Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Standard Anticoagulation Management and HIT Diagnosis under Impella 5+
2.3. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. HIT-Associated Clinical Features and Outcomes
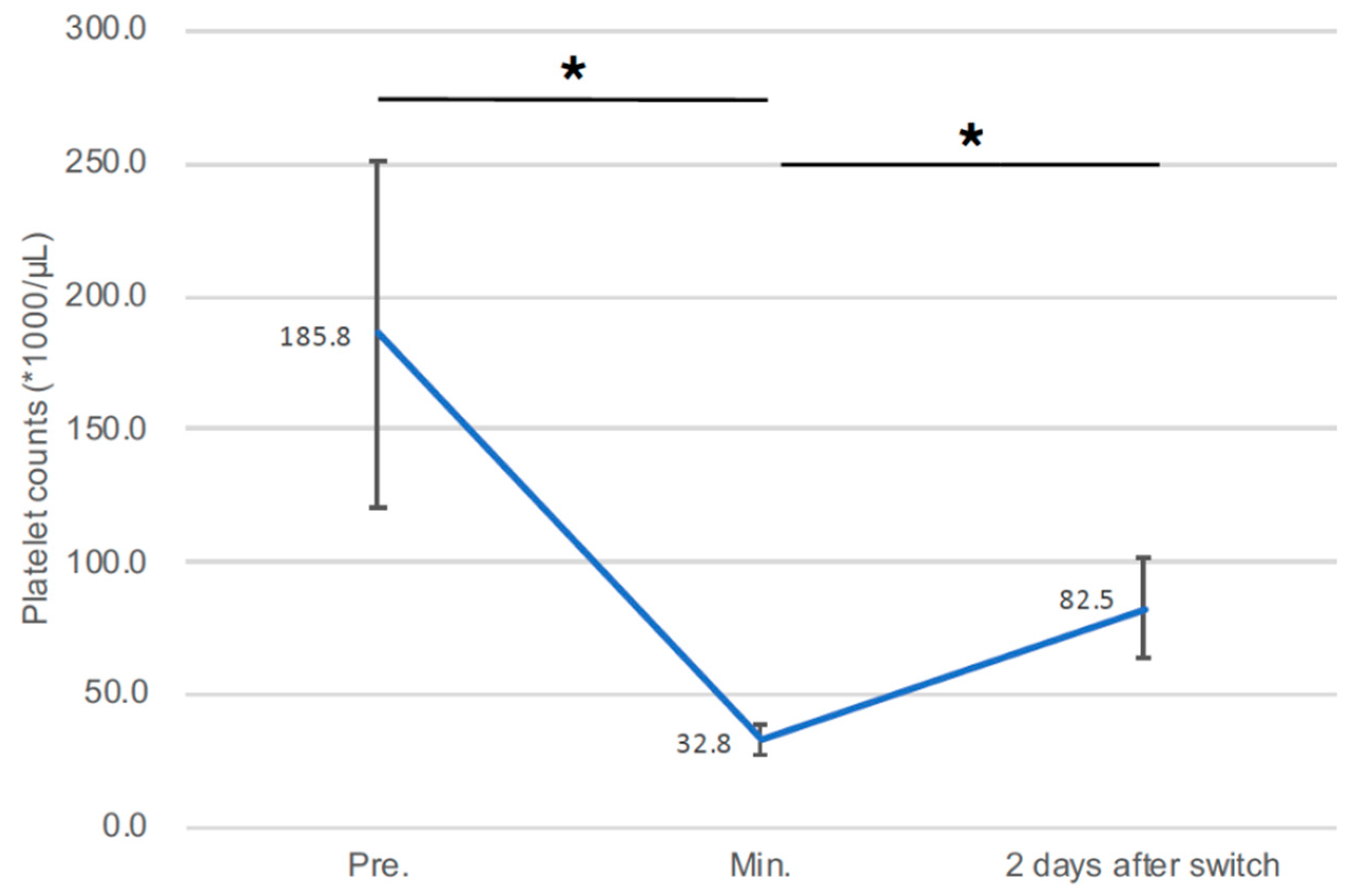
3.3. Changes in Platelet Count during Impella Support
3.4. Outcome of Survivors after Positive HIT Diagnostic
3.5. Impella Dysfunction Due to Pump Thrombosis Associated with HIT
3.6. LV Thrombus Associated with HIT under Impella 5.0 Support
4. Discussion
4.1. Diagnostic of HIT with Impella as a Part of MCS
4.2. Anticoagulation in HIT with Impella as a Part of MCS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greinacher, A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, T.W.; Weeks, P.A.; Lee, Y.; Kumar, S.; Castillo, B.; Kar, B.; Gregoric, I.D. Anticoagulation of Impella with a Bivalirudin Purge Solution. ASAIO J. 2020, 66, e117–e120. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.; Changal, K.H.; Smith, A.; Ambreen, S. Argatroban as Purge Solution in Patients With Heparin-Induced Thrombocytopenia on an Impella Device, a Case Review. Am. J. Ther. 2020, 28, e763–e765. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, H.; Milkovits, A.E. Use of Systemic Bivalirudin and an Anticoagulant-Free Purge Solution in a Percutaneous Left Ventricular Assist Device in a Patient With Heparin-Induced Thrombocytopenia. J. Pharm. Pract. 2020, 34, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Hohlfelder, B.; Militello, M.A.; Tong, M.Z.; Soltesz, E.G.; Wanek, M.R. Anticoagulation with temporary Impella device in patients with heparin-induced thrombocytopenia: A case series. Int. J. Artif. Organs 2020, 44, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Blum, E.C.; Martz, C.R.; Selektor, Y.; Nemeh, H.; Smith, Z.R.; To, L. Anticoagulation of Percutaneous Ventricular Assist Device Using Argatroban-Based Purge Solution: A Case Series. J. Pharm. Pract. 2018, 31, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, B.; Reed, B.N. Use of an argatroban-based purge solution in a percutaneous ventricular assist device. Am. J. Health Syst. Pharm. 2017, 74, e163–e169. [Google Scholar] [CrossRef] [PubMed]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Arepally, G.; Crowther, M.A.; Rice, L.; Datko, F.; Hook, K.; Propert, K.J.; Kuter, D.J.; Ortel, T.L.; Konkle, B.A.; et al. The HIT Expert Probability (HEP) Score: A novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J. Thromb. Haemost. 2010, 8, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Pishko, A.M.; Cuker, A. Heparin-Induced Thrombocytopenia in Cardiac Surgery Patients. Semin. Thromb. Hemost. 2017, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; El-Banayosy, A.; Prohaska, W.; Arusoglu, L.; Morshuis, M.; Koester-Eiserfunke, W.; Kizner, L.; Murray, E.; Eichler, P.; Koerfer, R.; et al. Heparin-induced thrombocytopenia in patients receiving mechanical circulatory support. J. Thorac. Cardiovasc. Surg. 2006, 131, 1373–1381.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuker, A.; Gimotty, P.A.; Crowther, M.A.; Warkentin, T.E. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: A systematic review and meta-analysis. Blood 2012, 120, 4160–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubenow, N.; Hinz, P.; Thomaschewski, S.; Lietz, T.; Vogler, M.; Ladwig, A.; Junger, M.; Nauck, M.; Schellong, S.; Wander, K.; et al. The severity of trauma determines the immune response to PF4/heparin and the frequency of heparin-induced thrombocytopenia. Blood 2010, 115, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Warkentin, T.E. Risk of heparin-induced thrombocytopenia in patients receiving thromboprophylaxis. Expert. Rev. Hematol. 2008, 1, 75–85. [Google Scholar] [CrossRef] [PubMed]


| All Cases (n = 56) | No HIT Test (n = 35) | HIT Positive (n = 6) | HIT Negative (n = 15) | p | |
|---|---|---|---|---|---|
| Age (year) | 61.8 ± 11.6 | 63.1 ± 12.2 | 57.0 ± 15.1 | 60.7 ± 8.17 | ns |
| Male, n (%) | 47 (83.9) | 29 (82.9) | 5 (83.3) | 13 (86.7) | ns |
| INTERMACS profiles I, n (%) | 25 (44.6) | 16 (45.7) | 1 (16.7) | 8 (53.3) | ns |
| Arterial hypertension, n (%) | 33 (58.9) | 23 (65.7) | 4 (66.7) | 6 (40.0) | ns |
| Hyperlipidemia, n (%) | 14 (25.0) | 10 (28.6) | 2 (33.3) | 2 (13.3) | ns |
| Diabetes, n (%) | 21 (37.5) | 14 (40.0) | 1 (16.7) | 6 (40.0) | ns |
| Peripheral vascular disease, n (%) | 6 (10.7) | 3 (8.6) | 0 (0.0) | 3 (20.0) | ns |
| Arrhythmia, n (%) | 19 (33.9) | 11 (31.4) | 0 (0.0) | 8 (53.3) | ns |
| COPD, n (%) | 3 (5.4) | 2 (5.7) | 0 (0.0) | 1 (6.7) | ns |
| Nicotine abuses, n (%) | 16 (28.6) | 10 (28.6) | 3 (50.0) | 3 (20.0) | ns |
| Drug abuses, n (%) | 2 (3.6) | 1 (2.9) | 1 (16.7) | 0 (0.0) | ns |
| Dialysis, n (%) | 2 (3.6) | 2 (5.7) | 0 (0.0) | 0 (0.0) | ns |
| History of PCI, n (%) | 19 (33.9) | 14 (40.0) | 1 (16.7) | 4 (26.7) | ns |
| post CPR, n (%) | 15 (26.8) | 9 (25.7) | 0 (0.0) | 6 (40.0) | ns |
| Biventricular failure, n (%) | 31 (55.4) | 17 (48.6) | 4 (66.7) | 10 (66.7) | ns |
| ACS/ICM, n (%) | 44 (78.0) | 31 (88.6) * | 3 (50.0) | 10 (66.7) | <0.05 |
| DCM, n (%) | 8 (14.3) | 3 (8.6) | 1 (16.7) | 4 (26.7) | ns |
| Myocarditis, n (%) | 2 (3.6) | 1 (2.9) | 1 (16.7) | 0 (0.0) | ns |
| CS after oHTX, n (%) | 2 (3.6) | 1 (2.9) | 0 (0.0) | 1 (6.7) | ns |
| Postoperative use, n (%) | 26 (46.4) | 17 (48.6) | 2 (33.3) | 7 (46.7) | ns |
| va-ECMO implantation, n (%) | 43 (76.8) | 26 (74.3) | 6 (100.0) | 11 (73.3) | ns |
| Pulmonary edema, n (%) | 36 (64.3) | 22 (62.9) | 5 (83.3) | 9 (60.0) | ns |
| Lactate (mmol/dL) | 2.33 ± 1.28 | 4.47 ± 4.76 | 1.70 ± 0.86 | 4.76 ± 4.21 | ns |
| Creatinine (mg/dL) | 1.69 ± 0.65 | 1.69 ± 0.91 | 1.45 ± 0.54 | 1.63 ± 0.96 | ns |
| Bilirubin (mg/dL) | 5.17 ± 4.21 | 2.70 ± 2.86 | 4.56 ± 4.60 | 2.39 ± 2.72 | ns |
| CRP (mg/dL) | 15.8 ± 12.9 | 12.8 ± 7.80 * | 17.7 ± 11.8 | 7.95 ± 8.27 | <0.05 |
| Mortality, n (%) | 33 (57.9) | 20 (57.1) | 2 (33.3) | 11 (73.3) | ns |
| Platelets | |||
|---|---|---|---|
| Maximum (* 1000/µL) | Minimum (* 1000/µL) | Rate of Decrease (%) | |
| Case 1 | 191 | 25 | 86.9 |
| Case 2 | 258 | 38 | 85.3 |
| Case 3 | 100 | 35 | 65.0 |
| Case 4 | 194 | 33 | 83.0 |
| Case 5 | 193 | 37 | 80.8 |
| Case 6 | 167 | 74 | 55.7 |
| average | 76.1 | ||
| HIT Positive (n = 6) | HIT Negative (n = 15) | p | |
|---|---|---|---|
| HIT test after first heparin (days) | 10.5 ± 2.89 | 8.00 ± 4.90 | 0.15 |
| Event for the first heparin administration since admission | |||
| va-ECMO implantation, n (%) | 3 (50.0) | 7 (46.7) | 1.0 |
| Open heart operation, n (%) | 3 (50.0) | 7 (46.7) | 1.0 |
| Impella, n (%) | 0 (0.0) | 1 (6.67) | 1.0 |
| HIT-associated thrombotic events under Impella support | |||
| Impella dysfunction, n (%) | 1 (16.7) | - | - |
| Left ventricular thrombus, n (%) | 1 (16.7) | - | - |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Case Nr. in Table 2. | Case 2 | Case 3 | Case 4 | Case 5 |
| Basis diagnosis | ACS/ICM | ACS/ICM | myocarditis | DCM |
| Postcardiotomy? | Yes (CABG) | No | No | No |
| Impella size | 5.0 | 5.0 | 5.0 | CP → 5.0 |
| va-ECMO? | Yes | Yes | Yes | Yes |
| Impella duration at HIT positive (d) | 1 | 4 | 10 | 11 |
| Successful Impella weaning? | Yes | Yes | Yes | Transition to oHTX |
| Total Impella duration (d) | 7 | 27 | 10 | 12 |
| Coagulopathy? | Yes | Yes | No | No |
| Systemic anticoagulation | ||||
| Before-HIT diagnostic | Heparin | None | Heparin | Heparin |
| Post-HIT diagnostic | Argatroban | None | Argatroban | Argatroban |
| Purge anticoagulation | ||||
| Before-HIT diagnostic | Heparin 20 U/mL | Heparin 50 U/mL | Heparin 50 U/mL | Heparin 50 U/mL |
| Post-HIT diagnostic | Argatroban 25 mg/L | Argatroban 20 mg/L | Argatroban 90 mg/L | Argatroban 40 mg/L |
| Neurological complications? | No | No | No | No |
| Discharge (d) | 55 | 63 | 27 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimura, Y.; Bauer, S.; Immohr, M.B.; Hermsen, D.F.; Westenfeld, R.; Boeken, U.; Aubin, H.; Tudorache, I.; Lichtenberg, A.; Akhyari, P. Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock. J. Cardiovasc. Dev. Dis. 2021, 8, 161. https://doi.org/10.3390/jcdd8120161
Sugimura Y, Bauer S, Immohr MB, Hermsen DF, Westenfeld R, Boeken U, Aubin H, Tudorache I, Lichtenberg A, Akhyari P. Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock. Journal of Cardiovascular Development and Disease. 2021; 8(12):161. https://doi.org/10.3390/jcdd8120161
Chicago/Turabian StyleSugimura, Yukiharu, Sebastian Bauer, Moritz Benjamin Immohr, Derik Franz Hermsen, Ralf Westenfeld, Udo Boeken, Hug Aubin, Igor Tudorache, Artur Lichtenberg, and Payam Akhyari. 2021. "Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock" Journal of Cardiovascular Development and Disease 8, no. 12: 161. https://doi.org/10.3390/jcdd8120161
APA StyleSugimura, Y., Bauer, S., Immohr, M. B., Hermsen, D. F., Westenfeld, R., Boeken, U., Aubin, H., Tudorache, I., Lichtenberg, A., & Akhyari, P. (2021). Heparin-Induced Thrombocytopenia under Mechanical Circulatory Support by Large Impella for Acute Cardiogenic Shock. Journal of Cardiovascular Development and Disease, 8(12), 161. https://doi.org/10.3390/jcdd8120161








