Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Jugular Vein Arterialization
2.2. Human Saphenous Vein
2.3. Ex Vivo Culture of Human Saphenous Vein
2.4. IL-1β Polymorphism Determination
2.5. Gene Expression Analysis
2.6. Immunohistochemistry
2.7. Statistical Analysis
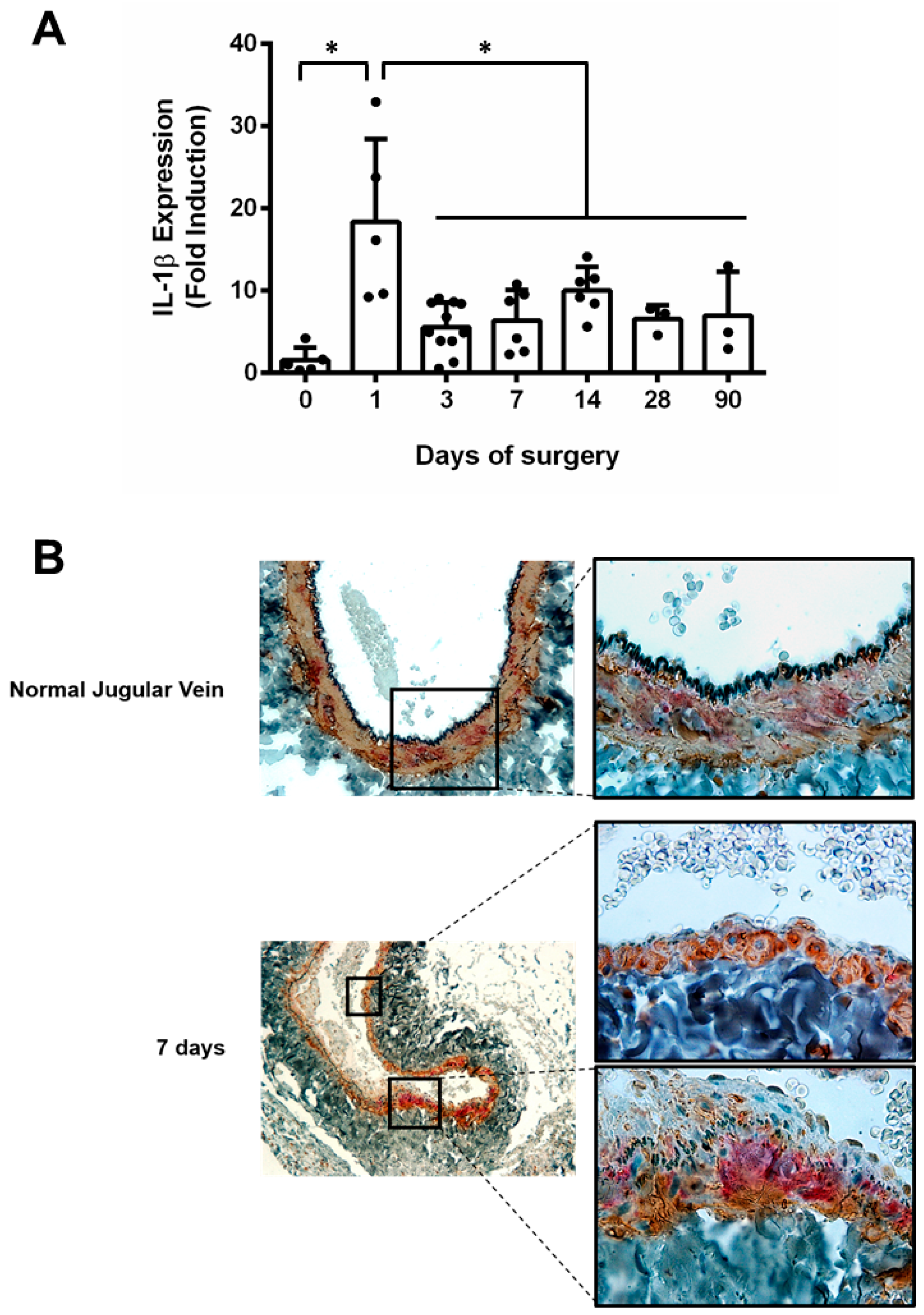
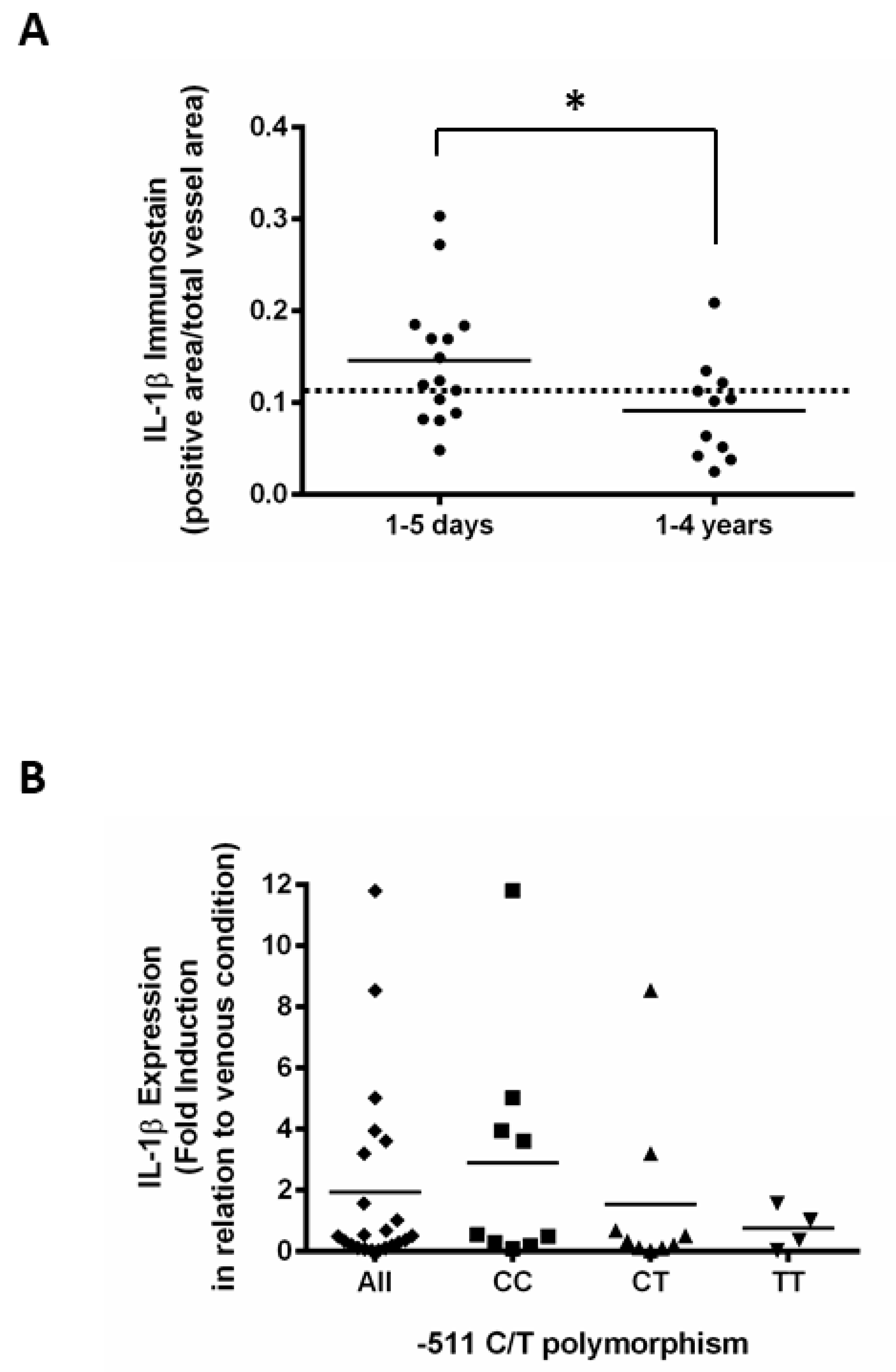
3. Result
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Owens, C.D.; Gasper, W.J.; Rahman, A.S.; Conte, M.S. Vein graft failure. J. Vasc. Surg. 2015, 61, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Inflammation or Atherogenesis. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.O.; Caputo, M.; Angelini, G.D.; George, S.J.; Zakkar, M. Activation and inflammation of the venous endothelium in vein graft disease. Atherosclerosis 2017, 265, 266–274. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.R.; Quax, P.H.A. Inflammation in Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 1–13. [Google Scholar] [CrossRef]
- Yayla, Ç.; Canpolat, U.; Akyel, A.; Yayla, K.G.; Yilmaz, S.; Açikgöz, S.K.; Özcan, F.; Turak, O.; Doʇan, M.; Yeter, E.; et al. Association between platelet to lymphocyte ratio and saphenous vein graft disease. Angiology 2016, 67, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kirii, H.; Niwa, T.; Yamada, Y.; Wada, H.; Saito, K.; Iwakura, Y.; Asano, M.; Moriwaki, H.; Seishima, M. Lack of interleukin-1ß decreases the severity of atherosclerosis in apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 656–660. [Google Scholar] [CrossRef]
- Thusen, J.H. Von; Kuiper, J.; van Berkel, T.J.C.; Biessen, E.A.L. Interleukins in Atherosclerosis: Molecular Pathways. Pharmacol. Rev. 2003, 55, 133–166. [Google Scholar] [CrossRef]
- Jiang, Z.; Berceli, S.A.; Pfahnl, C.L.; Wu, L.; Goldman, D.; Tao, M.; Kagayama, M.; Matsukawa, A.; Ozaki, C.K. Wall shear modulation of cytokines in early vein grafts. J. Vasc. Surg. 2004, 40, 345–350. [Google Scholar] [CrossRef]
- Wang, X.; Romanic, A.M.; Yue, T.L.; Feuerstein, G.Z.; Ohlstein, E.H. Expression of interleukin-1β, interleukin-1 receptor, and interleukin-1 receptor antagonist mRNA in rat carotid artery after balloon angioplasty. Biochem. Biophys. Res. Commun. 2000, 271, 138–143. [Google Scholar] [CrossRef]
- Ridker, P.M. Canakinumab for Residual Inflammatory Risk. Eur. Heart J. 2017, 38, 3545–3548. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2017, 319–328. [Google Scholar] [CrossRef]
- Yu, P.; Nguyen, B.T.; Tao, M.; Jiang, T.; Mauro, C.R.; Wang, Y.; Ozaki, C.K. Lack of interleukin-1 signaling results in perturbed early vein graft wall adaptations. Surgery 2013, 153, 63–69. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christiansen, J.F.; Hartwig, D.; Bechtel, J.F.M.; Klüter, H.; Sievers, H.H.; Schönbeck, U.; Bartels, C. Diseased vein grafts express elevated inflammatory cytokine levels compared with atherosclerotic coronary arteries. Ann. Thorac. Surg. 2004, 77, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Borin, T.F.; Miyakawa, A.A.; Cardoso, L.; De Figueiredo Borges, L.; Gonçalves, G.A.; Krieger, J.E. Apoptosis, cell proliferation and modulation of cyclin-dependent kinase inhibitor p21cip1in vascular remodelling during vein arterialization in the rat. Int. J. Exp. Pathol. 2009, 90, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, A.A.; Dallan, L.A.O.; Lacchini, S.; Borin, T.F.; Krieger, J.E. Human saphenous vein organ culture under controlled hemodynamic conditions. Clinics 2008, 63, 683–688. [Google Scholar] [CrossRef]
- Murillo, L.S.; Land, J.A.; Pleijster, J.; Bruggeman, C.A.; Salvador Peña, A.; Morré, S.A. Interleukin-1B (IL-1B) and interleukin-1 receptor antagonist (IL-1RN) gene polymorphisms are not associated with tubal pathology and Chlamydia trachomatis-related tubal factor subfertility. Hum. Reprod. 2003, 18, 2309–2314. [Google Scholar] [CrossRef][Green Version]
- Faries, P.L.; Marin, M.L.; Veith, F.J.; Ramirez, J.A.; Suggs, W.D.; Parsons, R.E.; Sanchez, L.A.; Lyon, R.T. Immunolocalization and temporal distribution of cytokine expression during the development of vein graft intimal hyperplasia in an experimental model. J. Vasc. Surg. 1996, 24, 463–471. [Google Scholar] [CrossRef]
- Rogus, J.; Beck, J.D.; Offenbacher, S.; Huttner, K.; Iacoviello, L.; Latella, M.C.; Gaetano, M.; Wang, H.Y.; Kornman, K.S.; Duff, G.W. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1β and C-reactive protein. Hum. Genet. 2008, 123, 387–398. [Google Scholar] [CrossRef]
- Iacoviello, L.; Di Castelnuovo, A.; Gattone, M.; Pezzini, A.; Assanelli, D.; Lorenzet, R.; Del Zotto, E.; Colombo, M.; Napoleone, E.; Amore, C.; et al. Polymorphisms of the interleukin-1β gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 222–227. [Google Scholar] [CrossRef]
- Huang, G.; Niu, T.; Peng, S.; Ling, D.; Liu, J.; Zhang, X.; Xu, X. Association between the interleukin-1β C(-511)T polymorphism and blood pressure in a Chinese hypertensive population. Immunol. Lett. 2004, 91, 159–162. [Google Scholar] [CrossRef]
- Marculescu, R.; Mlekusch, W.; Exner, M.; Sabeti, S.; Michor, S.; Rumpold, H.; Mannhalter, C.; Minar, E.; Wagner, O.; Schillinger, M. Interleukin-1 cluster combined genotype and restenosis after balloon angioplasty. Thromb. Haemost. 2003, 90, 491–500. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Epstein, H.; Grad, E.; Golomb, M.; Koroukhov, N.; Edelman, E.R.; Golomb, G.; Danenberg, H.D. Innate immunity has a dual effect on vascular healing: Suppression and aggravation of neointimal formation and remodeling post-endotoxin challenge. Atherosclerosis 2008, 199, 41–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomez, D.; Baylis, R.A.; Durgin, B.G.; Newman, A.A.C.; Alencar, G.F.; Mahan, S.; St Hilaire, C.; Müller, W.; Waisman, A.; Francis, S.E.; et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 2018, 24, 1418–1429. [Google Scholar] [CrossRef]
- Baylis, R.A.; Gomez, D.; Mallat, Z.; Pasterkamp, G.; Owens, G.K. The CANTOS trial one important step for clinical cardiology but a giant leap for vascular biology. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e174–e177. [Google Scholar] [CrossRef]
- Muehlschlegel, J.D.; Liu, K.Y.; Perry, T.E.; Fox, A.A.; Collard, C.D.; Shernan, S.K.; Body, S.C. Chromosome 9p21 variant predicts mortality after coronary artery bypass graft surgery. Circulation 2010, 122, 60–65. [Google Scholar] [CrossRef]
- Body, S.C.; Collard, C.D.; Shernan, S.K.; Fox, A.A.; Liu, K.Y.; Ritchie, M.D.; Perry, T.E.; Muehlschlegel, J.D.; Aranki, S.; Donahue, B.S.; et al. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ. Cardiovasc. Genet. 2009, 2, 499–506. [Google Scholar] [CrossRef]
- Toumpoulis, I.K.; Anagnostopoulos, C.E.; DeRose, J.J.; Swistel, D.G. European system for cardiac operative risk evaluation predicts long-term survival in patients with coronary artery bypass grafting. Eur. J. Cardio-Thorac. Surg. 2004, 25, 51–58. [Google Scholar] [CrossRef][Green Version]
- Nashef, S.A.M.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardio-Thorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Fukui, T.; Uchimuro, T.; Takanashi, S. EuroSCORE II with SYNTAX score to assess risks of coronary artery bypass grafting outcomes. Eur. J. Cardiothorac. Surg. 2015, 47, 66–71. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyakawa, A.A.; Borin, T.F.; Campos, L.C.G.; Girão-Silva, T.; Ribeiro-Silva, J.C.; Dallan, L.A.O.; Krieger, J.E. Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism. J. Cardiovasc. Dev. Dis. 2019, 6, 20. https://doi.org/10.3390/jcdd6020020
Miyakawa AA, Borin TF, Campos LCG, Girão-Silva T, Ribeiro-Silva JC, Dallan LAO, Krieger JE. Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism. Journal of Cardiovascular Development and Disease. 2019; 6(2):20. https://doi.org/10.3390/jcdd6020020
Chicago/Turabian StyleMiyakawa, Ayumi Aurea, Thaiz Ferraz Borin, Luciene Cristina Gastalho Campos, Thais Girão-Silva, Joao Carlos Ribeiro-Silva, Luis Alberto Oliveira Dallan, and Jose Eduardo Krieger. 2019. "Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism" Journal of Cardiovascular Development and Disease 6, no. 2: 20. https://doi.org/10.3390/jcdd6020020
APA StyleMiyakawa, A. A., Borin, T. F., Campos, L. C. G., Girão-Silva, T., Ribeiro-Silva, J. C., Dallan, L. A. O., & Krieger, J. E. (2019). Activation of Interleukin-1 Beta in Arterialized Vein Grafts and the Influence of the -511C/T IL-1β Gene Polymorphism. Journal of Cardiovascular Development and Disease, 6(2), 20. https://doi.org/10.3390/jcdd6020020




