Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Outcome Assessment
2.4. Study Selection Process and Data Extraction
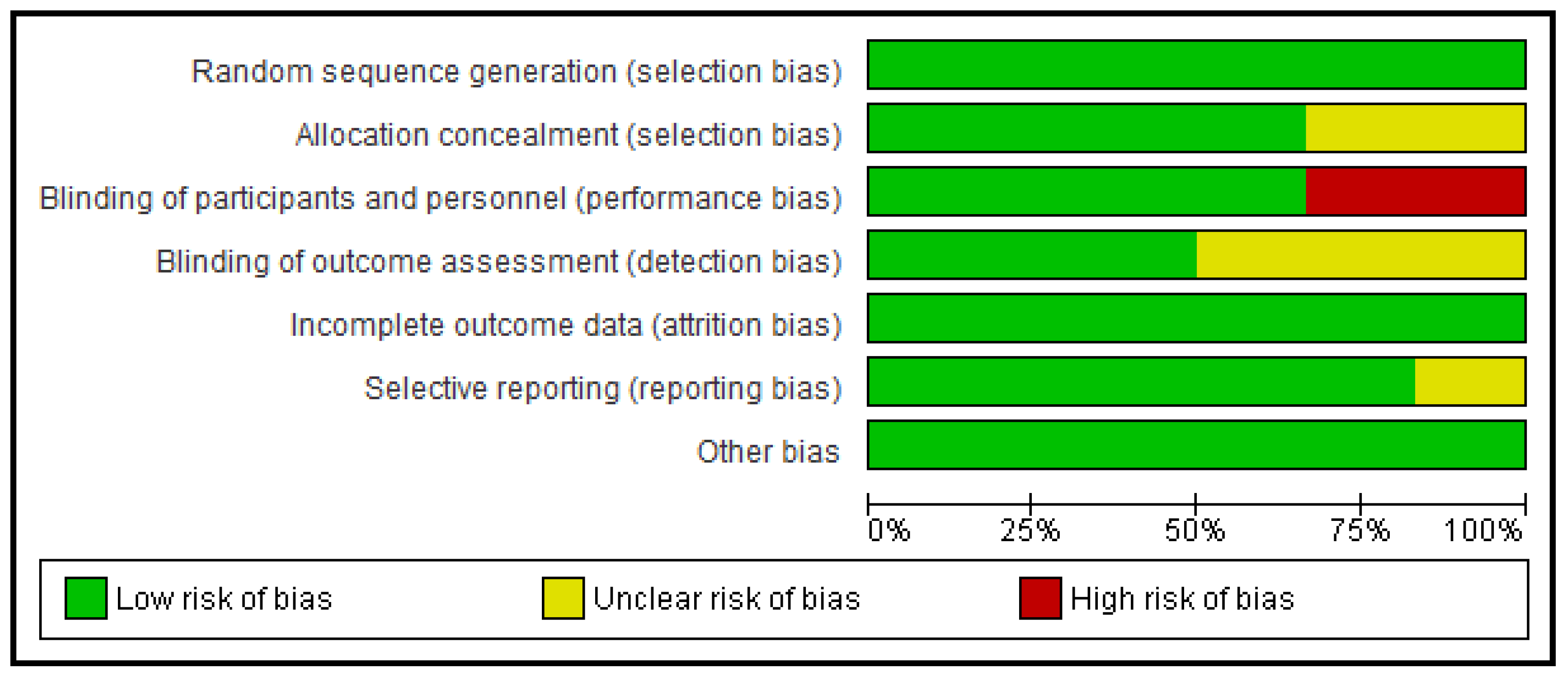
2.5. Risk of Bias Assessment
2.6. Statistical Analysis
3. Results
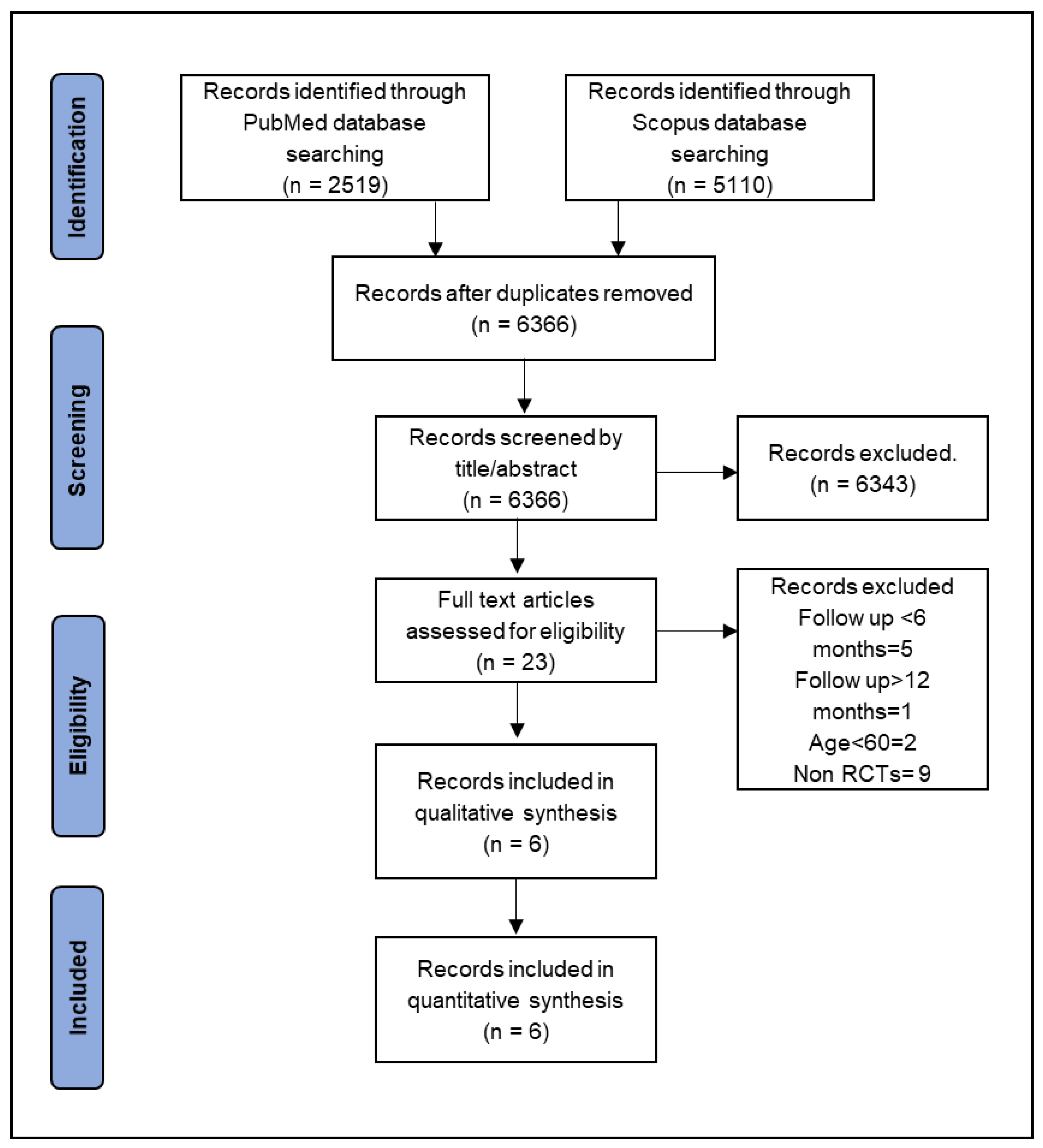
3.1. Study Selection
3.2. Characteristics of Studies
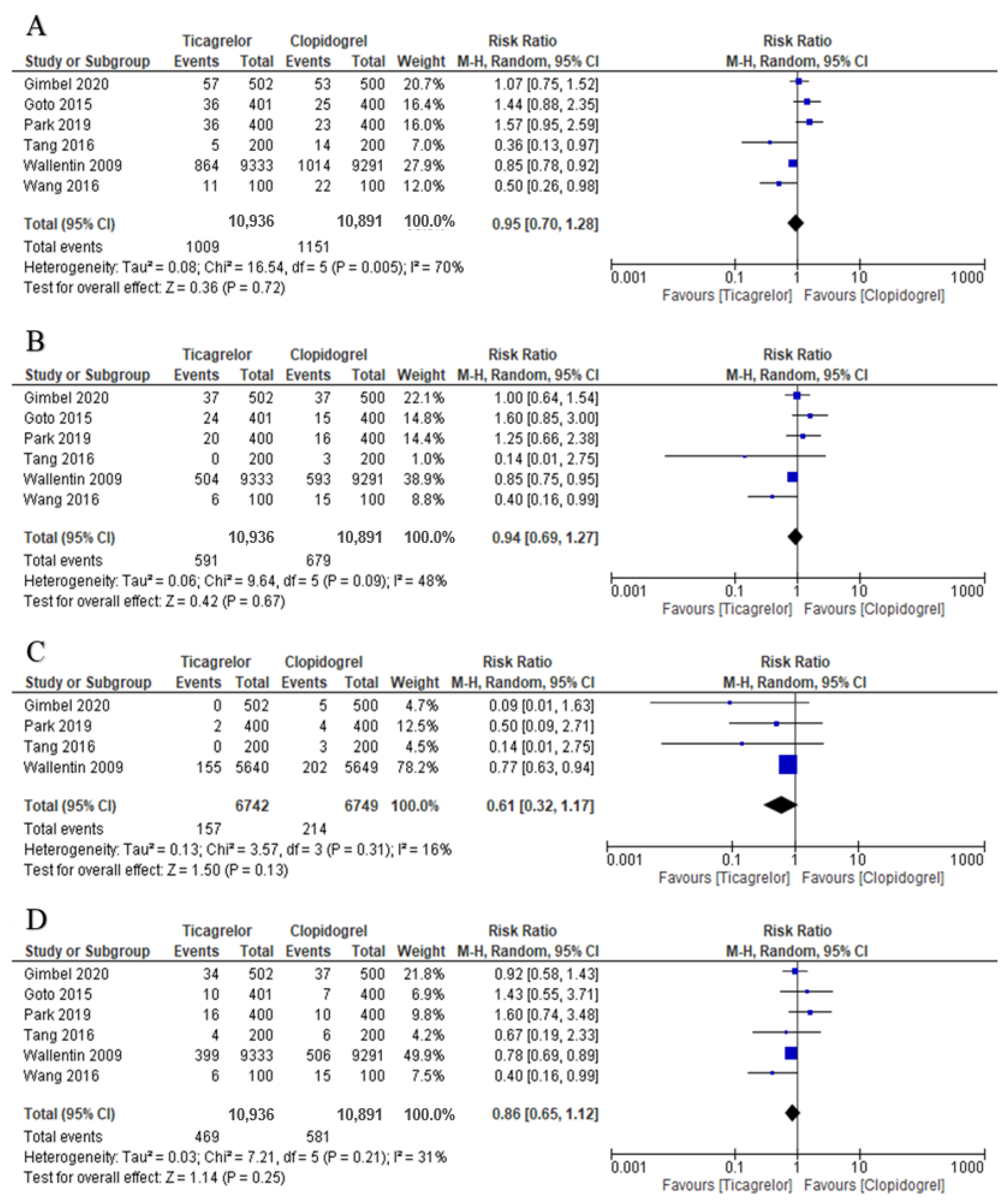
3.3. Primary Outcomes: MACE, MI, ST and All-Cause Death
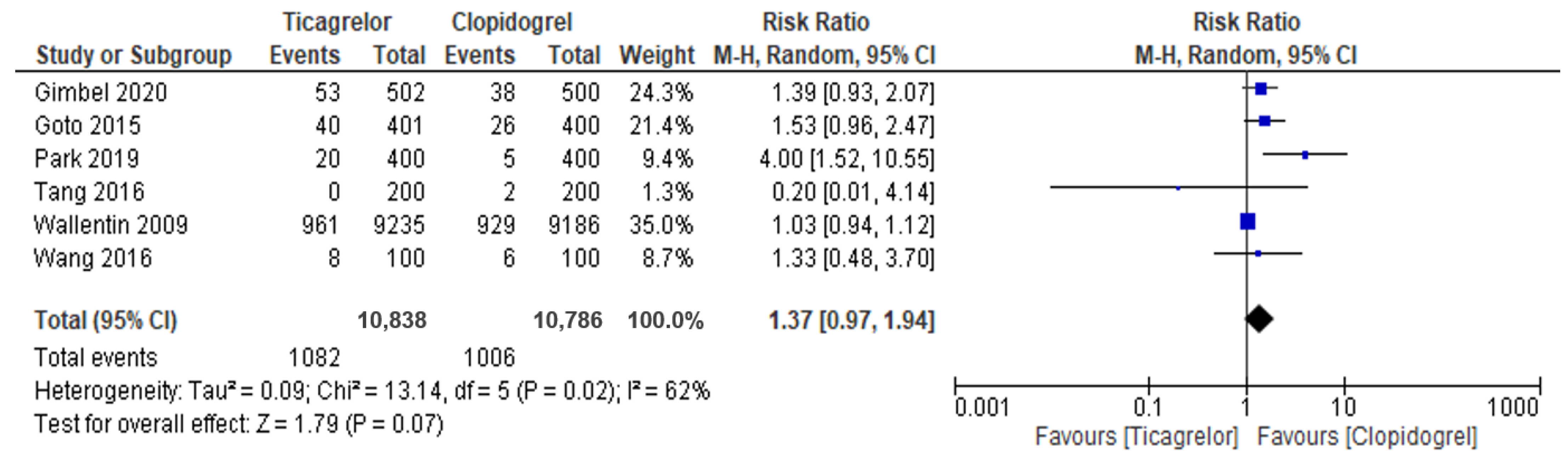
3.4. Secondary Outcomes: Major Bleeding
3.5. Sensitivity Analyses
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Roth, G.A.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, Á.; Goodman, S.G.; Flather, M.D.; Anderson, F.A. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehran, R.; Pocock, S.J.; Nikolsky, E.; Clayton, T.; Dangas, G.D.; Kirtane, A.J.; Parise, H.; Fahy, M.; Manoukian, S.V.; Feit, F. A risk score to predict bleeding in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2010, 55, 2556–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. J. Cardio-Thorac. Surg. 2018, 53, 34–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, S. Ticagrelor: A review of its use in adults with acute coronary syndromes. Am. J. Cardiovasc. Drugs 2015, 15, 51–68. [Google Scholar] [CrossRef]
- Akkaif, M.A.; Daud, N.A.A.; Sha’aban, A.; Ng, M.L.; Sk Abdul Kader, M.A.; Noor, D.A.M.; Ibrahim, B. The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 2021, 26, 1987. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, N.; Seong, J.M.; Gwak, H.S. Efficacy and safety of clopidogrel versus prasugrel and ticagrelor for coronary artery disease treatment in patients with CYP2C19 LoF alleles: A systemic review and meta-analysis. Br. J. Clin. Pharmacol. 2020, 86, 1489–1498. [Google Scholar] [CrossRef]
- Biswas, M.; Kali, M.S.K.; Biswas, T.K.; Ibrahim, B. Risk of major adverse cardiovascular events of CYP2C19 loss-of-function genotype guided prasugrel/ticagrelor vs. clopidogrel therapy for acute coronary syndrome patients undergoing percutaneous coronary intervention: A meta-analysis. Platelets 2021, 32, 591–600. [Google Scholar] [CrossRef]
- Husted, S.; James, S.; Becker, R.C.; Horrow, J.; Katus, H.; Storey, R.F.; Cannon, C.P.; Heras, M.; Lopes, R.D.; Morais, J. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: A substudy from the prospective randomized PLATelet inhibition and patient Outcomes (PLATO) trial. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 680–688. [Google Scholar] [CrossRef] [Green Version]
- Avezum, A.; Makdisse, M.; Spencer, F.; Gore, J.M.; Fox, K.A.; Montalescot, G.; Eagle, K.A.; White, K.; Mehta, R.H.; Knobel, E. Impact of age on management and outcome of acute coronary syndrome: Observations from the Global Registry of Acute Coronary Events (GRACE). Am. Heart J. 2005, 149, 67–73. [Google Scholar] [CrossRef]
- Gimbel, M.; Qaderdan, K.; Willemsen, L.; Hermanides, R.; Bergmeijer, T.; de Vrey, E.; Heestermans, T.; Gin, M.T.J.; Waalewijn, R.; Hofma, S. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): The randomised, open-label, non-inferiority trial. Lancet 2020, 395, 1374–1381. [Google Scholar] [CrossRef]
- Szummer, K.; Montez-Rath, M.E.; Alfredsson, J.; Erlinge, D.; Lindahl, B.; Hofmann, R.; Ravn-Fischer, A.; Svensson, P.; Jernberg, T. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: Insights from the SWEDEHEART registry. Circulation 2020, 142, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidelines, E.C.f.P.; Bax, J.J.; Baumgartner, H.; Ceconi, C.; Dean, V.; Deaton, C.; Fagard, R.; Funck-Brentano, C.; Hasdai, D.; Hoes, A. Third universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2012, 60, 1581–1598. [Google Scholar]
- Kikkert, W.J.; van Geloven, N.; van der Laan, M.H.; Vis, M.M.; Baan, J.; Koch, K.T.; Peters, R.J.; de Winter, R.J.; Piek, J.J.; Tijssen, J.G. The prognostic value of bleeding academic research consortium (BARC)-defined bleeding complications in ST-segment elevation myocardial infarction: A comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J. Am. Coll. Cardiol. 2014, 63, 1866–1875. [Google Scholar]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stare, J.; Maucort-Boulch, D. Odds ratio, hazard ratio and relative risk. Metodoloski Zv. 2016, 13, 59. [Google Scholar]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.; Lu, H.; Yang, K. Choosing between ticagrelor and clopidogrel following percutaneous coronary intervention: A systematic review and Meta-Analysis (2007–2017). Medicine 2018, 97, e12978. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther. Clin. Risk Manag. 2016, 12, 1101. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Li, R.; Jing, Q.; Wang, Q.; Liu, P.; Zhang, P.; Liu, Y. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Cardiovasc. Pharmacol. 2016, 68, 115–120. [Google Scholar] [CrossRef]
- Park, D.-W.; Kwon, O.; Jang, J.-S.; Yun, S.-C.; Park, H.; Kang, D.-Y.; Ahn, J.-M.; Lee, P.H.; Lee, S.-W.; Park, S.-W. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: A randomized clinical trial. Circulation 2019, 140, 1865–1877. [Google Scholar] [CrossRef]
- Goto, S.; Huang, C.-H.; Park, S.-J.; Emanuelsson, H.; Kimura, T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome–randomized, double-blind, phase III PHILO study. Circ. J. 2015, 79, 2452–2460. [Google Scholar] [CrossRef] [Green Version]
- Savi, P.; Nurden, P.; Nurden, A.; Levy-Toledano, S.; Herbert, J.-M. Clopidogrel: A review of its mechanism of action. Platelets 1998, 9, 251–255. [Google Scholar] [CrossRef]
- Wallentin, L. P2Y12 inhibitors: Differences in properties and mechanisms of action and potential consequences for clinical use. Eur. Heart J. 2009, 30, 1964–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkeland, K.; Parra, D.; Rosenstein, R. Antiplatelet therapy in acute coronary syndromes: Focus on ticagrelor. J. Blood Med. 2010, 1, 197. [Google Scholar]
- Akkaif, M.A.; Ng, M.L.; Kader, M.A.S.A.; Daud, N.A.A.; Sha’aban, A.; Ibrahim, B. A review of the effects of ticagrelor on adenosine concentration and its clinical significance. Pharmacol. Rep. 2021, 1–14. [Google Scholar] [CrossRef]
- Akkaif, M.A.; Sha’aban, A.; Daud, N.A.A.; Ng, M.L.; Ibrahim, B. Investigate the Strategy of Using Pharmacogenetics and Pharmacometabonomics to the Personalization of Ticagrelor Antiplatelet Therapy. Syst. Rev. Pharm. 2020, 11, 1100–1107. [Google Scholar]
- Kang, H.-J.; Clare, R.M.; Gao, R.; Held, C.; Himmelmann, A.; James, S.K.; Lim, S.T.; Santoso, A.; Yu, C.-M.; Wallentin, L. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: A retrospective analysis from the Platelet Inhibition and Patient Outcomes (PLATO) Trial. Am. Heart J. 2015, 169, 899–905.e891. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, T.; Kuno, T.; Ando, T.; Briasoulis, A.; Takagi, H.; Bangalore, S. Potent P2Y12 Inhibitors versus Clopidogrel in Elderly Patients with Acute Coronary Syndrome: Systematic Review and Meta-Analysis: P2Y12 inhibitors and elderly patients with ACS. Am. Heart J. 2021, 237, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Ueshima, D.; D’Amico, G.; Masiero, G.; Musumeci, G.; Stone, G.W.; Brener, S.J. Efficacy and safety of potent platelet P2Y12 receptor inhibitors in elderly versus nonelderly patients with acute coronary syndrome: A systematic review and meta-analysis. Am. Heart J. 2018, 195, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Crimi, G.; Morici, N.; Ferrario, M.; Ferri, L.A.; Piatti, L.; Grosseto, D.; Cacucci, M.; Mandurino Mirizzi, A.; Toso, A.; Piscione, F. Time course of ischemic and bleeding burden in elderly patients with acute coronary syndromes randomized to low-dose prasugrel or clopidogrel. J. Am. Heart Assoc. 2019, 8, e010956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuisset, T.; Deharo, P.; Quilici, J.; Johnson, T.W.; Deffarges, S.; Bassez, C.; Bonnet, G.; Fourcade, L.; Mouret, J.P.; Lambert, M. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: The TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur. Heart J. 2017, 38, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Franchi, F.; Rollini, F.; Rivas Rios, J.; Rivas, A.; Agarwal, M.; Kureti, M.; Nagaraju, D.; Wali, M.; Shaikh, Z.; Briceno, M. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease: Results of the SWAP-4 study. Circulation 2018, 137, 2450–2462. [Google Scholar] [CrossRef]
- Li, X.Y.; Su, G.H.; Wang, G.X.; Hu, H.Y.; Fan, C.J. Switching from ticagrelor to clopidogrel in patients with ST-segment elevation myocardial infarction undergoing successful percutaneous coronary intervention in real-world China: Occurrences, reasons, and long-term clinical outcomes. Clin. Cardiol. 2018, 41, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Pourdjabbar, A.; Hibbert, B.; Chong, A.-Y.; Le May, M.R.; Labinaz, M.; Simard, T.; Ramirez, F.D.; Lugomirski, P.; Maze, R.; Froeschl, M. A randomised study for optimising crossover from ticagrelor to clopidogrel in patients with acute coronary syndrome. Thromb. Haemost. 2017, 117, 303–310. [Google Scholar] [CrossRef]
| Authors | Location | Centres (N.) | Diagnosis | Design of the Study | Age Mean (SD) or Median (IQR) | Follow Up (Months) | Bleeding Classification | Outcome Indication | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | |||||||
| Gimbel et al., 2020 [14] | Netherlands | 12 | NSTE-ACS | RCTs | 77.00 (73–82) | 77.00 (73–81) | 12 | PLATO | MACE, MI, Mortality, Bleeding | MACE, MI, ST, Mortality, Bleeding |
| Goto et al., 2015 [27] | Japan | 110 | ACS | RCTs | 67.00 (12) | 66.00 (11) | 12 | PLATO | MACE, MI, Mortality, Bleeding | MACE, MI, Mortality, Bleeding |
| Park et al., 2019 [26] | Korea | 10 | ACS | RCTs | 62.50 (11.3) | 62.30 (11.5) | 12 | PLATO | MACE, MI, ST, Mortality, Bleeding | MACE, MI, ST, Mortality, Bleeding |
| Tang et al., 2016 [25] | China | 2 | STEMI | RCTs | 64.36 (11.4) | 64.18 (11.1) | 6 | TIMI | MACE, Mortality, Bleeding | MACE, MI, ST, Mortality, Bleeding |
| Wallentin et al., 2009 [6] | USA | 862 | ACS | RCTs | 62.00 | 62.00 | 12 | PLATO | MACE, MI, ST, Mortality, Bleeding | MACE, MI, ST, Mortality, Bleeding |
| Wang and Wang 2016 [24] | China | 1 | ACS | RCTs | 79.00 (76–85) | 80.00 (74–86) | 12 | PLATO | MACE, MI, ST, Mortality, Bleeding | MACE, MI, Mortality, Bleeding |
| Gimbel et al., 2020 [14] | Goto et al., 2015 [27] | Park et al., 2019 [26] | Tang et al., 2016 [25] | Wallentin et al., 2009 [6] | Wang and Wang 2016 [24] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel | Ticagrelor | Clopidogrel |
| Loading dose (mg) | 180 | 300,600 | 180 | 300 | 180 | 600 | 180 | 600 | 180 | 300 | 180 | 300 |
| Maintenance dose (mg) | 90 | 75 | 90 | 75 | 90 | 75 | 90 | 75 | 90 | 75 | 90 | 75 |
| Patient number | 502 | 500 | 401 | 400 | 400 | 400 | 200 | 200 | 9333 | 9291 | 100 | 100 |
| Male (%) | 325 (65) | 313 (63) | ND | ND | 297 (74.2) | 302 (75.5) | 142 (71) | 146 (73) | ND | ND | ND | ND |
| Female (%) | 177 (35) | 187 (37) | 95 (23.7) | 93 (23.3) | ND | ND | ND | ND | 2655 (28.4) | 2633 (28.3) | 31 (31) | 34 (34) |
| BMI (Kg/m2) | 26·9 (4·2) | 26·7 (4·0) | 23.7 (15.6–43.4)c | 23.6 (14.2–38.6) | 24.6 ± 3.0 | 24.9 ± 3.2 | ND | ND | 27 (13–68) | 27 (13–70) | ND | ND |
| Body weight (kg) | ND | ND | 63 (35–104) | 62 (36–109) | ND | ND | ND | ND | 80.0 (28–174) | 80.0 (29–180) | ND | ND |
| Weight < 60 kg (%) | 30 (6) | 35 (7) | 154 (38.4) | 152 (38.0) | ND | ND | ND | ND | 652 (7.0) | 660 (7.1) | ND | ND |
| Risk factors | ||||||||||||
| Diabetes mellitus (%) | 150 (30) | 146 (29) | 154 (38.4) | 124 (31.0) | 116 (29.0) | 100 (25.0) | 58 (29) | 42 (21) | 2326 (24.9) | 2336 (25.1) | 42 (42) | 39 (39) |
| Dyslipidemia (%) | 325 (65) | 323 (65) | 314 (78.3) | 289 (72.3) | 208 (52.0) | 194 (48.5) | 88 (44) | 74 (37) | 4347 (46.6) | 4342 (46.7) | 84 (84) | 79 (79) |
| Hypertension (%) | 365 (73) | 362 (73) | 305 (76.1) | 290 (72.5) | 223 (55.8) | 193 (48.2) | 122 (61) | 116 (58) | 6139 (65.8) | 6044 (65.1) | 79 (79) | 82 (82) |
| Smoking (%) | 62 (13) | 67 (14) | 151 (37.7) | 157 (39.3) | 146 (36.5) | 139 (34.8) | 116 (58) | 124 (62) | 3360 (36.0) | 3318 (35.7) | 37 (37) | 41 (41) |
| Previous medical history | ||||||||||||
| PCI (%) | 122 (24) | 98 (20) | 45 (11.2) | 42 (10.5) | 41 (10.2) | 31 (7.8) | ND | ND | 1272 (13.6) | 1220 (13.1) | 3 (3) | 6 (65) |
| MI (%) | 136 (27) | 121 (24) | 33 (8.2) | 31 (7.8) | 25 (6.2) | 20 (5.0) | 16 (8) | 10 (5) | 1900 (20.4) | 1924 (20.7) | 17 (17) | 15 (15) |
| Peripheral arterial disease (%) | 49 (10) | 62 (12) | 13 (3.2) | 14 (3.5) | 4 (1.0) | 2 (0.5) | 10 (5) | 8 (4) | 566 (6.1) | 578 (6.2) | 5 (5) | 7 (7) |
| Congestive heart failure (%) | ND | ND | 30 (7.5) | 28 (7.0) | 10 (2.5) | 6 (1.5) | ND | ND | 513 (5.5) | 537 (5.8) | 13 (13) | 9 (9) |
| Angina pectoris (%) | ND | ND | 102 (25.4) | 110 (27.5) | ND | ND | 114 (57) | 104 (52) | ND | ND | 40 (40) | 36 (36) |
| Atrial fibrillation/flutter (%) | ND | ND | ND | ND | ND | ND | 18 (9) | 22 (11) | ND | ND | ND | ND |
| Non-hemorrhagic stroke (%) | ND | ND | 27 (6.7) | 28 (7.0) | ND | ND | ND | ND | 353 (3.8) | 369 (4.0) | 11 (11) | 10 (10) |
| Peptic ulcer disease (%) | ND | ND | 37 (9.2) | 37 (9.3) | ND | ND | ND | ND | ND | ND | ND | ND |
| Gastrointestinal bleeding (%) | ND | ND | 6 (1.5) | 7 (1.8) | 1 (0.2) | 0 (0.0) | ND | ND | ND | ND | ND | ND |
| Asthma (%) | ND | ND | 12 (3.0) | 14 (3.5) | 12 (3.0) | 3 (0.8) | ND | ND | 267 (2.9) | 265 (2.9) | ND | ND |
| Dyspnea (%) | ND | ND | 32 (8.0) | 41 (10.3) | ND | ND | ND | ND | 1412 (15.1) | 1358 (14.6) | ND | ND |
| CABG (%) | 86 (17) | 84 (17) | 5 (1.2) | 1 (0.3) | 4 (1.0) | 3 (0.8) | ND | ND | 532 (5.7) | 574 (6.2) | 0 (0) | 0 (0) |
| Ischaemic stroke (%) | 25 (5) | 22 (4) | ND | ND | 24 (6.0) | 16 (4.0) | ND | ND | ND | ND | ND | ND |
| Transient ischaemic attack (%) | 38 (8) | 37 (7) | ND | ND | ND | ND | 32 (16) | 34 (17) | ND | ND | 16 (16) | 14 (14) |
| Chronic renal disease (%) | ND | ND | 18 (4.5) | 20 (5.0) | 6 (1.5) | 1 (0.2) | ND | ND | 379 (4.1) | 406 (4.4) | ND | ND |
| COPD (%) | 49 (10) | 61 (12) | 7 (1.7) | 10 (2.5) | ND | ND | ND | ND | 555 (5.9) | 530 (5.7) | ND | ND |
| Gout (%) | ND | ND | 23 (5.7) | 23 (5.7) | 5 (1.2) | 4 (1.0) | ND | ND | 272 (2.9) | 262 (2.8) | ND | ND |
| Diagnosis | ||||||||||||
| NSTEMI (%) | 424 (86) | 423 (86) | 66 (16.5) | 74 (18.5) | 148 (37.0) | 155 (38.8) | ND | ND | 4005 (42.9) | 3950 (42.5) | 44 (44) | 47 (47) |
| STEMI (%) | ND | ND | 205 (51.1) | 210 (52.5) | 170 (42.5) | 156 (39.0) | ND | ND | 3496 (37.5) | 3530 (38.0) | 37 (37) | 32 (32) |
| Unstable angina (%) | 52 (11) | 54 (11) | 119 (29.7) | 109 (27.3) | 82 (20.5) | 89 (22.2) | ND | ND | 1549 (16.6) | 1563 (16.8) | 19 (19) | 21 (21) |
| Positive troponin (%) | ND | ND | 309 (77.1) | 298 (74.5) | 338 (84.5) | 333 (83.3) | ND | ND | 7965/9333 (85.3) | 7999/9291 (86.1) | ND | ND |
| ECG findings | ||||||||||||
| Persistent ST-segment elevation (%) | ND | ND | 218 (54.4) | 225 (56.3) | ND | ND | ND | ND | 3497 (37.5) | 3511 (37.8) | ND | ND |
| ST-segment depression (%) | ND | ND | 188 (46.9) | 153 (38.3) | ND | ND | ND | ND | 4730 (50.7) | 4756 (51.2) | ND | ND |
| T-wave inversion (%) | ND | ND | 142 (35.4) | 126 (31.5) | ND | ND | ND | ND | 2970 (31.8) | 2975 (32.0) | ND | ND |
| Discharge medications | ||||||||||||
| Organic nitrate (%) | ND | ND | 344 (85.8) | 353 (88.3) | ND | ND | ND | ND | 7181 (76.9) | 7088 (76.3) | ND | ND |
| Vitamin K antagonist (%) | 62 (12) | 65 (13) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Proton pump inhibitor (%) | 446 (91) | 446 (90) | 167 (41.6) | 175 (43.8) | 12 (3.0) | 8 (2.0) | ND | ND | 4233 (45.4) | 4128 (44.4) | 31 (31) | 33 (33) |
| β-blocker (%) | ND | ND | 40 (10) | 44 (11.1) | 275 (68.8) | 297 (74.2) | 82 | 96 | 8339 (89.3) | 8336 (89.7) | 69 (69) | 74 (74) |
| ACE inhibitor (%) | ND | ND | 67 (16.7) | 64 (16.0) | ND | ND | ND | ND | 7090 (76.0) | 6986 (75.2) | 61 (61) | 67 (67) |
| Angiotensin receptor blocker (%) | ND | ND | 102 (25.4) | 95 (23.8) | 163 (40.8) | 171 (42.8) | ND | ND | 1143 (12.2) | 1125 (12.1) | ||
| Statin (%) | ND | ND | 215 (53.6) | 205 (51.3) | 354 (88.5) | 369 (92.2) | 198 | 199 | 8373 (89.7) | 8289 (89.2) | 83 (83) | 79 (79) |
| Calcium channel blocker (%) | ND | ND | 117 (29.2) | 109 (27.3) | 90 (22.5) | 90 (22.5) | ND | ND | 2769 (29.7) | 2789 (30.0) | 69 (69) | 63 (63) |
| Procedure | ||||||||||||
| Coronary angiography (%) | 452 (90) | 439 (88 | 385 (96.0) | 378 (95.4) | ND | ND | ND | ND | 7599 (81.4) | 7571 (81.5) | 86 (86) | 83 (83) |
| PCI (%) | 242 (48) | 232 (46) | 340 (84.8) | 338 (84.5) | ND | ND | ND | ND | 5978 (64.1) | 5999 (64.6) | 75 (75) | 71 (71) |
| BMS (%) | 6 (3) | 2 (1) | ND | ND | ND | ND | ND | ND | 3921 (42.0) | 3892 (41.9) | ND | ND |
| DES (%) | 224 (93) | 219 (94) | ND | ND | ND | ND | ND | ND | 1719 (18.4) | 1757 (18.9) | ND | ND |
| CABG (%) | 87 (17) | 78 (16) | 9 (2.2) | 3 (0.8) | ND | ND | ND | ND | 931 (10.0) | 968 (10.4) | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkaif, M.A.; Sha’aban, A.; Daud, N.A.A.; Yunusa, I.; Ng, M.L.; Sk Abdul Kader, M.A.; Noor, D.A.M.; Ibrahim, B. Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cardiovasc. Dev. Dis. 2021, 8, 123. https://doi.org/10.3390/jcdd8100123
Akkaif MA, Sha’aban A, Daud NAA, Yunusa I, Ng ML, Sk Abdul Kader MA, Noor DAM, Ibrahim B. Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Cardiovascular Development and Disease. 2021; 8(10):123. https://doi.org/10.3390/jcdd8100123
Chicago/Turabian StyleAkkaif, Mohammed Ahmed, Abubakar Sha’aban, Nur Aizati Athirah Daud, Ismaeel Yunusa, Mei Li Ng, Muhamad Ali Sk Abdul Kader, Dzul Azri Mohamed Noor, and Baharudin Ibrahim. 2021. "Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Cardiovascular Development and Disease 8, no. 10: 123. https://doi.org/10.3390/jcdd8100123
APA StyleAkkaif, M. A., Sha’aban, A., Daud, N. A. A., Yunusa, I., Ng, M. L., Sk Abdul Kader, M. A., Noor, D. A. M., & Ibrahim, B. (2021). Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Cardiovascular Development and Disease, 8(10), 123. https://doi.org/10.3390/jcdd8100123








