Biology and Biomechanics of the Heart Valve Extracellular Matrix
Abstract
1. Introduction
2. Extracellular Matrix Components
2.1. Collagens in Heart Valves
2.2. Proteoglycans in Heart Valves
2.3. Elastin in Heart Valves
2.4. Minor ECM Components of the Heart Valve
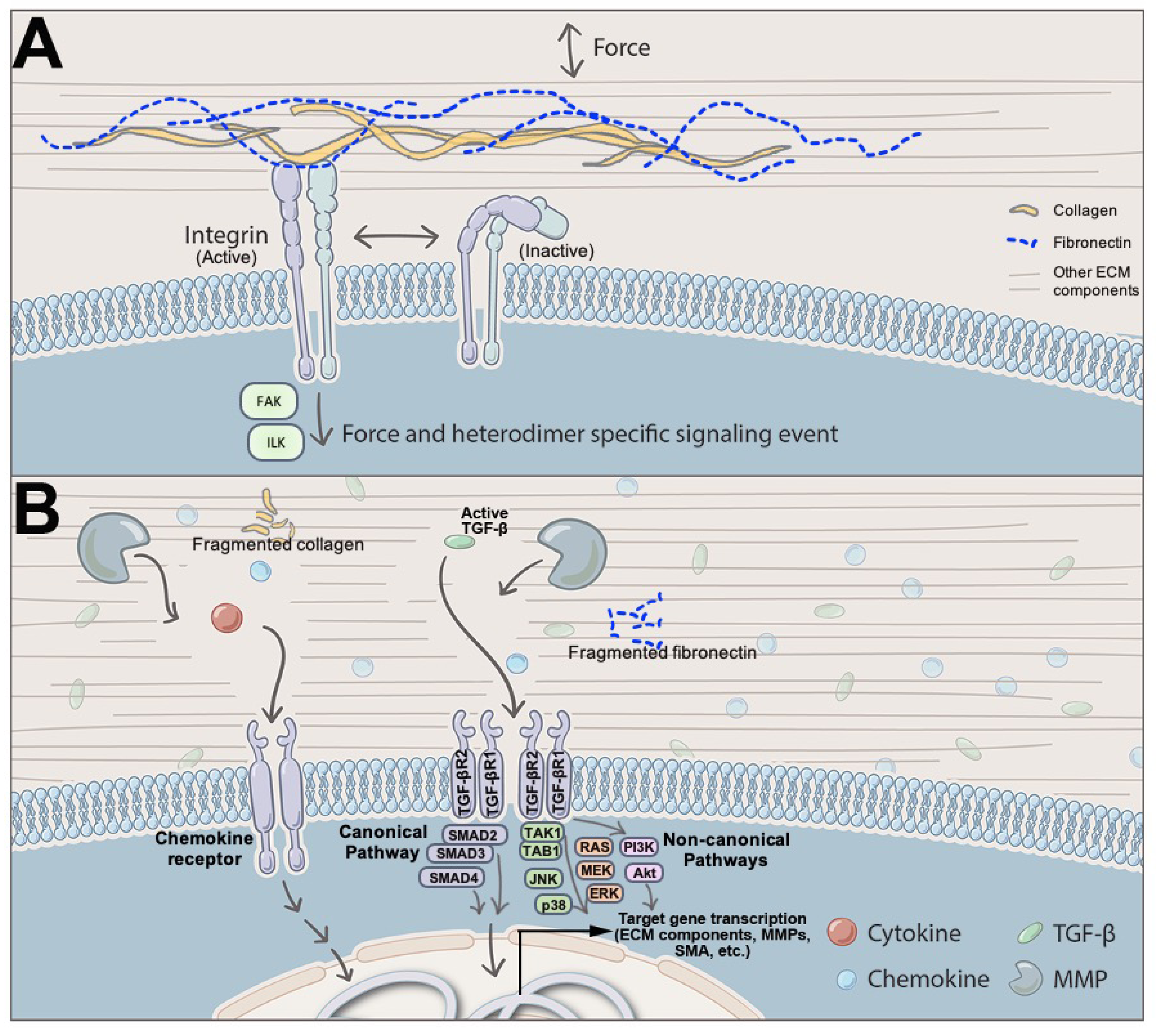
3. Signaling Pathways and ECM in Heart Valves
4. Heart Valve ECM Function
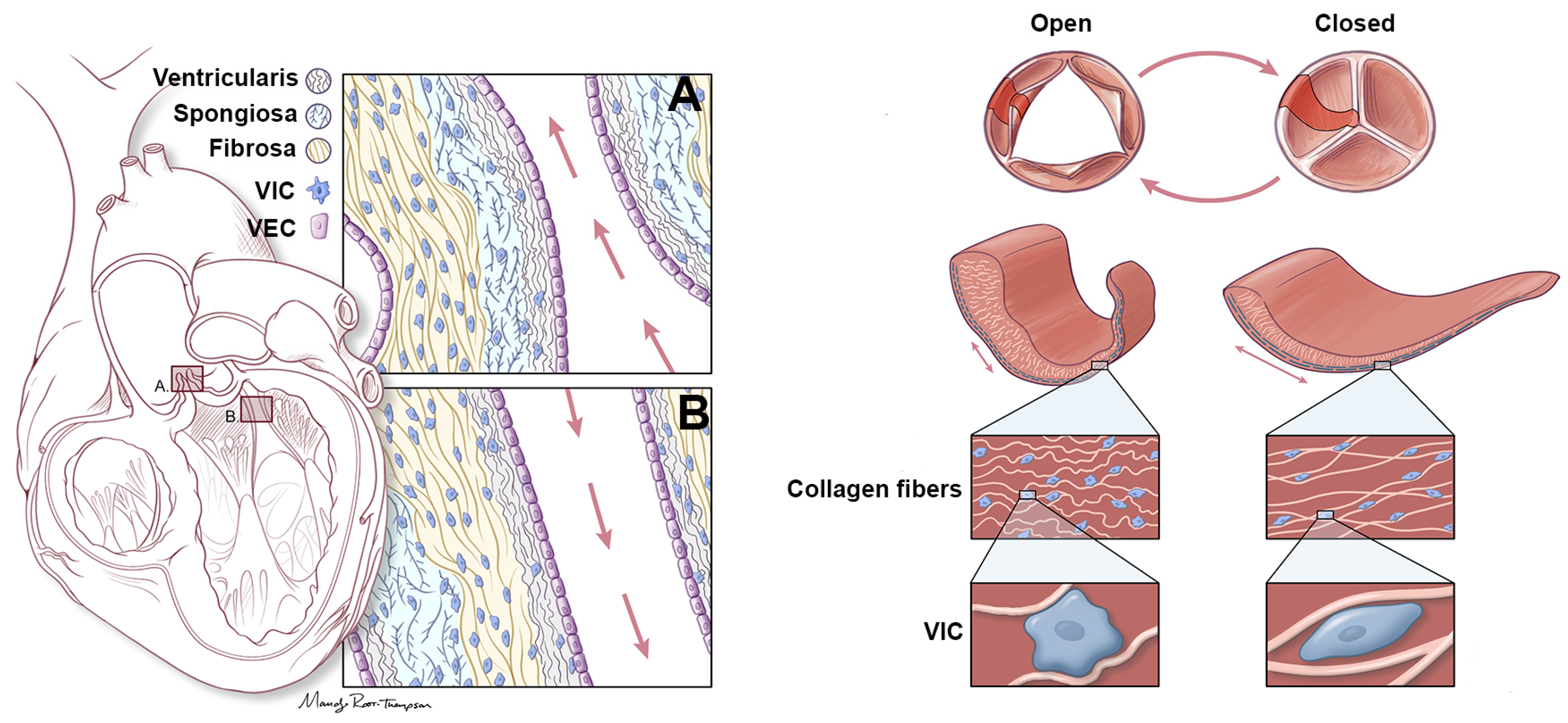
4.1. Heart Valve Biomechanics
4.2. VIC-Mediated Maintenance of ECM in Heart Valves
4.3. ECM Components as Signaling Mediators in Heart Valves
5. ECM and Heart Valve Disease
5.1. Histological Characteristics of Diseased, or Dyfunctional Heart Valves
5.2. Cellular Changes in Diseased, or Dysfunctional Heart Valves
5.3. Connective Tissue Disorders and Heart Valve Disease
6. ECM and Therapeutics
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tao, G.; Kotick, J.D.; Lincoln, J. Heart valve development, maintenance, and disease: The role of endothelial cells. Curr. Top. Dev. Biol. 2012, 100, 203–232. [Google Scholar]
- Hinton, R.B.; Lincoln, J.; Deutsch, G.H.; Osinska, H.; Manning, P.B.; Benson, D.W.; Yutzey, K.E. Extracellular Matrix Remodeling and Organization in Developing and Diseased Aortic Valves. Circ. Res. 2006, 98, 1431–1438. [Google Scholar] [CrossRef]
- Merryman, W.D.; Youn, I.; Lukoff, H.D.; Krueger, P.M.; Guilak, F.; Hopkins, R.A.; Sacks, M.S. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: Implications for collagen biosynthesis. Am. J. Physiol. Circ. Physiol. 2006, 290, H224–H231. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart Valve Structure and Function in Development and Disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef]
- Aikawa, E. Calcific Aortic Valve Disease; IntechOpen: London, UK, 2013. [Google Scholar]
- Buchanan, R.M.; Sacks, M.S. Interlayer micromechanics of the aortic heart valve leaflet. Biomech. Model. Mechanobiol. 2013, 13, 813–826. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rego, B.V.; Sacks, M.S. A functionally graded material model for the transmural stress distribution of the aortic valve leaflet. J. Biomech. 2017, 54, 88–95. [Google Scholar] [CrossRef]
- Horne, T.E.; VandeKopple, M.; Sauls, K.; Koenig, S.N.; Anstine, L.J.; Garg, V.; Norris, R.A.; Lincoln, J. Dynamic Heterogeneity of the Heart Valve Interstitial Cell Population in Mitral Valve Health and Disease. J. Cardiovasc. Dev. Dis. 2015, 2, 214–232. [Google Scholar] [CrossRef]
- Rabkin, E.; Aikawa, M.; Stone, J.R.; Fukumoto, Y.; Libby, P.; Schoen, F.J. Activated Interstitial Myofibroblasts Express Catabolic Enzymes and Mediate Matrix Remodeling in Myxomatous Heart Valves. Circulation 2001, 104, 2525–2532. [Google Scholar] [CrossRef]
- Rabkin-Aikawa, E.; Farber, M.; Aikawa, M.; Schoen, F.J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J. Heart Valve Dis. 2004, 13, 841–847. [Google Scholar] [PubMed]
- Helske-Suihko, S.; Kupari, M.; Lindstedt, K.A.; Kovanen, P.T. Aortic valve stenosis: An active atheroinflammatory process. Curr. Opin. Lipidol. 2007, 18, 483–491. [Google Scholar] [CrossRef]
- Anstine, L.J.; Bobba, C.; Ghadiali, S.N.; Lincoln, J. Growth and maturation of heart valves leads to changes in endothelial cell distribution, impaired function, decreased metabolism and reduced cell proliferation. J. Mol. Cell. Cardiol. 2016, 100, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bosse, K.; Hans, C.P.; Zhao, N.; Koenig, S.N.; Huang, N.; Guggilam, A.; Lahaye, S.; Tao, G.; Lucchesi, P.A.; Lincoln, J.; et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J. Mol. Cell. Cardiol. 2013, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Huk, D.J.; Austin, B.F.; Horne, T.E.; Hinton, R.B.; Ray, W.C.; Heistad, D.D.; Lincoln, J. Valve Endothelial Cell-Derived Tgfbeta1 Signaling Promotes Nuclear Localization of Sox9 in Interstitial Cells Associated With Attenuated Calcification. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 328–338. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Peacock, J.D.; Lu, Y.; Koch, M.; Kadler, K.E.; Lincoln, J. Temporal and spatial expression of collagens during murine atrioventricular heart valve development and maintenance. Dev. Dyn. 2008, 237, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, R.E.; Daniels, K.J.; Jensen, K.L.; Solursh, M. Type II collagen is transiently expressed during avian cardiac valve morphogenesis. Dev. Dyn. 1994, 200, 294–304. [Google Scholar] [CrossRef]
- Liu, Y.; Watanabe, H.; Nifuji, A.; Yamada, Y.; Olson, E.N.; Noda, M. Overexpression of a single helix-loop-helix-type transcription factor, scleraxis, enhances aggrecan gene expression in osteoblastic osteosarcoma ROS17/2.8 cells. J. Biol. Chem. 1997, 272, 29880–29885. [Google Scholar] [CrossRef]
- Wenstrup, R.J.; Florer, J.B.; Brunskill, E.W.; Bell, S.M.; Chervoneva, I.; Birk, D.E. Type V Collagen Controls the Initiation of Collagen Fibril Assembly. J. Biol. Chem. 2004, 279, 53331–53337. [Google Scholar] [CrossRef]
- Lincoln, J.; Florer, J.B.; Deutsch, G.H.; Wenstrup, R.J.; Yutzey, K.E. ColVa1 and ColXIa1 are required for myocardial morphogenesis and heart valve development. Dev. Dyn. 2006, 235, 3295–3305. [Google Scholar] [CrossRef]
- Klewer, S.E.; Krob, S.L.; Kolker, S.J.; Kitten, G.T. Expression of type VI collagen in the developing mouse heart. Dev. Dyn. 1998, 211, 248–255. [Google Scholar] [CrossRef]
- Cole, W.G.; Chan, D.; Hickey, A.J.; Wilcken, D.E. Collagen composition of normal and myxomatous human mitral heart valves. Biochem. J. 1984, 219, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Kunzelman, K.S.; Cochran, R.P.; Murphree, S.S.; Ring, W.S.; Verrier, E.D.; Eberhart, R.C. Differential collagen distribution in the mitral valve and its influence on biomechanical behaviour. J. Heart Valve Dis. 1993, 2, 236–244. [Google Scholar] [PubMed]
- Balguid, A.A.; Rubbens, M.P.; Driessen-Mol, A.A.; Bank, R.A.; Bogers, A.J.J.C.; Van Kats, J.P.; De Mol, B.A.J.M.; Baaijens, F.P.T.; Bouten, C.V.C. The Role of Collagen Cross-Links in Biomechanical Behavior of Human Aortic Heart Valve Leaflets—Relevance for Tissue Engineering. Tissue Eng. 2007, 13, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Pierlot, C.M.; Lee, J.M.; Amini, R.; Sacks, M.S.; Wells, S.M. Pregnancy-Induced Remodeling of Collagen Architecture and Content in the Mitral Valve. Ann. Biomed. Eng. 2014, 42, 2058–2071. [Google Scholar] [CrossRef] [PubMed]
- Pierlot, C.M.; Moeller, A.D.; Lee, J.M.; Wells, S.M. Pregnancy-induced remodeling of heart valves. Am. J. Physiol. Circ. Physiol. 2015, 309, H1565–H1578. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Liao, J.; Yang, L.; Grashow, J.; Sacks, M.S. The Relation Between Collagen Fibril Kinematics and Mechanical Properties in the Mitral Valve Anterior Leaflet. J. Biomech. Eng. 2006, 129, 78–87. [Google Scholar] [CrossRef]
- Zhang, W.; Ayoub, S.; Liao, J.; Sacks, M.S. A meso-scale layer-specific structural constitutive model of the mitral heart valve leaflets. Acta Biomater. 2016, 32, 238–255. [Google Scholar] [CrossRef]
- Ayoub, S.; Tsai, K.C.; Khalighi, A.H.; Sacks, M.S. The Three-Dimensional Microenvironment of the Mitral Valve: Insights into the Effects of Physiological Loads. Cell. Mol. Bioeng. 2018, 11, 291–306. [Google Scholar] [CrossRef]
- Billiar, K.L.; Sacks, M.S. A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 1997, 30, 753–756. [Google Scholar] [CrossRef]
- Billiar, K.L.; Sacks, M.S. Biaxial Mechanical Properties of the Natural and Glutaraldehyde Treated Aortic Valve Cusp—Part I: Experimental Results. J. Biomech. Eng. 2000, 122, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Billiar, K.L.; Sacks, M.S. Biaxial Mechanical Properties of the Native and Glutaraldehyde-Treated Aortic Valve Cusp: Part II—A Structural Constitutive Model. J. Biomech. Eng. 2000, 122, 327–335. [Google Scholar] [CrossRef]
- Goth, W.; Potter, S.; Allen, A.C.B.; Zoldan, J.; Sacks, M.S.; Tunnell, J.W. Non-Destructive Reflectance Mapping of Collagen Fiber Alignment in Heart Valve Leaflets. Ann. Biomed. Eng. 2019, 47, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Esko, J.D.; Kimata, K.; Lindahl, U. Proteoglycans and Sulfated Glycosaminoglycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbor: New York, NY, USA, 2009. [Google Scholar]
- Murata, K. Acidic glycosaminoglycans in human heart valves. J. Mol. Cell. Cardiol. 1981, 13, 281–292. [Google Scholar] [CrossRef]
- Stephens, E.H.; Kearney, D.L.; Grande-Allen, K.J. Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovasc. Pathol. 2012, 21, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Barzilla, J.E.; Mendez, J.S.; Stephens, E.H.; Lee, E.L.; Collard, C.D.; Laucirica, R.; Weigel, P.H.; Grande-Allen, K.J. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc. Pathol. 2009, 18, 191–197. [Google Scholar] [CrossRef]
- Kinsella, M.G.; Bressler, S.L.; Wight, T.N. The regulated synthesis of versican, decorin, and biglycan: Extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene Expr. 2004, 14, 203–234. [Google Scholar] [CrossRef]
- Grande-Allen, K.J.; Osman, N.; Ballinger, M.L.; Dadlani, H.; Marasco, S.; Little, P.J. Glycosaminoglycan synthesis and structure as targets for the prevention of calcific aortic valve disease. Cardiovasc. Res. 2007, 76, 19–28. [Google Scholar] [CrossRef]
- Rothenburger, M.; Völker, W.; Vischer, P.; Glasmacher, B.; Scheld, H.H.; Deiwick, M. Ultrastructure of Proteoglycans in Tissue-Engineered Cardiovascular Structures. Tissue Eng. 2002, 8, 1049–1056. [Google Scholar] [CrossRef]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Localization and pattern of expression of extracellular matrix components in human heart valves. J. Heart Valve Dis. 2005, 14, 218–227. [Google Scholar]
- Stephens, E.H.; Chu, C.-K.; Grande-Allen, K.J. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: Relevance to an age-specific tissue-engineered heart valve. Acta Biomater. 2008, 4, 1148–1160. [Google Scholar] [CrossRef]
- Stephens, E.H.; Saltarrelli, J.G.; Baggett, L.S.; Nandi, I.; Kuo, J.J.; Davis, A.R.; Olmsted-Davis, E.A.; Reardon, M.J.; Morrisett, J.D.; Grande-Allen, K.J. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc. Pathol. 2011, 20, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Danielson, K.G.; Baribault, H.; Holmes, D.F.; Graham, H.; Kadler, K.E.; Iozzo, R.V. Targeted Disruption of Decorin Leads to Abnormal Collagen Fibril Morphology and Skin Fragility. J. Cell Biol. 1997, 136, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Grande-Allen, K.J.; Calabro, A.; Gupta, V.; Wight, T.N.; Hascall, V.C.; Vesely, I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: Association with regions of tensile and compressive loading. Glycobiology 2004, 14, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Osinska, H.; Weinstein, M.B.; Hinton, R.B.; Kern, C.B. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J. Mol. Cell. Cardiol. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- Fox, P.R. Pathology of myxomatous mitral valve disease in the dog. J. Veter- Cardiol. 2012, 14, 103–126. [Google Scholar] [CrossRef]
- Stephens, E.H.; De Jonge, N.; McNeill, M.P.; Durst, C.A.; Grande-Allen, K.J. Age-Related Changes in Material Behavior of Porcine Mitral and Aortic Valves and Correlation to Matrix Composition. Tissue Eng. Part A 2010, 16, 867–878. [Google Scholar] [CrossRef]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef]
- Patel, A.; Fine, B.; Sandig, M.; Mequanint, K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc. Res. 2006, 71, 40–49. [Google Scholar] [CrossRef]
- Ayoub, S.; Ferrari, G.; Gorman, R.C.; Gorman, J.H.; Schoen, F.J.; Sacks, M.S. Heart Valve Biomechanics and Underlying Mechanobiology. Compr. Physiol. 2016, 6, 1743–1780. [Google Scholar] [CrossRef]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Fondard, O.; Detaint, D.; Iung, B.; Choqueux, C.; Adle-Biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.-P.; Henin, D.; Michel, J.-B.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef]
- Perrotta, I.; Sciangula, A.; Aquila, S.; Mazzulla, S. Matrix Metalloproteinase-9 Expression in Calcified Human Aortic Valves: A Histopathologic, Immunohistochemical, and Ultrastructural Study. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Fischer, C.S.; Vocke, D.C.; Kılıç, R.; Sarıkoç, A.; Piñol, R.; Hagl, S.; Lang, S.; et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 2005, 14, 80–87. [Google Scholar] [CrossRef]
- Platt, M.O.; Xing, Y.; Jo, H.; Yoganathan, A.P. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J. Heart Valve Dis. 2006, 15, 622–629. [Google Scholar]
- Bigg, P.W.; Baldo, G.; Sleeper, M.M.; O’Donnell, P.A.; Bai, H.; Rokkam, V.R.; Liu, Y.; Wu, S.; Giugliani, R.; Casal, M.L.; et al. Pathogenesis of mitral valve disease in mucopolysaccharidosis VII dogs. Mol. Genet. Metab. 2013, 110, 319–328. [Google Scholar] [CrossRef][Green Version]
- Mahimkar, R.; Nguyen, A.; Mann, M.; Yeh, C.-C.; Zhu, B.-Q.; Karliner, J.S.; Lovett, D.H. Cardiac transgenic matrix metalloproteinase-2 expression induces myxomatous valve degeneration: A potential model of mitral valve prolapse disease. Cardiovasc. Pathol. 2009, 18, 253–261. [Google Scholar] [CrossRef]
- Spadaccio, C.; Mozetic, P.; Nappi, F.; Nenna, A.; Sutherland, F.; Trombetta, M.; Chello, M.; Rainer, A. Cells and extracellular matrix interplay in cardiac valve disease: Because age matters. Basic Res. Cardiol. 2016, 111, 16. [Google Scholar] [CrossRef]
- Wang, H.; Leinwand, L.A.; Anseth, K.S. Cardiac valve cells and their microenvironment—insights from in vitro studies. Nat. Rev. Cardiol. 2014, 11, 715–727. [Google Scholar] [CrossRef]
- Lincoln, J.; Lange, A.W.; Yutzey, K.E. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev. Biol. 2006, 294, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Joag, V.R.; Gotlieb, A.I. The Emerging Role of Valve Interstitial Cell Phenotypes in Regulating Heart Valve Pathobiology. Am. J. Pathol. 2007, 171, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Lincoln, J. Calcific Aortic Valve Disease: A Developmental Biology Perspective. Curr. Cardiol. Rep. 2018, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Masters, K.S.; Shah, D.N.; Anseth, K.S.; Leinwand, L.A. Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 2004, 95, 253–260. [Google Scholar] [CrossRef]
- Dye, B.K.; Butler, C.; Lincoln, J. Smooth Muscle α-Actin Expression in Mitral Valve Interstitial Cells is Important for Mediating Extracellular Matrix Remodeling. J. Cardiovasc. Dev. Dis. 2020, 7, 32. [Google Scholar] [CrossRef]
- Lincoln, J.; Alfieri, C.M.; Yutzey, K.E. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev. Dyn. 2004, 230, 239–250. [Google Scholar] [CrossRef]
- Espira, L.; Lamoureux, L.; Jones, S.C.; Gerard, R.D.; Dixon, I.M.; Czubryt, M.P. The basic helix–loop–helix transcription factor scleraxis regulates fibroblast collagen synthesis. J. Mol. Cell. Cardiol. 2009, 47, 188–195. [Google Scholar] [CrossRef]
- Lincoln, J.; Alfieri, C.M.; Yutzey, K.E. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev. Biol. 2006, 292, 290–302. [Google Scholar] [CrossRef]
- Levay, A.; Peacock, J.D.; Lu, Y.; Koch, M.; Hinton, R.B.; Kadler, K.E.; Lincoln, J. Scleraxis Is Required for Cell Lineage Differentiation and Extracellular Matrix Remodeling During Murine Heart Valve Formation In Vivo. Circ. Res. 2008, 103, 948–956. [Google Scholar] [CrossRef]
- Barnette, D.N.; Hulin, A.; Ishtiaq Ahmed, A.S.; Colige, A.C.; Azhar, M.; Lincoln, J. Tgfbeta-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves. J. Mol. Cell. Cardiol. 2013, 65, 137–146. [Google Scholar] [CrossRef]
- Amini, R.; Eckert, C.E.; Koomalsingh, K.; McGarvey, J.; Minakawa, M.; Gorman, J.H.; Gorman, R.C.; Sacks, M.S. On the In Vivo Deformation of the Mitral Valve Anterior Leaflet: Effects of Annular Geometry and Referential Configuration. Ann. Biomed. Eng. 2012, 40, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.E.; Zubiate, B.; Vergnat, M.; Gorman, J.H.; Gorman, R.C.; Sacks, M.S. In Vivo Dynamic Deformation of the Mitral Valve Annulus. Ann. Biomed. Eng. 2009, 37, 1757–1771. [Google Scholar] [CrossRef] [PubMed]
- Rajput, F.A.; Zeltser, R. Aortic Valve Replacement; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Aggarwal, A.; Pouch, A.M.; Lai, E.; Lesicko, J.; Yushkevich, P.A.; Iii, J.H.G.; Gorman, R.C.; Sacks, M.S. In-vivo heterogeneous functional and residual strains in human aortic valve leaflets. J. Biomech. 2016, 49, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Brazile, B.; Wang, B.; Wang, G.; Bertucci, R.; Prabhu, R.; Patnaik, S.S.; Butler, J.R.; Claude, A.; Brinkman-Ferguson, E.; Williams, L.N.; et al. On the Bending Properties of Porcine Mitral, Tricuspid, Aortic, and Pulmonary Valve Leaflets. J. Autom. Inf. Sci. 2015, 25, 41–53. [Google Scholar] [CrossRef] [PubMed]
- VeDepo, M.; Buse, E.E.; Quinn, R.W.; Williams, T.D.; Detamore, M.S.; Hopkins, R.A.; Converse, G.L. Species-specific effects of aortic valve decellularization. Acta Biomater. 2017, 50, 249–258. [Google Scholar] [CrossRef]
- Lee, C.-H.; Carruthers, C.A.; Ayoub, S.; Gorman, R.C.; Gorman, J.H.; Sacks, M.S. Quantification and simulation of layer-specific mitral valve interstitial cells deformation under physiological loading. J. Theor. Biol. 2015, 373, 26–39. [Google Scholar] [CrossRef]
- Blaser, M.C.; Aikawa, E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front. Cardiovasc. Med. 2018, 5, 187. [Google Scholar] [CrossRef]
- Pierlot, C.M.; Moeller, A.D.; Lee, J.M.; Wells, S.M. Biaxial Creep Resistance and Structural Remodeling of the Aortic and Mitral Valves in Pregnancy. Ann. Biomed. Eng. 2015, 43, 1772–1785. [Google Scholar] [CrossRef]
- Rego, B.V.; Wells, S.M.; Lee, C.-H.; Sacks, M.S. Mitral valve leaflet remodelling during pregnancy: Insights into cell-mediated recovery of tissue homeostasis. J. R. Soc. Interface 2016, 13, 20160709. [Google Scholar] [CrossRef]
- Khang, A.; Buchanan, R.M.; Ayoub, S.; Rego, B.V.; Lee, C.-H.; Ferrari, G.; Anseth, K.S.; Sacks, M.S. Mechanobiology of the heart valve interstitial cell: Simulation, experiment, and discovery. Mechanobiol. Health Dis. 2018, 249–283. [Google Scholar] [CrossRef]
- Bowler, M.A.; Bersi, M.R.; Ryzhova, L.M.; Jerrell, R.J.; Parekh, A.; Merryman, W.D. Cadherin-11 as a regulator of valve myofibroblast mechanobiology. Am. J. Physiol. Circ. Physiol. 2018, 315, H1614–H1626. [Google Scholar] [CrossRef] [PubMed]
- Merryman, W.D.; Huang, H.-Y.S.; Schoen, F.J.; Sacks, M.S. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J. Biomech. 2006, 39, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vootukuri, S.; Meyer, A.; Ahamed, J.; Coller, B.S. Association between shear stress and platelet-derived transforming growth factor-beta1 release and activation in animal models of aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Buchanan, R.M.; Sanchez-Adams, J.; Guilak, F.; Sacks, M.S. On the Functional Role of Valve Interstitial Cell Stress Fibers: A Continuum Modeling Approach. J. Biomech. Eng. 2017, 139, 021007–02100713. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.G.; Schroeder, M.E.; Walker, C.J.; Anseth, K.S. FGF-2 inhibits contractile properties of valvular interstitial cell myofibroblasts encapsulated in 3D MMP-degradable hydrogels. APL Bioeng. 2018, 2, 046104. [Google Scholar] [CrossRef] [PubMed]
- Khang, A.; Gonzalez Rodriguez, A.; Schroeder, M.E.; Sansom, J.; Lejeune, E.; Anseth, K.S.; Sacks, M.S. Quantifying heart valve interstitial cell contractile state using highly tunable poly(ethylene glycol) hydrogels. Acta Biomater. 2019, 96, 354–367. [Google Scholar] [CrossRef]
- Mabry, K.M.; Lawrence, R.L.; Anseth, K.S. Dynamic stiffening of poly(ethylene glycol)-based hydrogels to direct valvular interstitial cell phenotype in a three-dimensional environment. Biomaterials 2015, 49, 47–56. [Google Scholar] [CrossRef]
- Masoumi, N.; Howell, M.C.; Johnson, K.L.; Niesslein, M.J.; Gerber, G.; Engelmayr, G.C. Design and testing of a cyclic stretch and flexure bioreactor for evaluating engineered heart valve tissues based on poly(glycerol sebacate) scaffolds. Proc. Inst. Mech. Eng. Part H 2014, 228, 576–586. [Google Scholar] [CrossRef]
- Eslami, M.; Javadi, G.R.; Agdami, N.; Shokrgozar, M.A. Expression of COLLAGEN 1 and ELASTIN Genes in Mitral Valvular Interstitial Cells within Microfiber Reinforced Hydrogel. Cell J. 2015, 17, 478–488. [Google Scholar]
- Duan, B.; Kapetanovic, E.; Hockaday, L.A.; Cheung, D.Y. Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater. 2014, 10, 1836–1846. [Google Scholar] [CrossRef]
- Miller, J.D.; Weiss, R.M.; Heistad, D.D. Calcific Aortic Valve Stenosis: Methods, Models, and Mechanisms. Circ. Res. 2011, 108, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.; Lee, C.-H.; Driesbaugh, K.H.; Anselmo, W.; Hughes, C.T.; Ferrari, G.; Gorman, R.C.; Gorman, J.H.; Sacks, M.S. Regulation of valve interstitial cell homeostasis by mechanical deformation: Implications for heart valve disease and surgical repair. J. R. Soc. Interface 2017, 14, 20170580. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Simmons, C.A. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: Critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 2011, 108, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Colognato, H.; Yurchenco, P.D. Form and function: The laminin family of heterotrimers. Dev. Dyn. 2000, 218, 213–234. [Google Scholar] [CrossRef]
- Ruoslahti, E. Fibronectin and its receptors. Annu. Rev. Biochem. 1988, 57, 375–413. [Google Scholar] [CrossRef]
- Latif, N.; Sarathchandra, P.; Taylor, P.M.; Antoniw, J.; Yacoub, M.H. Molecules Mediating Cell–ECM and Cell–Cell Communication in Human Heart Valves. Cell Biophys. 2005, 43, 275–288. [Google Scholar] [CrossRef]
- Yan, J.; Stringer, S.E.; Hamilton, A.; Charlton-Menys, V.; Gotting, C.; Muller, B.; Aeschilimann, D.; Yvonne, M. Decorin GAG synthesis and TGF-beta signaling mediate Ox-LDL-induced mineralization of human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 608–615. [Google Scholar] [CrossRef]
- Matsuo, I.; Kimura-Yoshida, C. Extracellular modulation of Fibroblast Growth Factor signaling through heparan sulfate proteoglycans in mammalian development. Curr. Opin. Genet. Dev. 2013, 23, 399–407. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Lin, X.; Beenken, A.; Eliseenkova, A.V.; Mohammadi, M.; Linhardt, R.J. Compositional Analysis of Heparin/Heparan Sulfate Interacting with Fibroblast Growth Factor·Fibroblast Growth Factor Receptor Complexes. Biochemistry 2009, 48, 8379–8386. [Google Scholar] [CrossRef]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Benton, J.A.; Kern, H.B.; Anseth, K.S. Substrate properties influence calcification in valvular interstitial cell culture. J. Heart Valve Dis. 2008, 17, 689–699. [Google Scholar]
- Duan, B.; Yin, Z.; Kang, L.H.; Magin, R.L.; Cheung, D.Y. Active tissue stiffness modulation controls valve interstitial cell phenotype and osteogenic potential in 3D culture. Acta Biomater. 2016, 36, 42–54. [Google Scholar] [CrossRef]
- Pho, M.; Lee, W.; Watt, D.R.; Laschinger, C.; Simmons, C.A.; McCulloch, C.A. Cofilin is a marker of myofibroblast differentiation in cells from porcine aortic cardiac valves. Am. J. Physiol. Circ. Physiol. 2008, 294, H1767–H1778. [Google Scholar] [CrossRef]
- Yip, C.Y.Y.; Chen, J.-H.; Zhao, R.; Simmons, C.A. Calcification by Valve Interstitial Cells Is Regulated by the Stiffness of the Extracellular Matrix. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 936–942. [Google Scholar] [CrossRef]
- Stephens, E.H.; Durst, C.A.; West, J.L.; Grande-Allen, K.J. Mitral valvular interstitial cell responses to substrate stiffness depend on age and anatomic region. Acta Biomater. 2011, 7, 75–82. [Google Scholar] [CrossRef]
- Butcher, J.T.; Simmons, C.A.; Warnock, J.N. Mechanobiology of the aortic heart valve. J. Heart Valve Dis. 2008, 17, 62–73. [Google Scholar]
- Metzler, S.A.; Pregonero, C.A.; Butcher, J.T.; Burgess, S.C.; Warnock, J.N. Cyclic strain regulates pro-inflammatory protein expression in porcine aortic valve endothelial cells. J. Heart Valve Dis. 2008, 17, 571-7. [Google Scholar]
- Smith, K.E.; Metzler, S.A.; Warnock, J.N. Cyclic strain inhibits acute pro-inflammatory gene expression in aortic valve interstitial cells. Biomech. Model. Mechanobiol. 2009, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, K.; Sucosky, P.; Jo, H.; Yoganathan, A.P. Elevated Cyclic Stretch Induces Aortic Valve Calcification in a Bone Morphogenic Protein-Dependent Manner. Am. J. Pathol. 2010, 177, 49–57. [Google Scholar] [CrossRef]
- Sacks, M.S.; Merryman, W.D.; Schmidt, D.E. On the biomechanics of heart valve function. J. Biomech. 2009, 42, 1804–1824. [Google Scholar] [CrossRef] [PubMed]
- Cheek, J.D.; Wirrig, E.E.; Alfieri, C.M.; James, J.F.; Yutzey, K.E. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J. Mol. Cell. Cardiol. 2012, 52, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Wirrig, E.E.; Hinton, R.B.; Yutzey, K.E. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J. Mol. Cell. Cardiol. 2011, 50, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Oncology, F.T.A.F.C.T.I.; Mulkey, F.; Hasserjian, R.P.; Sanford, B.L.; Vij, R.; Hurd, D.D.; Odenike, O.M.; Bloomfield, C.D.; Owzar, K.; et al. A phase II study of the oral VEGF receptor tyrosine kinase inhibitor vatalanib (PTK787/ZK222584) in myelodysplastic syndrome: Cancer and Leukemia Group B study 10105 (Alliance). Investig. New Drugs 2013, 31, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Alfieri, C.M.; Yutzey, K.E. Endothelial Cell Lineage Analysis Does Not Provide Evidence for EMT in Adult Valve Homeostasis and Disease. Anat. Rec. 2019, 302, 125–135. [Google Scholar] [CrossRef]
- Lu, C.-C.; Liu, M.-M.; Clinton, M.; Culshaw, G.; Argyle, D.J.; Corcoran, B.M. Developmental pathways and endothelial to mesenchymal transition in canine myxomatous mitral valve disease. Vet. J. 2015, 206, 377–384. [Google Scholar] [CrossRef]
- Hjortnaes, J.; Camci-Unal, G.; Hutcheson, J.D.; Jung, S.M.; Schoen, F.J.; Kluin, J.; Aikawa, E.; Khademhosseini, A. Directing Valvular Interstitial Cell Myofibroblast-Like Differentiation in a Hybrid Hydrogel Platform. Adv. Health Mater. 2015, 4, 121–130. [Google Scholar] [CrossRef]
- Dietz, H.C.; Cutting, C.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nat. Cell Biol. 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Colombi, M.; Dordoni, C.; Chiarelli, N.; Ritelli, M. Differential diagnosis and diagnostic flow chart of joint hypermobility syndrome/ehlers-danlos syndrome hypermobility type compared to other heritable connective tissue disorders. Am. J. Med Genet. Part C Semin. Med Genet. 2015, 169, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Altayó, F.; Ortiz-Romero, P.; Puertas-Umbert, L.; Dantas, A.P.; Pérez, B.; Vila, E.; D’Ocon, P.; Campuzano, V. Stenosis coexists with compromised alpha1-adrenergic contractions in the ascending aorta of a mouse model of Williams-Beuren syndrome. Sci. Rep. 2020, 10, 889. [Google Scholar]
- Thacoor, A. Mitral valve prolapse and Marfan syndrome. Congenit. Hear. Dis. 2017, 12, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Cheng, A.; Myers, L.A.; Martinez-Murillo, F.; Jie, C.; Bedja, D.; Gabrielson, K.L.; Hausladen, J.M.W.; Mecham, R.P.; Judge, D.P.; et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Investig. 2004, 114, 1586–1592. [Google Scholar] [CrossRef]
- Dietz, H. Marfan Syndrome; Adam, M.P., Ed.; GeneReviews: Seattle, WA, USA, 1993. [Google Scholar]
- Wozniak-Mielczarek, L.; Sabiniewicz, R.; Drezek-Nojowicz, M.; Nowak, R.; Gilis-Malinowska, N.; Mielczarek, M.; Łabuc, A.; Waldoch, A.; Wierzba, J. Differences in Cardiovascular Manifestation of Marfan Syndrome Between Children and Adults. Pediatr. Cardiol. 2018, 40, 393–403. [Google Scholar] [CrossRef]
- Kim, A.J.; Xu, N.; Umeyama, K.; Hulin, A.; Ponny, S.R.; Vagnozzi, R.J.; Green, E.A.; Hanson, P.; McManus, B.M.; Nagashima, H.; et al. Deficiency of Circulating Monocytes Ameliorates the Progression of Myxomatous Valve Degeneration in Marfan Syndrome. Circulation 2020, 141, 132–146. [Google Scholar] [CrossRef]
- Détaint, D.; Faivre, L.; Collod-Beroud, G.; Child, A.H.; Loeys, B.L.; Binquet, C.; Gautier, E.; Arbustini, E.; Mayer, K.; Arslan-Kirchner, M.; et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur. Heart J. 2010, 31, 2223–2229. [Google Scholar] [CrossRef]
- Malfait, F. Vascular aspects of the Ehlers-Danlos Syndromes. Matrix Biol. 2018, 71–72, 380–395. [Google Scholar] [CrossRef]
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef]
- Byers, P.H.; Belmont, J.; Black, J.; De Backer, J.; Frank, M.; Jeunemaitre, X.; Johnson, D.; Pepin, M.; Robert, L.; Sanders, L.; et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 40–47. [Google Scholar] [CrossRef]
- Wenstrup, R.J.; Florer, J.B.; Davidson, J.M.; Phillips, C.L.; Pffeifer, B.J.; Menezes, D.W.; Chervoneva, I.; Birk, D.E. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J. Biol. Chem. 2006, 281, 12888–12895. [Google Scholar] [CrossRef]
- Palomo, T.; Vilaca, T.; Lazaretti-Castro, M. Osteogenesis imperfecta: Diagnosis and treatment. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 381–388. [Google Scholar] [CrossRef]
- Valadares, E.R.; Carneiro, T.B.; Santos, P.M.; Oliveira, A.C.; Zabel, B. What is new in genetics and osteogenesis imperfecta classification? J. Pediatr. (Rio J.) 2014, 90, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, F.; Sillence, D. Osteogenesis imperfecta: Clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. Part A 2014, 164, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Chipman, S.D.; Sweet, H.O.; McBride, D.J.; Davisson, M.T.; Marks, S.C.; Shuldiner, A.R.; Wenstrup, R.J.; Rowe, D.W.; Shapiro, J.R. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1993, 90, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, A.; Fayers, T.; Clarke, S.; Parsonage, W.A. Valvular and Aortic Diseases in Osteogenesis Imperfecta. Heart Lung Circ. 2013, 22, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Symoens, S.; Coucke, P.; Nunes, L.; De Almeida, S.; De Paepe, A. Total absence of the 2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J. Med. Genet. 2005, 43, e36. [Google Scholar] [CrossRef]
- Bonita, R.E.; Cohen, I.S.; Berko, B.A. Valvular Heart Disease in Osteogenesis Imperfecta: Presentation of a Case and Review of the Literature. Echocardiography 2010, 27, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.M.; Loch, D.C.; Habashi, J.P.; Calderon, J.F.; Chen, Y.; Bedja, D.; Van Erp, C.; Gerber, E.E.; Parker, S.J.; Sauls, K.; et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J. Clin. Investig. 2014, 124, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Hara, H.; Fujiwara, T.; Kanaya, T.; Maemura, S.; Komuro, I. TGF-beta Signaling-Related Genes and Thoracic Aortic Aneurysms and Dissections. Int. J. Mol. Sci. 2018, 19, 2125. [Google Scholar] [CrossRef] [PubMed]
- Van der Pluijm, I.; Burger, J.; Van Heijningen, P.M.; Ijpma, A.; Van Vliet, N.; Milanese, C.; Schoonderwoerd, K.; Sluiter, W.; Ringuette, L.-J.; Dekkers, D.H.W.; et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc. Res. 2018, 114, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Vermeij, M.; Garinis, G.A.; De Waard, M.C.; Kunen, M.G.S.; Myers, L.; Maas, A.; Duncker, D.J.; Meijers, C.; Dietz, H.C.; et al. Perturbations of Vascular Homeostasis and Aortic Valve Abnormalities in Fibulin-4 Deficient Mice. Circ. Res. 2007, 100, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Munjal, C.; Jegga, A.; Opoka, A.M.; Stoilov, I.; Norris, R.A.; Thomas, C.J.; Smith, J.M.; Mecham, R.P.; Bressan, G.M.; Hinton, R.B. Inhibition of MAPK-Erk pathway in vivo attenuates aortic valve disease processes in Emilin1-deficient mouse model. Physiol. Rep. 2017, 5, e13152. [Google Scholar] [CrossRef] [PubMed]
- Capuano, A.; Bucciotti, F.; Farwell, K.D.; Davis, B.T.; Mroske, C.; Hulick, P.J.; Weissman, S.M.; Gao, Q.; Spessotto, P.; Colombatti, A.; et al. Diagnostic Exome Sequencing Identifies a Novel Gene, EMILIN1, Associated with Autosomal-Dominant Hereditary Connective Tissue Disease. Hum. Mutat. 2016, 37, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.M.; Drews, J.D.; Breuer, C.K. Tissue-Engineered Heart Valves: A Call for Mechanistic Studies. Tissue Eng. Part B Rev. 2018, 24, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J.; Gotlieb, A.I. Heart valve health, disease, replacement, and repair: A 25-year cardiovascular pathology perspective. Cardiovasc. Pathol. 2016, 25, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Fioretta, E.; Von Boehmer, L.; Motta, S.; Lintas, V.; Hoerstrup, S.; Emmert, M.Y. Cardiovascular tissue engineering: From basic science to clinical application. Exp. Gerontol. 2019, 117, 1–12. [Google Scholar] [CrossRef]
- Zhang, B.L.; Bianco, R.W.; Schoen, F.J. Preclinical Assessment of Cardiac Valve Substitutes: Current Status and Considerations for Engineered Tissue Heart Valves. Front. Cardiovasc. Med. 2019, 6, 72. [Google Scholar] [CrossRef]
- Chester, A.H.; Grande-Allen, K.J. Which Biological Properties of Heart Valves Are Relevant to Tissue Engineering? Front. Cardiovasc. Med. 2020, 7, 63. [Google Scholar] [CrossRef]
| Human Disease | Associated ECM Gene | Mouse Model | Human Valvular Defects Recapitulated in the Mouse Model | References |
|---|---|---|---|---|
| Williams-Beuren Syndrome | Elastin | Deletion (from Gtf2i to Fkbp6) (includes ELN) (most common deletion found in humans with WBS) | Supravalvular aortic stenosis (SVAS) | [125] |
| Loeys-Dietz Syndrome | TGF-βR1 TGF-βr2R2 TGF-β1 SMAD3 | Loss-of-function mutations: -Tgfβr1M318R/+−Tgfβr2G357W/+ -Transgenic Tgfβr2 | Aortic aneurysm Mitral valve prolapse | [143] [144] [145] [146] |
| Cutis Laxa | FBNL4 FBNL5 | Fibulin-4R/R (reduced expression allele) | Thickened aortic valve leaflets with stenosis and insufficiency | [145] [146] |
| Marfan Syndrome | FBN1 | Fbn1C1039G/+ | Mitral valve prolapse | [127] |
| Ehlers-Danlos Syndrome | COLVA1 | ColVa1+/− | No gross of functional valve defects | [20] |
| Stickler Syndrome | COLXIA1 | ColXIa1−/− | Thickened valve leaflets | [20] |
| Osteogenesis Imperfecta | COL1A2 | Col1a2−/− (oim/oim) | Myxomatous aortic valve leaflets | [117] |
| Fibrotic aortic valve disease-autosomal-dominant connective tissue disease | EMILIN-1 | Emilin1−/− | Marked progression of valve pathology that shows severe fibrosis, neovascularization and inflammation | [147] [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodigepalli, K.M.; Thatcher, K.; West, T.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. https://doi.org/10.3390/jcdd7040057
Kodigepalli KM, Thatcher K, West T, Howsmon DP, Schoen FJ, Sacks MS, Breuer CK, Lincoln J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. Journal of Cardiovascular Development and Disease. 2020; 7(4):57. https://doi.org/10.3390/jcdd7040057
Chicago/Turabian StyleKodigepalli, Karthik M., Kaitlyn Thatcher, Toni West, Daniel P. Howsmon, Frederick J. Schoen, Michael S. Sacks, Christopher K. Breuer, and Joy Lincoln. 2020. "Biology and Biomechanics of the Heart Valve Extracellular Matrix" Journal of Cardiovascular Development and Disease 7, no. 4: 57. https://doi.org/10.3390/jcdd7040057
APA StyleKodigepalli, K. M., Thatcher, K., West, T., Howsmon, D. P., Schoen, F. J., Sacks, M. S., Breuer, C. K., & Lincoln, J. (2020). Biology and Biomechanics of the Heart Valve Extracellular Matrix. Journal of Cardiovascular Development and Disease, 7(4), 57. https://doi.org/10.3390/jcdd7040057






