Left Ventricular Blood Flow Kinetic Energy Assessment by 4D Flow Cardiovascular Magnetic Resonance: A Systematic Review of the Clinical Relevance †
Abstract
1. Introduction
2. Methods
2.1. Systematic Review Registration
2.2. Search Strategy
2.3. Article Screening
3. Results
4. Discussion
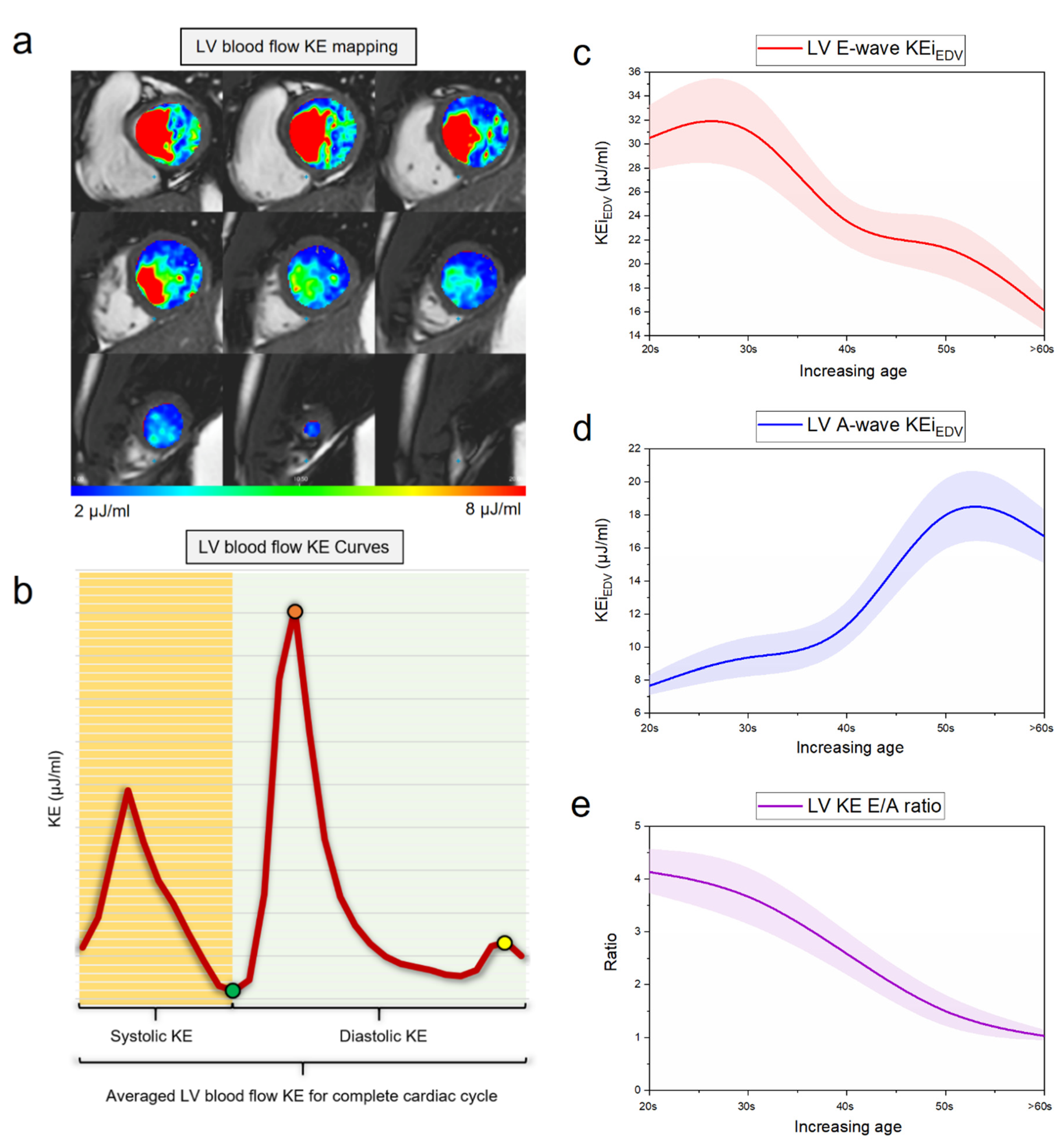
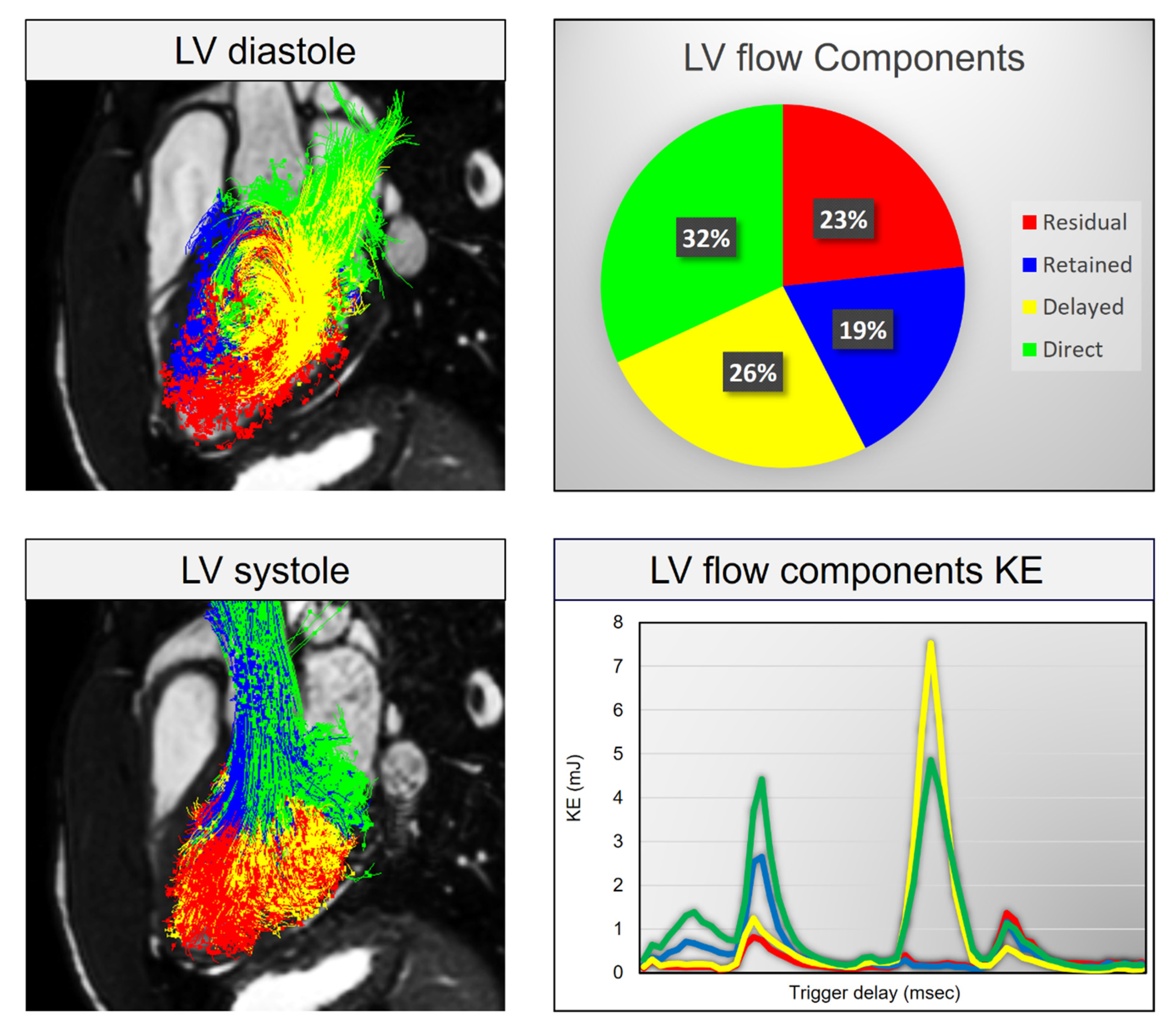
4.1. Aging and LV Diastolic Function
4.2. Ischemic Heart Disease and Myocardial Infarction
4.3. Heart Failure Including Cardiomyopathies
4.4. Valvular Heart Disease
4.5. Clinical Perspective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| 4D | four-dimensional |
| CMR | cardiac magnetic resonance |
| DCM | dilated cardiomyopathy |
| DI | dissipation index |
| EDV | end-diastolic volume |
| ESV | end-systolic volume |
| EF | ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| HDAS | healthcare databases advanced search |
| IHD | ischemic heart disease |
| KE | kinetic energy |
| KE/CI | mean kinetic energy/cardiac index |
| KEiEDV | kinetic energy indexed end-diastolic volume |
| LBBB | left bundle branch block |
| LV | left ventricle/left ventricular |
| LVEDV | left ventricular end-diastolic volume |
| LVEDVi | left ventricular end-diastolic volume index |
| LVESVi | left ventricular end-systolic volume index |
| LVM | left ventricular mass |
| LVOT | left ventricular outflow tract |
| MI | myocardial infarction |
| mJ | millijoules |
| MR | mitral regurgitation |
| MRI | magnetic resonance imaging |
| MV | mitral valve |
| PRISMA | preferred reporting items for systematic reviews and meta-analyses |
| PROSPERO | international prospective register of systematic reviews |
| RV | right ventricle |
| SV | stroke volume |
| TD | time difference |
| TKE | turbulent kinetic energy |
References
- Sengupta, P.P.; Pedrizzetti, G.; Kilner, P.J.; Kheradvar, A.; Ebbers, T.; Tonti, G.; Fraser, A.G.; Narula, J. Emerging trends in CV flow visualization. JACC Cardiovasc. Imaging 2012, 5, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Doost, S.N.; Ghista, D.; Su, B.; Zhong, L.; Morsi, Y.S. Heart blood flow simulation: A perspective review. Biomed. Eng. Online 2016, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Kass, D.A. Invasive hemodynamic assessment in heart failure. Heart Fail Clin. 2009, 5, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reason. J. Soc. Cardiovasc. Magn. Reson. 2015, 10, 17–72. [Google Scholar] [CrossRef]
- Van der Geest, R.J.; Garg, P. Advanced Analysis Techniques for Intra-cardiac Flow Evaluation from 4D Flow MRI. Curr. Radiol. Rep. 2016, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Walker, P.G.; Pedersen, E.M.; Poulsen, J.K.; Oyre, S.; Houlind, K.; Yoganathan, A.P. Left ventricular blood flow patterns in normal subjects: A quantitative analysis by three-dimensional magnetic resonance velocity mapping. J. Am. Coll. Cardiol. 1995, 26, 224–238. [Google Scholar] [CrossRef]
- Harjinder, K.; Garg, P. The Clinical Benefit of Four-Dimensional Cardiac Magnetic Resonance Derived Left Ventricle Energetics: A Systematic Review; CRD42019117855; PROSPERO: York, UK, 2019; Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019117855 (accessed on 20 August 2020).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 21, e1000097. [Google Scholar] [CrossRef]
- Crandon, S.; Westenberg, J.J.; Swoboda, P.P.; Fent, G.J.; Foley, J.R.; Chew, P.G.; Brown, L.A.; Saunderson, C.; Al-Mohammad, A.; Greenwood, J.P.; et al. Impact of Age and Diastolic Function on Novel, 4D flow CMR Biomarkers of Left Ventricular Blood Flow Kinetic Energy. Sci. Rep. 2018, 26, 14436. [Google Scholar] [CrossRef]
- Garg, P.; Crandon, S.; Swoboda, P.P.; Fent, G.J.; Foley, J.R.; Chew, P.G.; Brown, L.A.; Vijayan, S.; Hassell, M.E.; Nijveldt, R.; et al. Left ventricular blood flow kinetic energy after myocardial infarction—insights from 4D flow cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reason. J. Soc. Cardiovasc. Magn. Reson. 2018, 30, 61. [Google Scholar] [CrossRef]
- Garg, P.; Van Der Geest, R.J.; Swoboda, P.P.; Crandon, S.; Fent, G.J.; Foley, J.R.; Dobson, L.E.; Al Musa, T.; Onciul, S.; Vijayan, S.; et al. Left ventricular thrombus formation in myocardial infarction is associated with altered left ventricular blood flow energetics. Eur. Heart J. Cardiovasc. Imaging 2018, 22, 108–117. [Google Scholar] [CrossRef]
- Carlsson, M.; Heiberg, E.; Toger, J.; Arheden, H. Quantification of left and right ventricular kinetic energy using four-dimensional intracardiac magnetic resonance imaging flow measurements. Am. J. Physiol. Heart Circ. Physiol. 2012, 15, H893–H900. [Google Scholar] [CrossRef] [PubMed]
- AlGhatrif, M.; Strait, J.B.; Morrell, C.H.; Canepa, M.; Wright, J.; Elango, P.; Scuteri, A.; Najjar, S.S.; Ferrucci, L.; Lakatta, E.G. Longitudinal trajectories of arterial stiffness and the role of blood pressure: The Baltimore Longitudinal Study of Aging. Hypertension 2013, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Föll, D.; Taeger, S.; Bode, C.; Jung, B.; Markl, M. Age, gender, blood pressure, and ventricular geometry influence normal 3D blood flow characteristics in the left heart. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 366–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eriksson, J.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Four-dimensional blood flow-specific markers of LV dysfunction in dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Chabiniok, R.; Devecchi, A.; Dedieu, N.; Sammut, E.; Schaeffter, T.; Razavi, R. Age-related changes in intraventricular kinetic energy: A physiological or pathological adaptation? Am. J. Physiol. Heart Circ. Physiol. 2016, 15, H747–H755. [Google Scholar] [CrossRef]
- De Souza, R.R. Aging of myocardial collagen. Biogerontology 2002, 3, 325–335. [Google Scholar] [CrossRef]
- Zajac, J.; Eriksson, J.; Dyverfeldt, P.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.-J. Turbulent kinetic energy in normal and myopathic left ventricles. J. Magn. Reason. Imaging 2015, 41, 1021–1029. [Google Scholar] [CrossRef]
- Steding-Ehrenborg, K.; Arvidsson, P.M.; Töger, J.; Rydberg, M.; Heiberg, E.; Carlsson, M.; Arheden, H. Determinants of kinetic energy of blood flow in the four-chambered heart in athletes and sedentary controls. Am. J. Physiol. Heart Circ. Physiol. 2016, H113–H122. [Google Scholar] [CrossRef]
- Chan, B.T.; Yeoh, H.K.; Liew, Y.M.; Dokos, S.; Al Abed, A.; Chee, K.H.; Abdul Aziz, Y.F.; Sridhar, G.S.; Chinna, K.; Lim, E. Quantitative analysis of intraventricular flow-energetics and vortex in ischaemic hearts. Coron. Artery Dis. 2018, 29, 316–324. [Google Scholar] [CrossRef]
- Svalbring, E.; Fredriksson, A.; Eriksson, J.; Dyverfeldt, P.; Ebbers, T.; Bolger, A.F.; Engvall, J.; Carlhäll, C.J. Altered Diastolic Flow Patterns and Kinetic Energy in Subtle Left Ventricular Remodeling and Dysfunction Detected by 4D Flow MRI. PLoS ONE 2016, 11, e0161391. [Google Scholar] [CrossRef]
- Zajac, J.; Eriksson, J.; Alehagen, U.; Ebbers, T.; Bolger, A.F.; Carlhäll, C.-J. Mechanical dyssynchrony alters left ventricular flow energetics in failing hearts with LBBB: A 4D flow CMR pilot study. Int. J. Cardiovasc. Imaging 2018, 34, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.; Dyverfeldt, P.; Engvall, J.; Bolger, A.F.; Ebbers, T.; Carlhäll, C.J. Quantification of presystolic blood flow organization and energetics in the human left ventricle. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2135–H2141. [Google Scholar] [CrossRef] [PubMed]
- Al-Wakeel, N.; Fernandes, J.F.; Amiri, A.; Siniawski, H.; Goubergrits, L.; Berger, F.; Kuehne, T. Hemodynamic and energetic aspects of the left ventricle in patients with mitral regurgitation before and after mitral valve surgery. J. Magn. Reason. Imaging 2015, 42, 1705–1712. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Method | N | Reproducibility | Disease | |
|---|---|---|---|---|---|---|
| Aging and LV diastolic function | ||||||
| 1 | Wong et al. | 2016 | LV KE | 45 | − | Aging and LV diastolic assessment |
| 2 | Crandon et al. | 2018 | LV KE | 53 | + | Aging and LV diastolic assessment |
| 3 | Zajac et el. | 2015 | LV TKE | 20 | − | LV diastolic assessment |
| 4 | Steding-Ehrenborg et al. | 2015 | LV KE | 28 | − | Athletes vs. sedentary |
| 5 | Carlsson et al. | 2011 | LV KE | 9 | + | Healthy—mechanistic |
| 6 | Kim et al. | 1995 | Vortex | 26 | + | Healthy—mechanistic |
| Ischemic heart disease and myocardial infarction | ||||||
| 7 | Garg et al. | 2018 | LV KE MI | 58 | + | MI |
| 8 | Garg et al. | 2018 | LV KE thrombus | 108 | + | LV thrombus |
| 9 | Chan et al. | 2018 | LV KE and vortex | 50 | − | STEMI |
| Heart failure including cardiomyopathies | ||||||
| 10 | Svalbring et al. | 2016 | LV DF KE | 36 | − | HFrEF |
| 11 | Eriksson et al. | 2012 | LV PT + KE | 20 | − | DCM |
| 12 | Kanski et al. | 2015 | LV KE | 41 | − | HFrEF |
| 13 | Zajac et al. | 2017 | LV PT + KE | 22 | − | HF with LBBB |
| 14 | Eriksson et al. | 2011 | LV PT + KE | 13 | − | DCM |
| 15 | Bolger et al. | 2007 | LV PT + KE | 18 | − | DCM |
| Valvular heart disease | ||||||
| 16 | Al-Wakeel et al. | 2015 | LV KE in MR | 17 | − | MV—mitral regurgitation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Assadi, H.; Alabed, S.; Cameron, D.; Vassiliou, V.S.; Westenberg, J.J.M.; van der Geest, R.; Zhong, L.; Dastidar, A.; Swift, A.J.; et al. Left Ventricular Blood Flow Kinetic Energy Assessment by 4D Flow Cardiovascular Magnetic Resonance: A Systematic Review of the Clinical Relevance. J. Cardiovasc. Dev. Dis. 2020, 7, 37. https://doi.org/10.3390/jcdd7030037
Kaur H, Assadi H, Alabed S, Cameron D, Vassiliou VS, Westenberg JJM, van der Geest R, Zhong L, Dastidar A, Swift AJ, et al. Left Ventricular Blood Flow Kinetic Energy Assessment by 4D Flow Cardiovascular Magnetic Resonance: A Systematic Review of the Clinical Relevance. Journal of Cardiovascular Development and Disease. 2020; 7(3):37. https://doi.org/10.3390/jcdd7030037
Chicago/Turabian StyleKaur, Harjinder, Hosamadin Assadi, Samer Alabed, Donnie Cameron, Vassilios S. Vassiliou, Jos J. M. Westenberg, Rob van der Geest, Liang Zhong, Amardeep Dastidar, Andrew J. Swift, and et al. 2020. "Left Ventricular Blood Flow Kinetic Energy Assessment by 4D Flow Cardiovascular Magnetic Resonance: A Systematic Review of the Clinical Relevance" Journal of Cardiovascular Development and Disease 7, no. 3: 37. https://doi.org/10.3390/jcdd7030037
APA StyleKaur, H., Assadi, H., Alabed, S., Cameron, D., Vassiliou, V. S., Westenberg, J. J. M., van der Geest, R., Zhong, L., Dastidar, A., Swift, A. J., & Garg, P. (2020). Left Ventricular Blood Flow Kinetic Energy Assessment by 4D Flow Cardiovascular Magnetic Resonance: A Systematic Review of the Clinical Relevance. Journal of Cardiovascular Development and Disease, 7(3), 37. https://doi.org/10.3390/jcdd7030037





