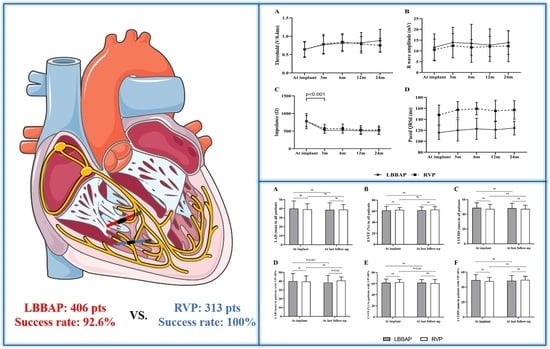
Medium- and Long-Term Lead Stability and Echocardiographic Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Procedures
2.3. Follow-Up and Echocardiographic Evaluation
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Procedural and Electrophysiological Parameters
3.3. Pacing Parameters and Lead Stability during Follow-Up
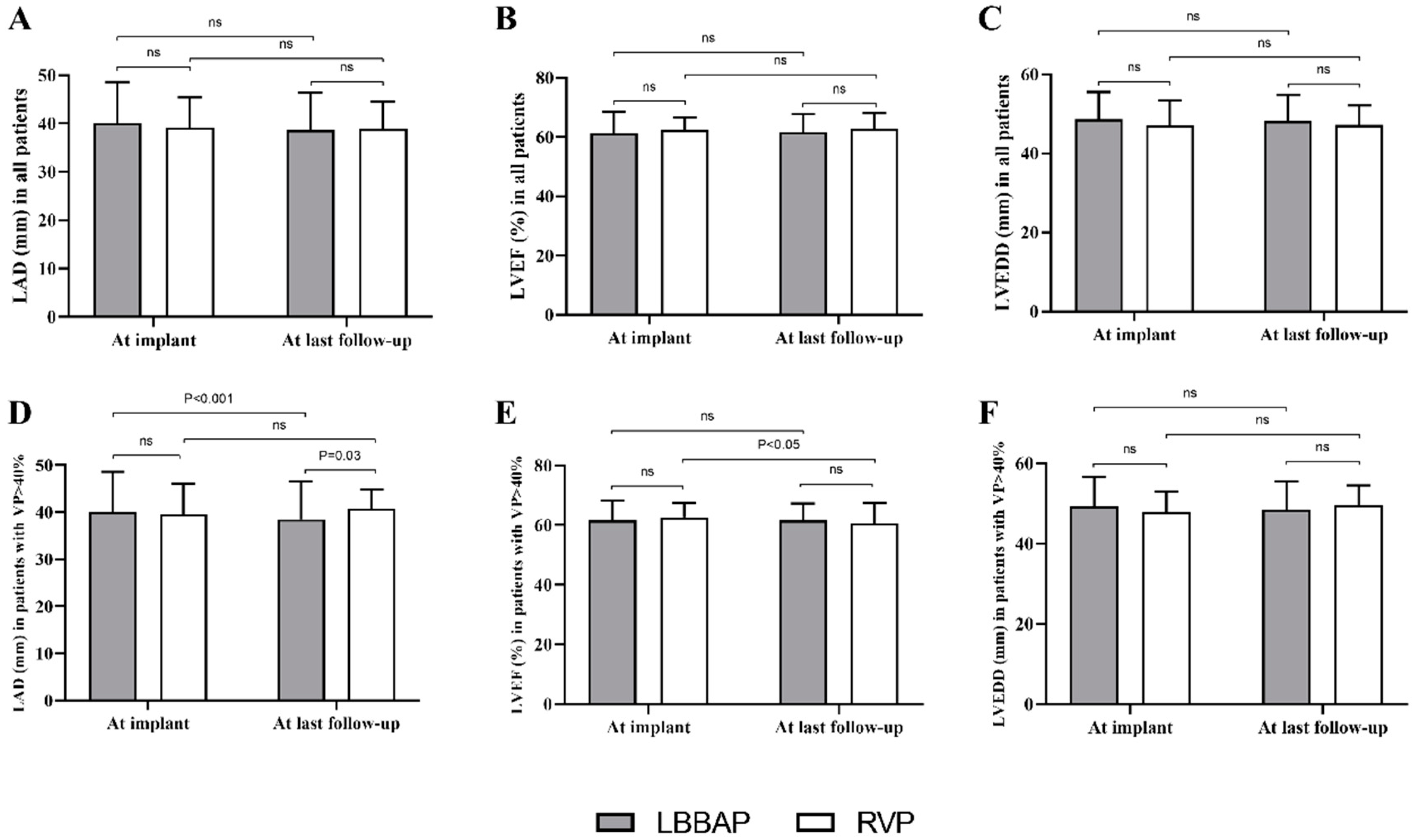
3.4. Echocardiographic Outcomes during Follow-Up
3.5. Procedure-Related Complications during Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy with Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, 1736.e1731–1736.e1733. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Fan, X.; Li, X.; Niu, H.; Gu, M.; Ning, X.; Hu, Y.; Gold, M.R.; Zhang, S. Comparison of Left Bundle Branch and His Bundle Pacing in Bradycardia Patients. JACC Clin. Electrophysiol. 2020, 6, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.O.; Hellkamp, A.S.; Ellenbogen, K.A.; Greenspon, A.J.; Freedman, R.A.; Lee, K.L.; Lamas, G.A.; Investigators MOST. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003, 107, 2932–2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimony, A.; Eisenberg, M.J.; Filion, K.B.; Amit, G. Beneficial effects of right ventricular non-apical vs. apical pacing: A systematic review and meta-analysis of randomized controlled trials. Europace 2012, 14, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, X.; Su, L.; Wu, S.; Xia, X.; Vijayaraman, P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019, 16, 1791–1796. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Wang, S.; Wu, S.; Xu, L.; Huang, Z.; Chen, X.; Zheng, R.; Jiang, L.; Ellenbogen, K.A.; Whinnett, Z.I.; et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythm. Electrophysiol. 2021, 14, e009261. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, Q.; Bai, J.; Wang, W.; Qin, S.; Wang, J.; Liang, Y.; Su, Y.; Ge, J. The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: A single-centre experience. Europace 2020, 22, ii36–ii44. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e382–e482. [Google Scholar] [PubMed]
- Li, X.; Li, H.; Ma, W.; Ning, X.; Liang, E.; Pang, K.; Yao, Y.; Hua, W.; Zhang, S.; Fan, X. Permanent left bundle branch area pacing for atrioventricular block: Feasibility, safety, and acute effect. Heart Rhythm 2019, 16, 1766–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Hou, X.; Qian, Z.; Wang, Y.; Tang, L.; Qiu, Y.; Jiang, Z.; Chen, X.; Li, K.; Zou, J. A novel 9-partition method using fluoroscopic images for guiding left bundle branch pacing. Heart Rhythm 2020, 17, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Zu, L.; Cheng, L.; Su, R.; Wang, X.; Liang, Z.; Chen, J.; Hang, F.; Du, J.; et al. Simplifying Physiological Left Bundle Branch Area Pacing Using a New Nine-Partition Method. Can. J. Cardiol. 2021, 37, 329–338. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Li, H.; Ning, X.; Liang, E.; Ma, W.; Wang, H.; Liu, Z.; Yao, Y. ECG patterns of successful permanent left bundle branch area pacing in bradycardia patients with typical bundle branch block. Pacing Clin. Electrophysiol. PACE 2020, 43, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Pastore, G.; Aggio, S.; Baracca, E.; Fraccaro, C.; Picariello, C.; Roncon, L.; Corbucci, G.; Noventa, F.; Zanon, F. Hisian area and right ventricular apical pacing differently affect left atrial function: An intra-patients evaluation. Europace 2014, 16, 1033–1039. [Google Scholar] [CrossRef]
- Pastore, G.; Zanon, F.; Baracca, E.; Aggio, S.; Corbucci, G.; Boaretto, G.; Roncon, L.; Noventa, F.; Barold, S.S. The risk of atrial fibrillation during right ventricular pacing. Europace 2016, 18, 353–358. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Bolun, Z.; Pei, M.; Ma, B.; Tong, Q.; Yin, H.; Zhang, Y.; You, L.; Xie, R. Comparison of cardiac function between left bundle branch pacing and right ventricular outflow tract septal pacing in the short-term: A registered controlled clinical trial. Int. J. Cardiol. 2021, 322, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, L.; Bai, J.; Wang, W.; Qin, S.; Wang, J.; Liang, Y.; Su, Y.; Ge, J. Procedure-Related Complications of Left Bundle Branch Pacing: A Single-Center Experience. Front. Cardiovasc. Med. 2021, 8, 645947. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A.; Panikkath, R.; John, K.; Mascarenhas, V.; Bauch, T.D.; Huang, W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019, 16, 1774–1782. [Google Scholar] [CrossRef] [Green Version]

| Variables | LBBAP (n = 406) | RVP (n = 313) | p Value |
|---|---|---|---|
| Age, years | 64.9 ± 14.3 | 67.5 ± 12.2 | 0.080 |
| Male, n (%) | 197 (48.5%) | 150 (47.9%) | 0.554 |
| Hypertension, n (%) | 244 (60.1%) | 200 (63.9%) | 0.329 |
| Diabetes, n (%) | 79 (19.5%) | 72 (23.0%) | 0.292 |
| Atrial fibrillation, n (%) | 178 (43.8%) | 129 (41.2%) | 0.534 |
| CAD, n (%) | 76 (18.7%) | 66 (21.1%) | 0.470 |
| Valvular heart disease, n (%) | 35 (8.6%) | 24 (7.7%) | 0.748 |
| Baseline Electrocardiogram | |||
| Heart rate, bpm | 54.7 ± 17.5 | 61.1 ± 17.0 | 0.236 |
| QRS duration, ms | 112.4 ± 24.1 | 98.0 ± 18.3 | 0.405 |
| LBBB, n (%) | 43 (10.5%) | 1 (0.3%) | <0.001 |
| RBBB, n (%) | 95 (23.4%) | 14 (4.5%) | <0.001 |
| Baseline Echocardiography | |||
| LAD, mm | 40.2 ± 8.45 | 39.1 ± 6.30 | 0.060 |
| LVEDD, mm | 48.6 ± 6.91 | 47.1 ± 6.25 | 0.224 |
| LVEF, mm | 61.2 ± 7.27 | 62.5 ± 4.14 | 0.203 |
| IVS, mm | 9.8 ± 1.93 | 10.4 ± 4.27 | 0.360 |
| Moderate or severe MR, n (%) | 40 (9.9%) | 28 (8.9%) | 0.702 |
| Moderate or severe TR, n (%) | 38 (9.4%) | 32 (10.2%) | 0.705 |
| Pacing indications | <0.001 | ||
| AVB, n (%) | 245 (60.3%) | 86 (27.5%) | |
| SND, n (%) | 161 (39.7%) | 227 (72.5%) | |
| Type of device | <0.001 | ||
| Double-chamber PM, n (%) | 341(84.0%) | 297 (94.9%) | |
| Single-chamber PM, n (%) | 65 (16.0%) | 16 (5.1%) | |
| Medications | |||
| Beta blockers, n (%) | 41 (10.1%) | 30 (9.6%) | 0.819 |
| ACEI/ARBs, n (%) | 188 (46.3%) | 157 (50.2%) | 0.305 |
| CCB, n (%) | 221 (54.4%) | 184 (58.8%) | 0.243 |
| Antiarrhythmic drugs *, n (%) | 107 (26.4%) | 72 (23.0%) | 0.303 |
| NOACs, n (%) | 26 (6.4%) | 20 (6.4%) | 0.560 |
| Warfarin, n (%) | 31 (7.6%) | 24 (7.7%) | 0.548 |
| Antiplatelet agents, n (%) | 34 (8.4%) | 28 (8.9%) | 0.790 |
| Variables | LBBAP (n = 376) | RVP (n = 313) | p Value |
|---|---|---|---|
| LBB potential, n (%) | 256 (68.1%) | - | - |
| P-V interval, ms | 27.7 ± 4.7 | - | - |
| Sti-LVAT at 5 V/0.4 ms, ms | 73.9 ± 13.4 | - | - |
| Sti-LVAT at 2 V/0.4 ms, ms | 76.7 ± 15.4 | - | - |
| Ring capture at 2 V/0.4 ms, n (%) | 366 (97.3%) | - | - |
| Ring capture threshold, V/0.4 ms | 1.04 ± 0.65 | - | - |
| Capture threshold, V/0.4 ms | 0.64 ± 0.22 | 0.64 ± 0.20 | 0.573 |
| Paced QRSd, ms | 114 ± 10.7 | 148 ± 18.0 | <0.001 |
| Pacing impedance, Ω | 783 ± 154 | 782 ± 217 | 0.231 |
| R wave amplitude, mV | 11.7 ± 6.1 | 10.6 ± 4.9 | 0.142 |
| Procedural duration, min | 11.0 (7.0, 18.8) | 6.7 (5.8, 7.8) | <0.001 |
| Fluoroscopy duration, min | 5.0 (3.0, 8.0) | 2.8 (1.9, 3.5) | <0.001 |
| Variables | β | 95% CI | p Value |
|---|---|---|---|
| Age | 0.045 | 0.003, 0.087 | 0.035 |
| Female (vs. Male) | 0.055 | −1.062, 1.173 | 0.923 |
| LBBAP (vs. RVP) | −1.601 | −3.094, −0.109 | 0.036 |
| Hypertension | 0.429 | −0.724, 1.581 | 0.465 |
| Diabetes | −1.207 | −2.613, 0.200 | 0.092 |
| CAD | 0.417 | −1.060, 1.894 | 0.579 |
| Atrial fibrillation | 2.113 | 0.900, 3.325 | 0.001 |
| Valvular heart disease | 1.010 | −0.907, 2.927 | 0.301 |
| AVB | 0.185 | −1.433, 1.802 | 0.822 |
| SND | −0.588 | −1.880, 0.705 | 0.372 |
| Device type (DDD vs VVI) | 0.040 | −0.941, 1.020 | 0.936 |
| Baseline LAD | −0.433 | −0.517, −0.349 | <0.001 |
| Baseline LVEDD | 0.019 | −0.073, 0.112 | 0.683 |
| Baseline LVEF | −0.128 | −0.216, −0.040 | 0.004 |
| VP% ≥ 40% | 0.116 | −1.237, 1.469 | 0.866 |
| Beta blockers | 0.026 | −0.006, 0.058 | 0.113 |
| ACEI/ARBs | 0.022 | −0.006, 0.049 | 0.128 |
| CCB | −0.247 | −2.290, 1.795 | 0.812 |
| Antiarrhythmic drugs * | −0.849 | −2.299, 0.600 | 0.250 |
| Procedure-Related Complications | LBBAP (n = 376) | RVP (n = 313) |
|---|---|---|
| At implant | ||
| Lead dislodgement, n (%) | 1 (0.27%) | 2 (0.64%) |
| Lead perforation during procedure, n (%) | 1 (0.27%) | 0 (0%) |
| Transient RBB injury, n (%) | 30 (7.98%) | 0 (0%) |
| Persistent RBB injury, n (%) | 8 (2.13%) | 0 (0%) |
| Pericardial effusion, n (%) | 0 (0%) | 0 (0%) |
| Pacing system infection, n (%) | 0 (0%) | 0 (0%) |
| Pocket hematoma, n (%) | 0 (0%) | 0 (0%) |
| Pneumothorax/hemothorax, n (%) | 0 (0%) | 0 (0%) |
| During follow-up | ||
| Lead dislodgement, n (%) | 0 (0%) | 2 (0.64%) |
| Lead perforation, n (%) | 0 (0%) | 0 (0%) |
| Pocket hematoma, n (%) | 0 (0%) | 0 (0%) |
| Pacing threshold > 2.0 V/0.4 ms, n (%) | 0 (0%) | 0 (0%) |
| Pacing system infection, n (%) | 0 (0%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Wang, Z.; Li, X.; Yao, Y.; Liu, Z.; Fan, X. Medium- and Long-Term Lead Stability and Echocardiographic Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing. J. Cardiovasc. Dev. Dis. 2021, 8, 168. https://doi.org/10.3390/jcdd8120168
Zhu H, Wang Z, Li X, Yao Y, Liu Z, Fan X. Medium- and Long-Term Lead Stability and Echocardiographic Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing. Journal of Cardiovascular Development and Disease. 2021; 8(12):168. https://doi.org/10.3390/jcdd8120168
Chicago/Turabian StyleZhu, Haojie, Zhao Wang, Xiaofei Li, Yan Yao, Zhimin Liu, and Xiaohan Fan. 2021. "Medium- and Long-Term Lead Stability and Echocardiographic Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing" Journal of Cardiovascular Development and Disease 8, no. 12: 168. https://doi.org/10.3390/jcdd8120168
APA StyleZhu, H., Wang, Z., Li, X., Yao, Y., Liu, Z., & Fan, X. (2021). Medium- and Long-Term Lead Stability and Echocardiographic Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing. Journal of Cardiovascular Development and Disease, 8(12), 168. https://doi.org/10.3390/jcdd8120168