Clinical Assessment of Ventricular Wall Stress in Understanding Compensatory Hypertrophic Response and Maladaptive Ventricular Remodeling
Abstract
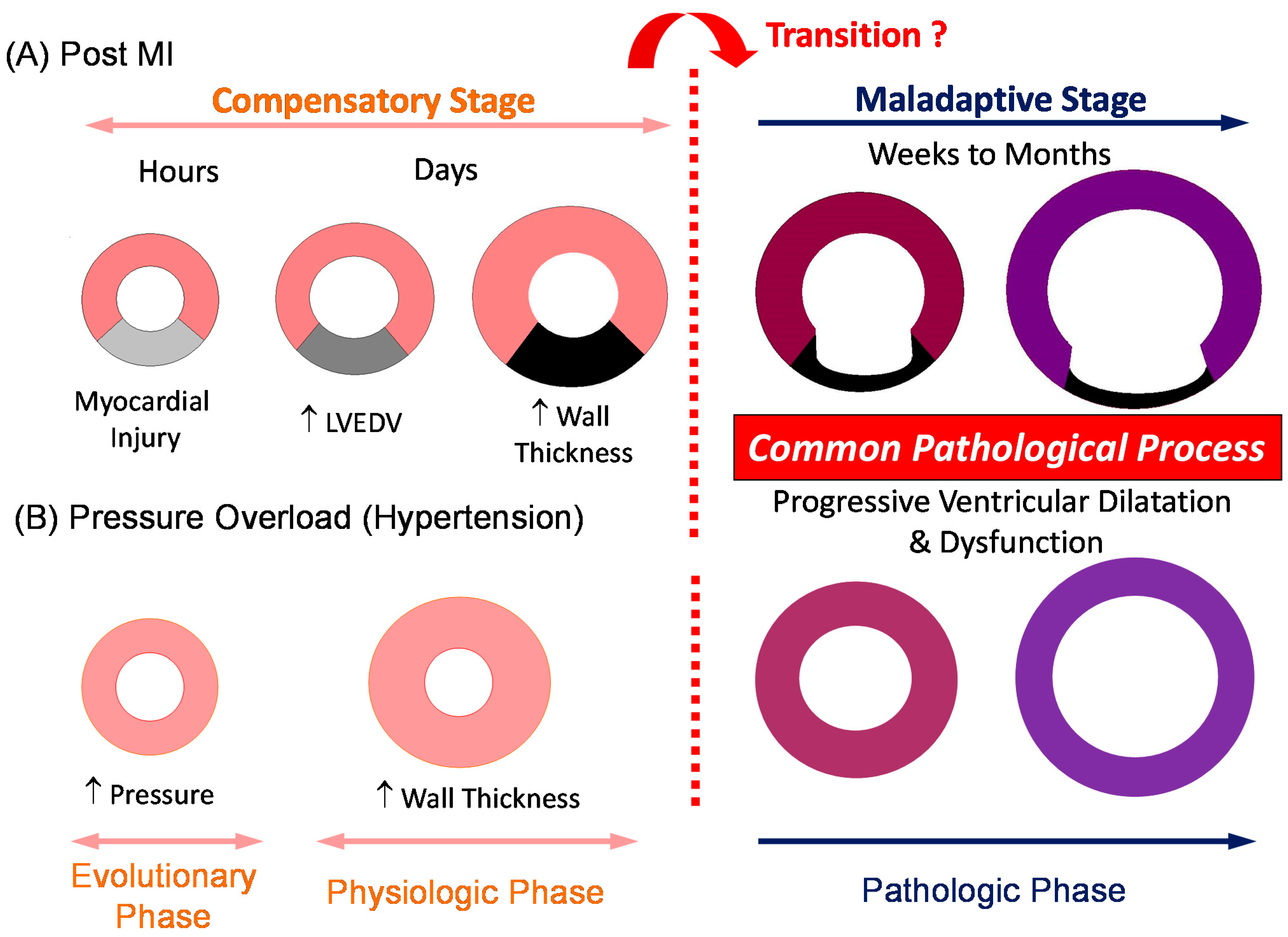
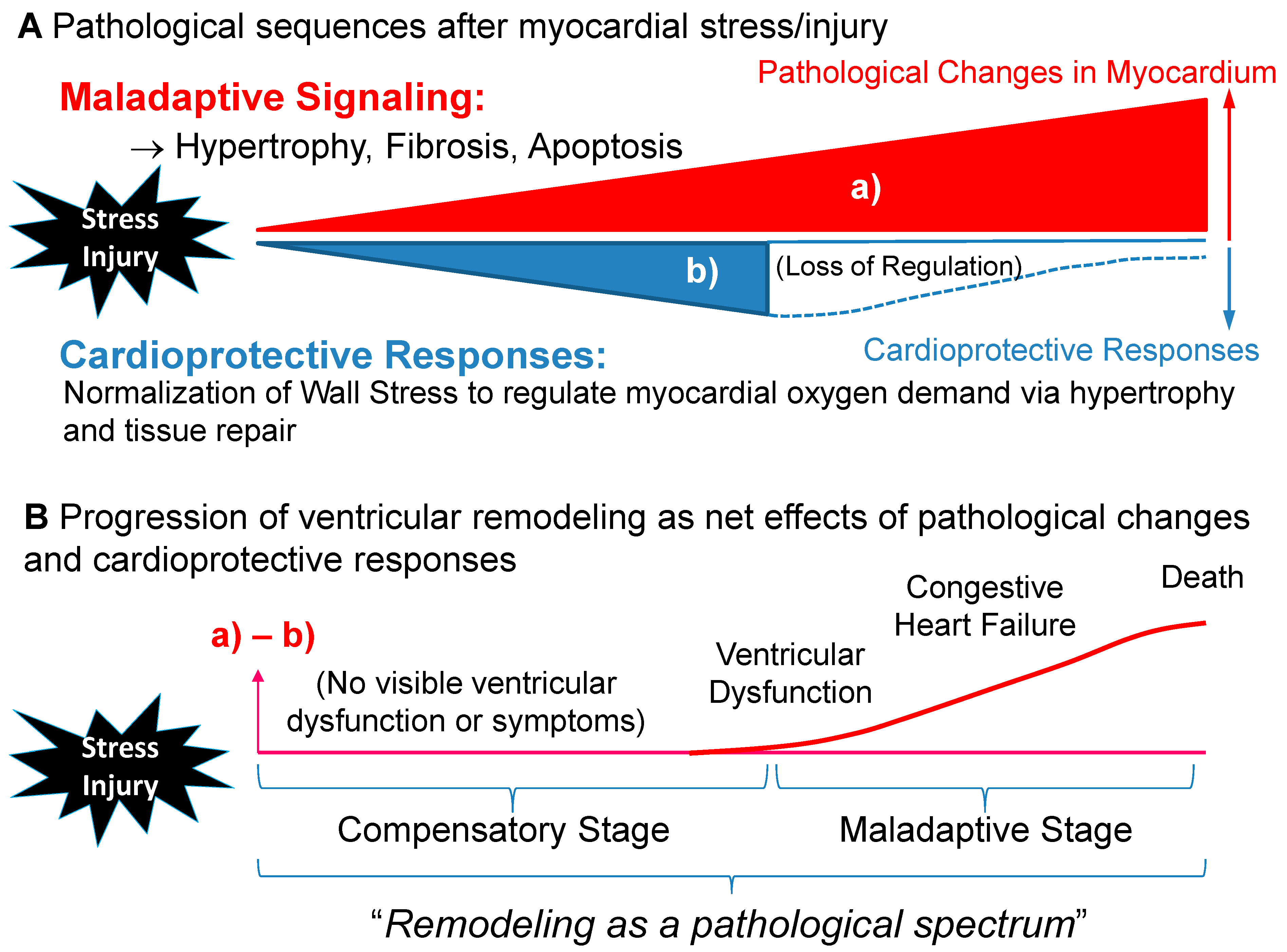
1. Ventricular Remodeling as a Dynamic, Pathological Process Leading to Symptomatic Heart Failure
2. Physiological Significance of Ventricular Wall Stress
3. Transition for Compensatory Hypertrophy to Maladaptive Remodeling: Is Compensatory Hypertrophy Always Benign?
4. Clinical Significance of Ventricular Wall Stress
5. Noninvasive Measurement of Wall Stress as a Biomarker for Ventricular Remodeling
6. Assessing Global and Regional Ventricular Wall Stress by Magnetic Resonance Imaging
7. Conclusions
Funding
Conflicts of Interest
References
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, 187–197. [Google Scholar]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological ventricular remodeling: Mechanisms: Part 1 of 2. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Swynghedauw, B. Molecular mechanisms of myocardial remodeling. Physiol. Rev. 1999, 79, 215–262. [Google Scholar] [CrossRef]
- Grossman, W. Cardiac hypertrophy: Useful adaptation or pathologic process? Am. J. Med. 1980, 69, 576–584. [Google Scholar] [CrossRef]
- Opie, L.H.; Lopaschuk, G.D. Overload hypertrophy and its molecular biology. In Heart Physiology: From Cell to Circulation, 4th ed.; Opie, L.H., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; pp. 402–427. [Google Scholar]
- Olson, E.N. A decade of discoveries in cardiac biology. Nat. Med. 2004, 10, 467–474. [Google Scholar] [CrossRef]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef]
- Katz, A.M. Proliferative signaling and disease progression in heart failure. Circ. J. 2002, 66, 225–231. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Mann, D.L. Basic mechanisms of left ventricular remodeling: The contribution of wall stress. J. Card. Fail. 2004, 10, S202–S206. [Google Scholar] [CrossRef]
- McKay, R.G.; Pfeffer, M.A.; Pasternak, R.C.; Markis, J.E.; Come, P.C.; Nakao, S.; Alderman, J.D.; Ferguson, J.J.; Safian, R.D.; Grossman, W. Left ventricular remodeling after myocardial infarction: A corollary to infarct expansion. Circulation 1986, 74, 693–702. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Pfeffer, J.M. Ventricular enlargement and reduced survival after myocardial infarction. Circulation 1987, 75, IV93–IV97. [Google Scholar]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Clark, W.A.; Janicki, J.S.; Shroff, S.G. Physiologic versus pathologic hypertrophy and the pressure-overloaded myocardium. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. 6), S37–S50. [Google Scholar] [CrossRef]
- Force, T.; Michael, A.; Kilter, H.; Haq, S. Stretch-activated pathways and left ventricular remodeling. J. Card. Fail. 2002, 8, S351–S358. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef]
- Dujardin, K.S.; Enriquez-Sarano, M.; Rossi, A.; Bailey, K.R.; Seward, J.B. Echocardiographic assessment of left ventricular remodeling: Are left ventricular diameters suitable tools? J. Am. Coll. Cardiol. 1997, 30, 1534–1541. [Google Scholar] [CrossRef]
- Braunwald, E. Control of myocardial oxygen consumption: Physiologic and clinical considerations. Am. J. Cardiol. 1971, 27, 416–432. [Google Scholar] [CrossRef]
- Strauer, B.E. Left ventricular dynamics, energetics and coronary hemodynamics in hypertrophic heart disease. Eur. Heart J. 1983, 4 (Suppl. A), 137–142. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Beer, K.; Heitlinger, K.; Hofling, B. Left ventricular systolic wall stress as a primary determinant of myocardial oxygen consumption: Comparative studies in patients with normal left ventricular function, with pressure and volume overload and with coronary heart disease. Basic Res. Cardiol. 1977, 72, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.C. Ventricular wall stress. Circ. Res. 1981, 49, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Gao, E.; Evangelisti, L.; Markova, D.; Ma, X.; Chu, M.L. Post-ischemic myocardial fibrosis occurs independent of hemodynamic changes. Cardiovasc. Res. 2003, 59, 926–933. [Google Scholar] [CrossRef]
- Olivetti, G.; Capasso, J.M.; Meggs, L.G.; Sonnenblick, E.H.; Anversa, P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ. Res. 1991, 68, 856–869. [Google Scholar] [CrossRef]
- Grossman, W.; Jones, D.; McLaurin, L.P. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd. The fuzzy logic of physiological cardiac hypertrophy. Hypertension 2007, 49, 962–970. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Hill, J.A. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation 2015, 131, 1435–1447. [Google Scholar] [CrossRef]
- Opie, L.H.; Commerford, P.J.; Gersh, B.J.; Pfeffer, M.A. Controversies in ventricular remodelling. Lancet 2006, 367, 356–367. [Google Scholar] [CrossRef]
- de Simone, G.; Devereux, R.B.; Daniels, S.R.; Meyer, R.A. Gender differences in left ventricular growth. Hypertension 1995, 26, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Maron, B.J.; Spataro, A.; Proschan, M.A.; Spirito, P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N. Engl. J. Med. 1991, 324, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Baggish, A.L. Differentiating exercise-induced cardiac adaptations from cardiac pathology: The “grey zone” of clinical uncertainty. Can. J. Cardiol. 2016, 32, 429–437. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; De Luca, R.; Di Paolo, F.M.; Spataro, A.; Culasso, F. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 2002, 105, 944–949. [Google Scholar] [CrossRef]
- Duvekot, J.J.; Peeters, L.L. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet. Gynecol. Surv. 1994, 49, S1–S14. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, M.; Wang, Y.; Toro, L.; Stefani, E. Heart hypertrophy during pregnancy: A better functioning heart? Trends Cardiovasc. Med. 2006, 16, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Ro, A.; Frishman, W.H. Peripartum cardiomyopathy. Cardiol. Rev. 2006, 14, 35–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ho, K.K.; Pinsky, J.L.; Kannel, W.B.; Levy, D. The epidemiology of heart failure: The Framingham study. J. Am. Coll. Cardiol. 1993, 22, 6A–13A. [Google Scholar] [CrossRef]
- Kannel, W.B. Incidence and epidemiology of heart failure. Heart Fail. Rev. 2000, 5, 167–173. [Google Scholar] [CrossRef]
- Berenji, K.; Drazner, M.H.; Rothermel, B.A.; Hill, J.A. Does load-induced ventricular hypertrophy progress to systolic heart failure? Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H8–H16. [Google Scholar] [CrossRef]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef]
- Hill, J.A.; Karimi, M.; Kutschke, W.; Davisson, R.L.; Zimmerman, K.; Wang, Z.; Kerber, R.E.; Weiss, R.M. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 2000, 101, 2863–2869. [Google Scholar] [CrossRef]
- Esposito, G.; Rapacciuolo, A.; Prasad, S.V.N.; Takaoka, H.; Thomas, S.A.; Koch, W.J.; Rockman, H.A. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 2002, 105, 85–92. [Google Scholar] [CrossRef]
- Sandler, H.; Dodge, H.T. Left ventricular tension and stress in man. Circ. Res. 1963, 13, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Reichek, N.; Wilson, J.; St John Sutton, M.; Plappert, T.A.; Goldberg, S.; Hirshfeld, J.W. Noninvasive determination of left ventricular end-systolic stress: Validation of the method and initial application. Circulation 1982, 65, 99–108. [Google Scholar] [CrossRef]
- Quinones, M.A.; Mokotoff, D.M.; Nouri, S.; Winters, W.L., Jr.; Miller, R.R. Noninvasive quantification of left ventricular wall stress. Validation of method and application to assessment of chronic pressure overload. Am. J. Cardiol. 1980, 45, 782–790. [Google Scholar] [CrossRef]
- Douglas, P.S.; Reichek, N.; Plappert, T.; Muhammad, A.; St John Sutton, M.G. Comparison of echocardiographic methods for assessment of left ventricular shortening and wall stress. J. Am. Coll. Cardiol. 1987, 9, 945–951. [Google Scholar] [CrossRef]
- Greim, C.A.; Roewer, N.; Am Esch, J.S. Assessment of changes in left ventricular wall stress from the end-systolic pressure-area product. Br. J. Anaesth. 1995, 75, 583–587. [Google Scholar] [CrossRef][Green Version]
- Colan, S.D.; Borow, K.M.; MacPherson, D.; Sanders, S.P. Use of the indirect axillary pulse tracing for noninvasive determination of ejection time, upstroke time, and left ventricular wall stress throughout ejection in infants and young children. Am. J. Cardiol. 1984, 53, 1154–1158. [Google Scholar] [CrossRef]
- Colan, S.D.; Borow, K.M.; Neumann, A. Effects of loading conditions and contractile state (methoxamine and dobutamine) on left ventricular early diastolic function in normal subjects. Am. J. Cardiol. 1985, 55, 790–796. [Google Scholar] [CrossRef]
- Zile, M.R.; Gaasch, W.H.; Carroll, J.D.; Levine, H.J. Chronic mitral regurgitation: Predictive value of preoperative echocardiographic indexes of left ventricular function and wall stress. J. Am. Coll. Cardiol. 1984, 3, 235–242. [Google Scholar] [CrossRef]
- Fontanet, H.L.; Perez, J.E.; Davila-Roman, V.G. Diminished contractile reserve in patients with left ventricular hypertrophy and increased end-systolic stress during dobutamine stress echocardiography. Am. J. Cardiol. 1996, 78, 1029–1035. [Google Scholar] [CrossRef]
- Nivatpumin, T.; Katz, S.; Scheuer, J. Peak left ventricular systolic pressure/end-systolic volume ratio: A sensitive detector of left ventricular disease. Am. J. Cardiol. 1979, 43, 969–974. [Google Scholar] [CrossRef]
- Davila-Roman, V.G.; Creswell, L.L.; Rosenbloom, M.; Perez, J.E. Myocardial contractile state in dogs with chronic mitral regurgitation: Echocardiographic approach to the peak systolic pressure/end-systolic area relationship. Am. Heart J. 1993, 126, 155–160. [Google Scholar] [CrossRef]
- Colan, S.D.; Borow, K.M.; Neumann, A. Left ventricular end-systolic wall stress-velocity of fiber shortening relation: A load-independent index of myocardial contractility. J. Am. Coll. Cardiol. 1984, 4, 715–724. [Google Scholar] [CrossRef]
- Hoit, B.D.; Khan, Z.U.; Pawloski-Dahm, C.M.; Walsh, R.A. In vivo determination of left ventricular wall stress-shortening relationship in normal mice. Am. J. Physiol. 1997, 272, H1047–H1052. [Google Scholar] [CrossRef] [PubMed]
- Lamers, L.; Ensing, G.; Pignatelli, R.; Goldberg, C.; Bezold, L.; Ayres, N.; Gajarski, R. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J. Am. Coll. Cardiol. 2006, 47, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Massie, B.; Thomas, D.; Bristow, J.D.; Cheitlin, M.; Broudy, D.; Szlachcic, J.; Krishnamurthy, G. Association between the exercise ejection fraction response and systolic wall stress in patients with chronic aortic insufficiency. Circulation 1985, 71, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.; Taylor, D.; Teo, K.; Quinney, A.; Humen, D. Left ventricular wall stress during leg-press exercise performed with a brief Valsalva maneuver. Chest 2001, 119, 150–154. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Rietzschel, E.R.; De Buyzere, M.L.; Raja, M.W.; Claessens, T.; Gillebert, T.C. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: The Asklepios study. Hypertension 2013, 61, 296–303. [Google Scholar] [CrossRef][Green Version]
- Mondillo, S.; Galderisi, M.; Mele, D.; Cameli, M.; Lomoriello, V.S.; Zaca, V.; Badano, L. Speckle-tracking echocardiography: A new technique for assessing myocardial function. J. Ultrasound Med. 2011, 30, 71–83. [Google Scholar] [CrossRef]
- Hurlburt, H.M.; Aurigemma, G.P.; Hill, J.C.; Narayanan, A.; Gaasch, W.H.; Vinch, C.S.; Meyer, T.E.; Tighe, D.A. Direct ultrasound measurement of longitudinal, circumferential, and radial strain using 2-dimensional strain imaging in normal adults. Echocardiography 2007, 24, 723–731. [Google Scholar] [CrossRef]
- Murai, D.; Yamada, S.; Hayashi, T.; Okada, K.; Nishino, H.; Nakabachi, M.; Yokoyama, S.; Abe, A.; Ichikawa, A.; Ono, K.; et al. Relationships of left ventricular strain and strain rate to wall stress and their afterload dependency. Heart Vessels 2017, 32, 574–583. [Google Scholar] [CrossRef]
- Di Napoli, P.; Taccardi, A.A.; Grilli, A.; Felaco, M.; Balbone, A.; Angelucci, D.; Barsotti, A. Left ventricular wall stress as a direct correlate of cardiomyocyte apoptosis in patients with severe dilated cardiomyopathy. Am. Heart J. 2003, 146, 1105–1111. [Google Scholar] [CrossRef]
- Rohde, L.E.; Aikawa, M.; Cheng, G.C.; Sukhova, G.; Solomon, S.D.; Libby, P.; Pfeffer, J.; Pfeffer, M.A.; Lee, R.T. Echocardiography-derived left ventricular end-systolic regional wall stress and matrix remodeling after experimental myocardial infarction. J. Am. Coll. Cardiol. 1999, 33, 835–842. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Goethals, M.; Verstreken, S.; De Bruyne, B.; Muller, K.; Van Schuerbeeck, E.; Bartunek, J. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J. Am. Coll. Cardiol. 2004, 44, 2349–2354. [Google Scholar] [CrossRef]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: Comparison between systolic and diastolic heart failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef]
- Dong, H.; Mosca, H.; Gao, E.; Akins, R.E.; Gidding, S.S.; Tsuda, T. Integrated wall stress: A new methodological approach to assess ventricular workload and myocardial contractile reserve. J. Transl. Med. 2013, 11, 183. [Google Scholar] [CrossRef]
- Devereux, R.B.; Roman, M.J.; Palmieri, V.; Okin, P.M.; Boman, K.; Gerdts, E.; Dahlöf, B. Left ventricular wall stresses and wall stress-mass-heart rate products in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE study. losartan intervention for endpoint reduction in hypertension. J. Hypertens. 2000, 18, 1129–1138. [Google Scholar] [CrossRef]
- Gerdts, E.; Saeed, S.; Midtbø, H.; Rossebø, A.; Chambers, J.B.; Einarsen, E.; Bahlmann, E.; Devereux, R. Higher left ventricular mass-wall stress-heart rate product and outcome in aortic valve stenosis. Heart 2019, 105, 1629–1633. [Google Scholar] [CrossRef]
- Auffermann, W.; Wagner, S.; Holt, W.W.; Buser, P.T.; Kircher, B.; Schiller, N.B.; Lim, T.H.; Wolfe, C.L.; Higgins, C.B. Noninvasive determination of left ventricular output and wall stress in volume overload and in myocardial disease by cine magnetic resonance imaging. Am. Heart J. 1991, 121, 1750–1758. [Google Scholar] [CrossRef]
- Alter, P.; Rupp, H.; Rominger, M.B.; Vollrath, A.; Czerny, F.; Figiel, J.; Adams, P.; Stoll, F.; Klose, K.J.; Maisch, B. B-type natriuretic peptide and wall stress in dilated human heart. Mol. Cell. Biochem. 2008, 314, 179–191. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Boonyasirinant, T.; Saiviroonporn, P.; Thanapiboonpol, P.; Nakyen, S.; Udompunturak, S. Correlation between NT-pro BNP levels and left ventricular wall stress, sphericity index and extent of myocardial damage: A magnetic resonance imaging study. J. Card. Fail. 2008, 14, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Alter, P.; Rupp, H.; Adams, P.; Stoll, F.; Figiel, J.; Klose, K.J.; Rominger, M.B.; Maisch, B. Occurrence of late gadolinium enhancement is associated with increased left ventricular wall stress and mass in patients with non-ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2011, 13, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Carter-Storch, R.; Moller, J.E.; Christensen, N.L.; Rasmussen, L.M.; Pecini, R.; Søndergård, E.; Videbæk, L.M.; Dahl, J.S. End-systolic wall stress in aortic stenosis: Comparing symptomatic and asymptomatic patients. Open Heart 2019, 6, e001021. [Google Scholar] [CrossRef]
- Vohringer, M.; Mahrholdt, H.; Yilmaz, A.; Sechtem, U. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR). Herz 2007, 32, 129–137. [Google Scholar] [CrossRef]
- Genet, M.; Lee, L.C.; Nguyen, R.; Haraldsson, H.; Acevedo-Bolton, G.; Zhang, Z.; Guccione, J.M. Distribution of normal human left ventricular myofiber stress at end diastole and end systole, a target for in silico design of heart failure treatments. J. Appl. Physiol. 2014, 117, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Scardulla, F.; Rinaudo, A.; Pasta, S.; Scardulla, C. Evaluation of ventricular wall stress and cardiac function in patients with dilated cardiomyopathy. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wollmuth, J.R.; Bree, D.R.; Cupps, B.P.; Krock, M.D.; Pomerantz, B.J.; Pasque, R.P.; Pasque, M.K. Left ventricular wall stress in patients with severe aortic insufficiency with finite element analysis. Ann. Thorac. Surg. 2006, 82, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Su, Y.; Gobeawan, L.; Sola, S.; Tan, R.-S.; Navia, J.L.; Ghista, D.N.; Chua, T.; Guccione, J.; Kassab, G.S. Impact of surgical ventricular restoration on ventricular shape, wall stress, and function in heart failure patients. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1653–H1660. [Google Scholar] [CrossRef] [PubMed]
- Alter, P.; Rupp, H.; Stoll, F.; Adams, P.; Figiel, J.H.; Klose, K.J.; Maisch, B. Increased end diastolic wall stress precedes left ventricular hypertrophy in dilative heart failure—Use of the volume-based wall stress index. Int. J. Cardiol. 2012, 157, 233–238. [Google Scholar] [CrossRef]
- Zhao, X.; Tan, R.-S.; Tang, H.-C.; Teo, S.-K.; Su, Y.; Wan, M.; Leng, S.; Zhang, J.-M.; Allen, J.; Kassab, G.S.; et al. Left ventricular wall stress is sensitive marker of hypertrophic cardiomyopathy with preserved ejection fraction. Front. Physiol. 2018, 9, 250. [Google Scholar] [CrossRef]
| 1. End-systolic WS (1): Ventricular afterload |
| -Inversely correlated with systolic performance |
| 2. End-systolic WS (2): Myocardial oxygen demand/consumption |
| -End-systolic WS represents primary determinant of myocardial oxygen demand (Strauer et al. 1977 [20]) |
| 3. Noninvasive determination of LV end-systolic WS |
| -Utilization of cuff systolic blood pressure: Well correlated with invasive LV pressure measurement [42,43] |
| 4. End-systolic WS-Vcf relationship (Colan et al. 1984 [52]; Hoit et al. 1997 [53]; Lamers et al. 2006 [54]) |
| -Vcf/HR is inversely related to end-systolic WS |
| -A marker for load-dependent myocardial contractile status |
| 5. Integrated WS (Dong et al. 2013 [65]) |
| -Average WS over a unit time |
| -Stable with dobutamine stimulation in healthy heart |
| -Good correlation with Peak-systolic WS × HR |
| 6. WS-HR-LV mass product (Devereux et al. 2000 [66]; Gerdts et al. 2019 [67]) |
| -Myocardial oxygen demand is estimated from LV mass × end-systolic WS × HR |
| -Associated with higher mortality and morbidity in patients with aortic stenosis |
| 7. Early and late systolic WS (Chirinos et al. 2013 [57]) |
| -Early systolic WS: Associated with greater longitudinal systolic function and enhanced early diastolic relaxation |
| -Late systolic WS: Associated with decreased diastolic relaxation and decreased longitudinal systolic function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuda, T. Clinical Assessment of Ventricular Wall Stress in Understanding Compensatory Hypertrophic Response and Maladaptive Ventricular Remodeling. J. Cardiovasc. Dev. Dis. 2021, 8, 122. https://doi.org/10.3390/jcdd8100122
Tsuda T. Clinical Assessment of Ventricular Wall Stress in Understanding Compensatory Hypertrophic Response and Maladaptive Ventricular Remodeling. Journal of Cardiovascular Development and Disease. 2021; 8(10):122. https://doi.org/10.3390/jcdd8100122
Chicago/Turabian StyleTsuda, Takeshi. 2021. "Clinical Assessment of Ventricular Wall Stress in Understanding Compensatory Hypertrophic Response and Maladaptive Ventricular Remodeling" Journal of Cardiovascular Development and Disease 8, no. 10: 122. https://doi.org/10.3390/jcdd8100122
APA StyleTsuda, T. (2021). Clinical Assessment of Ventricular Wall Stress in Understanding Compensatory Hypertrophic Response and Maladaptive Ventricular Remodeling. Journal of Cardiovascular Development and Disease, 8(10), 122. https://doi.org/10.3390/jcdd8100122




