Pseudoaneurysm Development after Free Wall Rupture Post Myocardial Infarction
Abstract
:1. Introduction
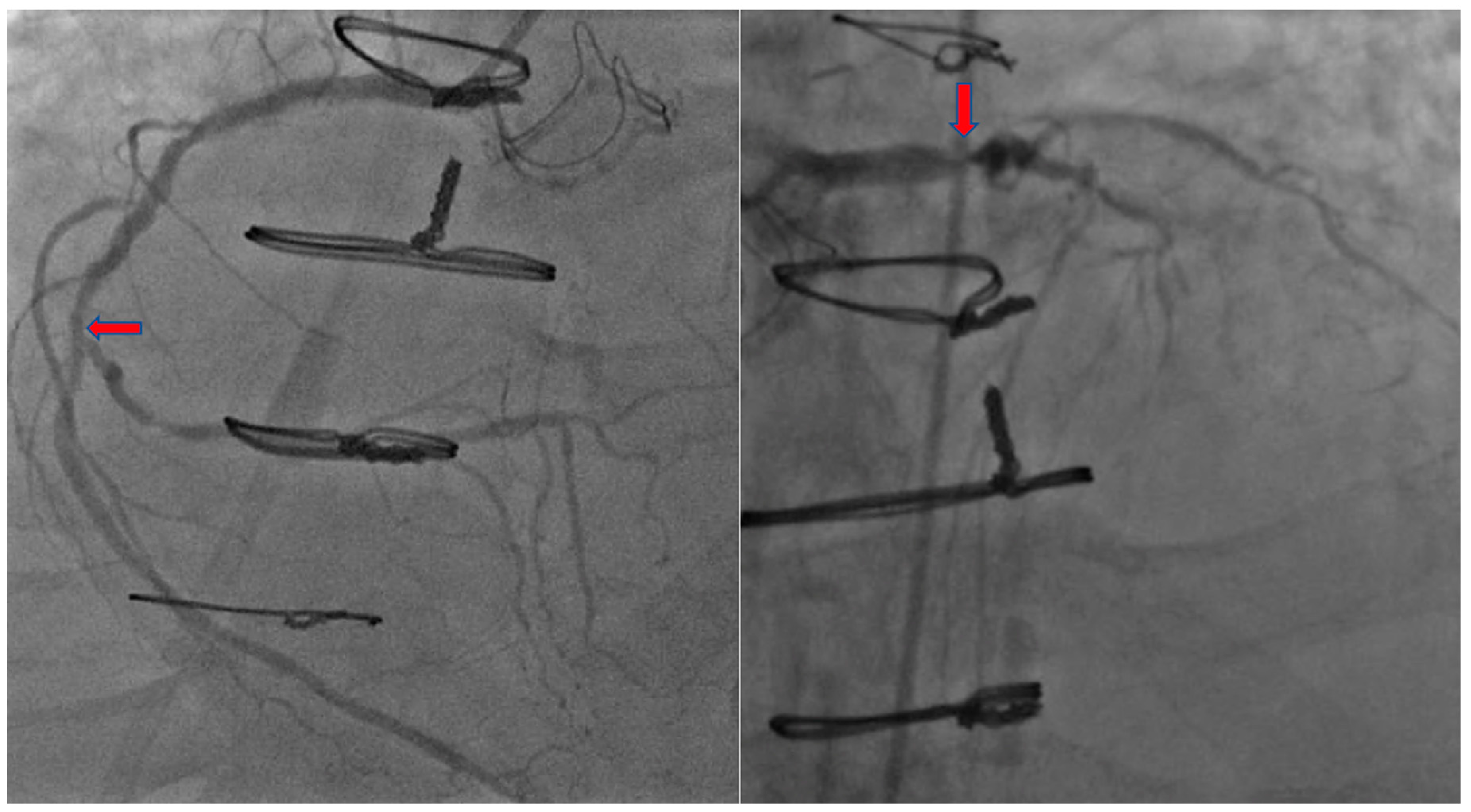
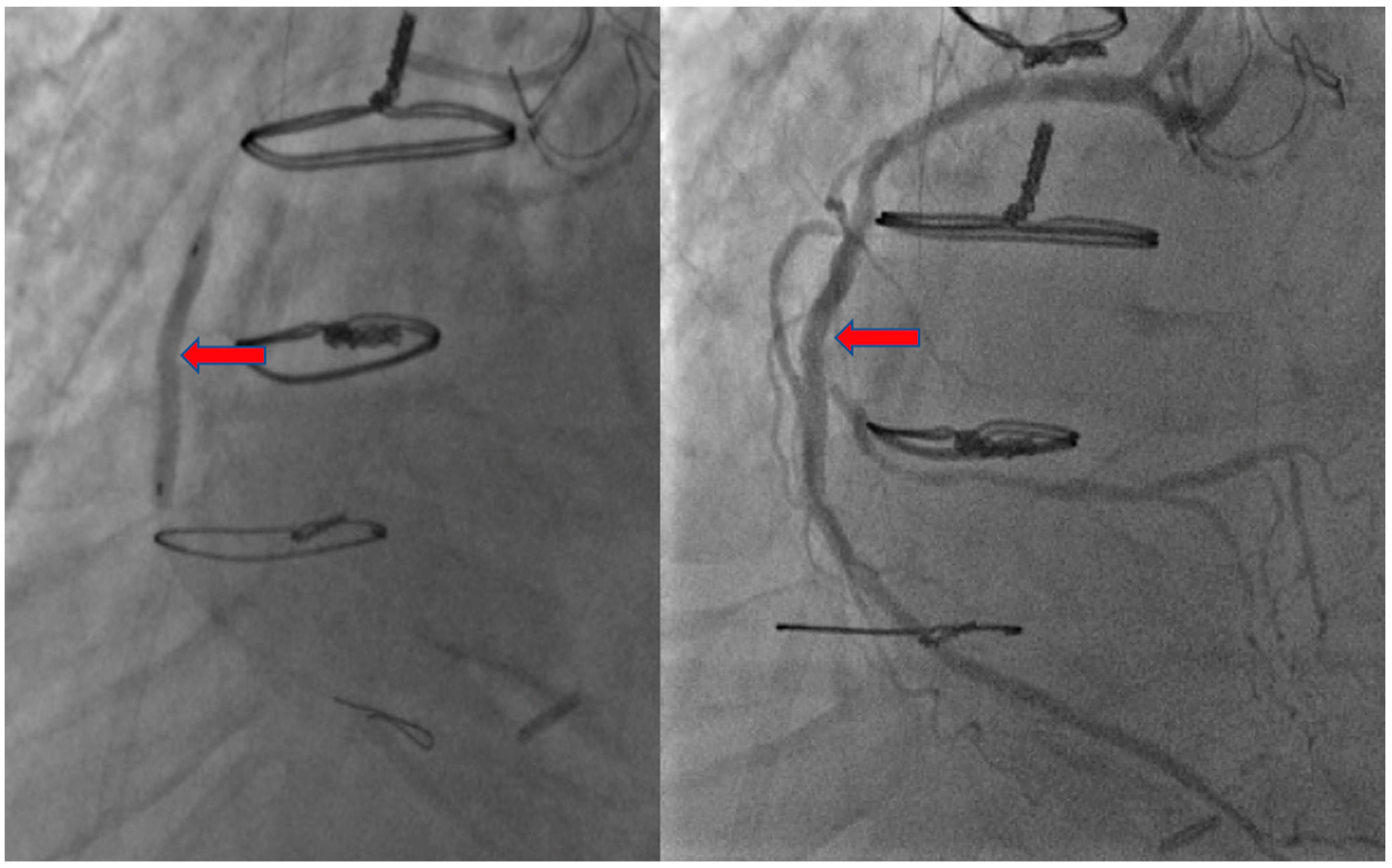
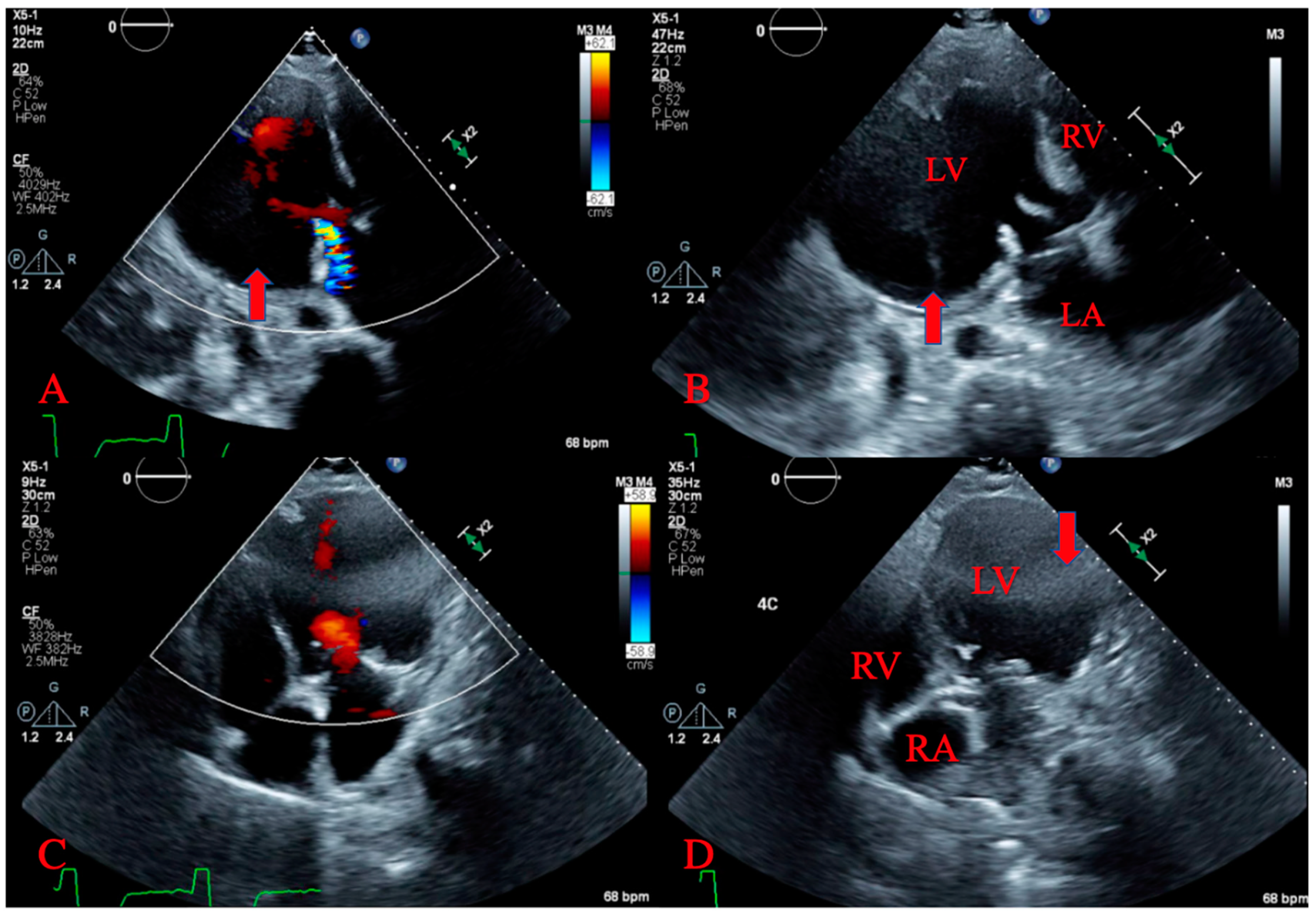
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Informed Consent
References
- Montrief, T.; Davis, W.T.; Koyfman, A.; Long, B. Mechanical, inflammatory, and embolic complications of myocardial infarction: An emergency medicine review. Am. J. Emerg. Med. 2019, 37, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergiya, R.; Gopi, A. A large left ventricular pseudo-aneurysm. Indian Hear. J. 2015, 67, 271–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorhees, A.P.; Han, H.-C. Biomechanics of Cardiac Function. Compr. Physiol. 2015, 5, 1623–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, A.; Sethi, A.; Rathor, P.; Suppogu, N.; Sethi, A. Acute Complications of Myocardial Infarction in the Current Era. J. Investig. Med. 2015, 63, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sendon, J.; Gurfinkel, E.P.; De Sa, E.L.; Agnelli, G.; Gore, J.M.; Steg, P.G.; Eagle, K.A.; Cantador, J.R.; Fitzgerald, G.; Granger, C.B.; et al. Factors related to heart rupture in acute coronary syndromes in the Global Registry of Acute Coronary Events. Eur. Hear. J. 2010, 31, 1449–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieczorek, J.; Mizia-Stec, K.; Rybicka-Musialik, A.; Janusiewicz, P.; Malinowski, M.; Deja, M.A. A large pseudoaneurysm of the left cardiac ventricle in a 57-year-old patient after urgent coronary artery bypass grafting and surgical mitral valve replacement due to acute myocardial infarction. Kardiochirurgia i Torakochirurgia Polska Polish J. Cardiol.-Thoracic Surg. 2014, 11, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergiya, R.; Kumar, A.; Rana, S.S.; Singh, H.; Puri, G.D.; Singhal, M. Post-myocardial infarction giant left ventricular pseudoaneurysm presenting with severe heart failure. World J. Cardiol. 2012, 4, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Soud, M.; Pacha, H.M.; Hritani, R.; Alraies, M.C. Post myocardial infarction left ventricular pseudoaneurysm. Cardiovasc. Revascularization Med. 2018, 19, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Mujanovic, E.; Bergsland, J.; Avdic, S.; Stanimirovic-Mujanovic, S.; Kovacevic-Preradovic, T.; Kabil, E. Surgical Treatment of Left Ventricular Pseudoaneurysm. Reliabil. Secur. Authent. Meta Med. Image Arch. Integr. Healthc. Enterp. 2014, 68, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catherwood, E.; Mintz, G.S.; Kotler, M.N.; Parry, W.R.; Segal, B.L. Two-dimensional echocardiographic recognition of left ventricular pseudoaneurysm. Circulation 1980, 62, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulten, E.; Blankstein, R. Pseudoaneurysms of the Heart. Circulation 2012, 125, 1920–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blażejewski, J.; Sinkiewicz, W.; Bujak, R.; Banach, J.; Karasek, D.; Balak, W. Giant post-infarction pseudoaneurysm of the left ventricle manifesting as severe heart failure. Kardiologia Polska 2012, 70, 85–87. [Google Scholar] [PubMed]
- Faustino, M.; Ranchordás, S.; Abecasis, J.; Freitas, A.; Ferreira, M.; Gil, V.; Morais, C.; Neves, J.F. Left ventricular pseudoaneurysm—A challenging diagnosis. Rev. Port. Cardiol. 2016, 35, 373.e1–373.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frances, C.; Romero, A.; Grady, D. Left ventricular pseudoaneurysm. J. Am. Coll. Cardiol. 1998, 32, 557–561. [Google Scholar] [CrossRef] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douedi, S.; Alfraji, N.; Upadhyaya, V.D.; Odak, M.; Meleka, M.; Raza, M.R. Pseudoaneurysm Development after Free Wall Rupture Post Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2020, 7, 34. https://doi.org/10.3390/jcdd7030034
Douedi S, Alfraji N, Upadhyaya VD, Odak M, Meleka M, Raza MR. Pseudoaneurysm Development after Free Wall Rupture Post Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2020; 7(3):34. https://doi.org/10.3390/jcdd7030034
Chicago/Turabian StyleDouedi, Steven, Nasam Alfraji, Vandan D. Upadhyaya, Mihir Odak, Matthew Meleka, and Muhammad R. Raza. 2020. "Pseudoaneurysm Development after Free Wall Rupture Post Myocardial Infarction" Journal of Cardiovascular Development and Disease 7, no. 3: 34. https://doi.org/10.3390/jcdd7030034
APA StyleDouedi, S., Alfraji, N., Upadhyaya, V. D., Odak, M., Meleka, M., & Raza, M. R. (2020). Pseudoaneurysm Development after Free Wall Rupture Post Myocardial Infarction. Journal of Cardiovascular Development and Disease, 7(3), 34. https://doi.org/10.3390/jcdd7030034




