chicago/Turabian StyleImamura, Teruhiko, Yohei Ueno, and Koichiro Kinugawa. 2021. "Impact of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Renal Function in Patient with Heart Failure" Journal of Cardiovascular Development and Disease 8, no. 12: 189. https://doi.org/10.3390/jcdd8120189
APA StyleImamura, T., Ueno, Y., & Kinugawa, K. (2021). Impact of Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitor on Renal Function in Patient with Heart Failure. Journal of Cardiovascular Development and Disease, 8(12), 189. https://doi.org/10.3390/jcdd8120189






