1. Introduction
In a recent review published in the Journal, Poelmann and Gittenberger de Groot discussed in depth the influence of hemodynamics on cardiac development [
1]. When describing their experiments, they explained how ligating the right vitelline vein in the developing chick embryo induced the formation of congenital cardiac malformations, including ventricular septal defects. Their findings revealed that the deficient ventricular septation was the result of failure of fusion and muscularisation of the proximal outflow cushions. In this context, they then showed that although the proximal components had failed to fuse, the aortic and pulmonary roots, along with the intrapericardial arterial trunks, had properly separated one from the other. In their discussion, they alluded to the fact that the structures separating the components of the outflow tract at one stage had been described as the “aortopulmonary septal complex” [
2]. They then indicated that the more appropriate term was “outflow tract septal complex”. In their subsequent discussion, they provide an exemplary account of the patterns of gene expression involved in mechanosensing and relate these changes to their experimental findings. They do not, however, discuss the potential clinical significance of their appropriate change in terminology from “aortopulmonary septal complex” to “outflow tract septal complex”. Although seemingly a minor change, its implications are crucial for the discussions which are ongoing regarding the most appropriate categorisation of ventricular septal defects when observed in the clinical situation [
3]. In our review, therefore, we point to the significance of appropriate understanding of the separation of the components of the developing outflow tract. We emphasise that in the postnatal setting, there are no septal structures interposing between the cavities of the right and left ventricular outflow tracts. These findings regarding the structure of the normal heart are based on the availability of computed tomographic datasets from otherwise normal individuals undergoing assessment at Kobe University for suspected coronary arterial disease. We have recently used these datasets to provide an extensive account of the overall anatomy of the normal heart, including again details of the structural arrangement of the outflow tracts [
4]. We have also examined in detail the normal heart specimens held in the cardiac archive of Cincinnati Children’s Hospital. We interrogated one heart using high-resolution magnetic resonance imaging prior to its subsequent dissection. As we show, this permitted us to demonstrate the precise relationships of the components of the ventricular mass. Our findings related to cardiac development are based on our analysis of a large number of datasets obtained from developing mice and prepared using episcopic microscopy [
5]. For the key stages of development, involving the period from embryonic days 11.5 through 14.5, we have access to at least 40 datasets for each day of murine development. These datasets were all prepared by Dr Timothy Mohun, and we are grateful to him for making them available to us for these studies. The details regarding the datasets are provided on the website of “Deciphering the Mechanisms of Developmental Disorders” (DMDD). As explained by Dr Mohun on his own website, “
all DMDD data is available to view and study online via our database, which is an ever-expanding resource for developmental biologists and clinicians”. 2. Significance of Developmental Concepts to Categorisation of Ventricular Septal Defects
One of the systems currently used for the categorisation of ventricular septal defects [
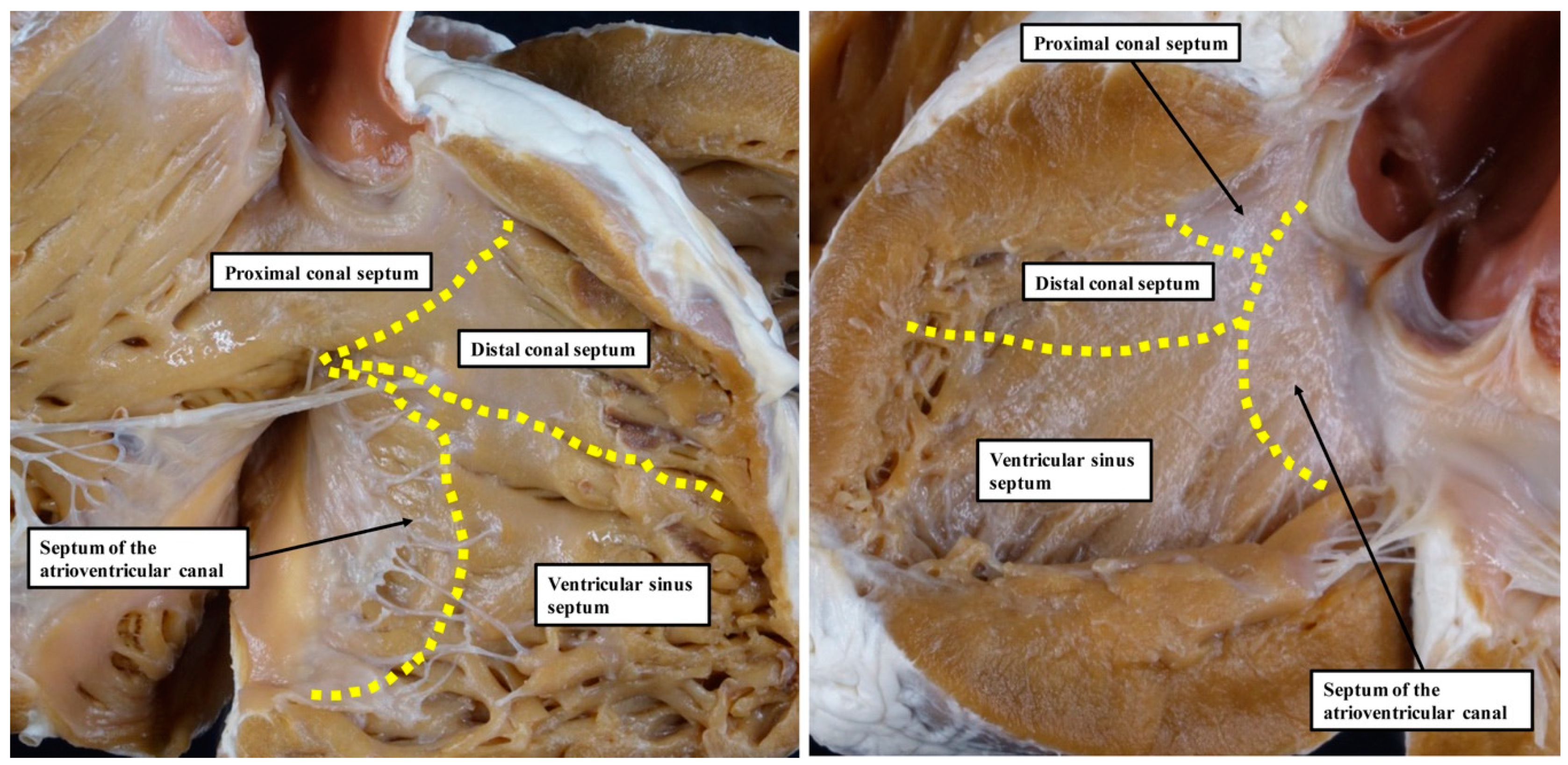
6] is based on the notion that, during development, the ventricular septum is derived from four different sources. These components were described as the septum of the atrioventricular canal, the ventricular sinus septum, and the proximal and distal components of the outlet septum (
Figure 1).
Our own studies, based on extensive examination of normal hearts in the autopsy room (
Figure 2), do not support these interpretations.
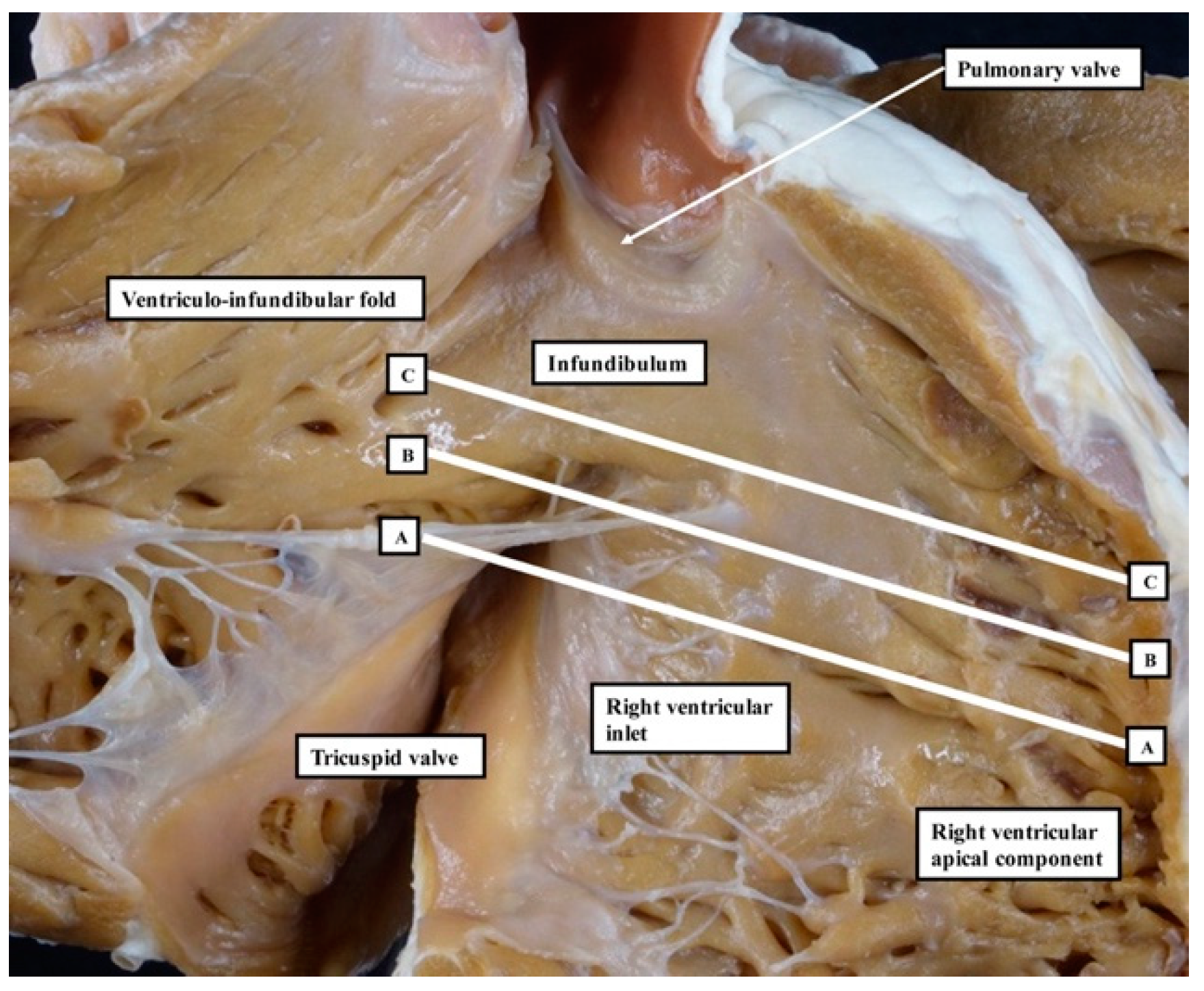
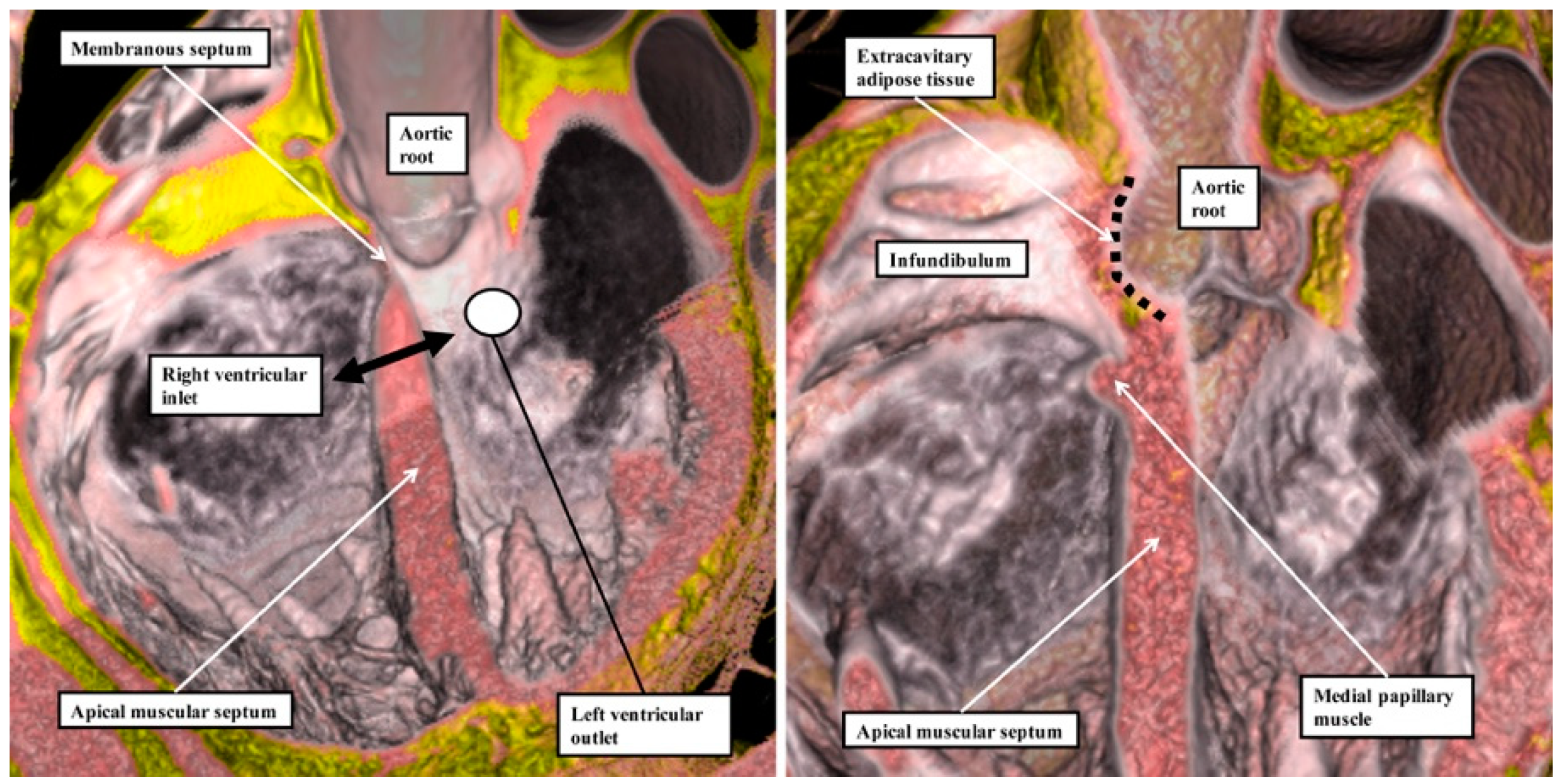
We have now validated our examinations made on the basis of dissection by using high-resolution magnetic resonance imaging of an intact autopsy specimen prior to its subsequent dissection. Thus, in
Figure 3, we show the view of the right ventricle of the same heart we used to produce
Figure 1. In
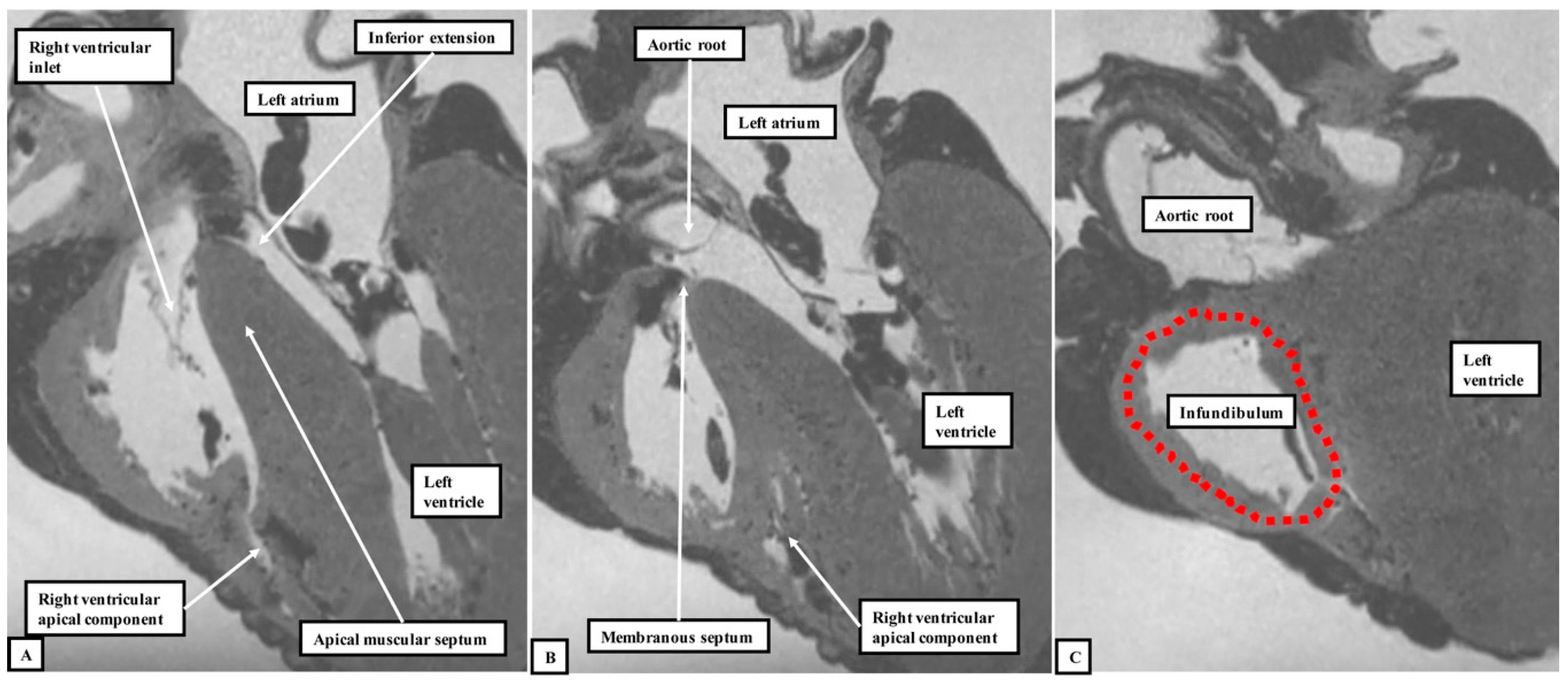
Figure 4, we show sectional images through the heart obtained from the three-dimensional dataset of the heart produced by interrogation using a 7 Tesla magnetic resonance scanner prior to dissection.
We then provided further information regarding the inter-relationships of the ventricular components by interrogating computed tomographic datasets obtained during life from individuals undergoing assessment for suspected coronary arterial disease (
Figure 5).
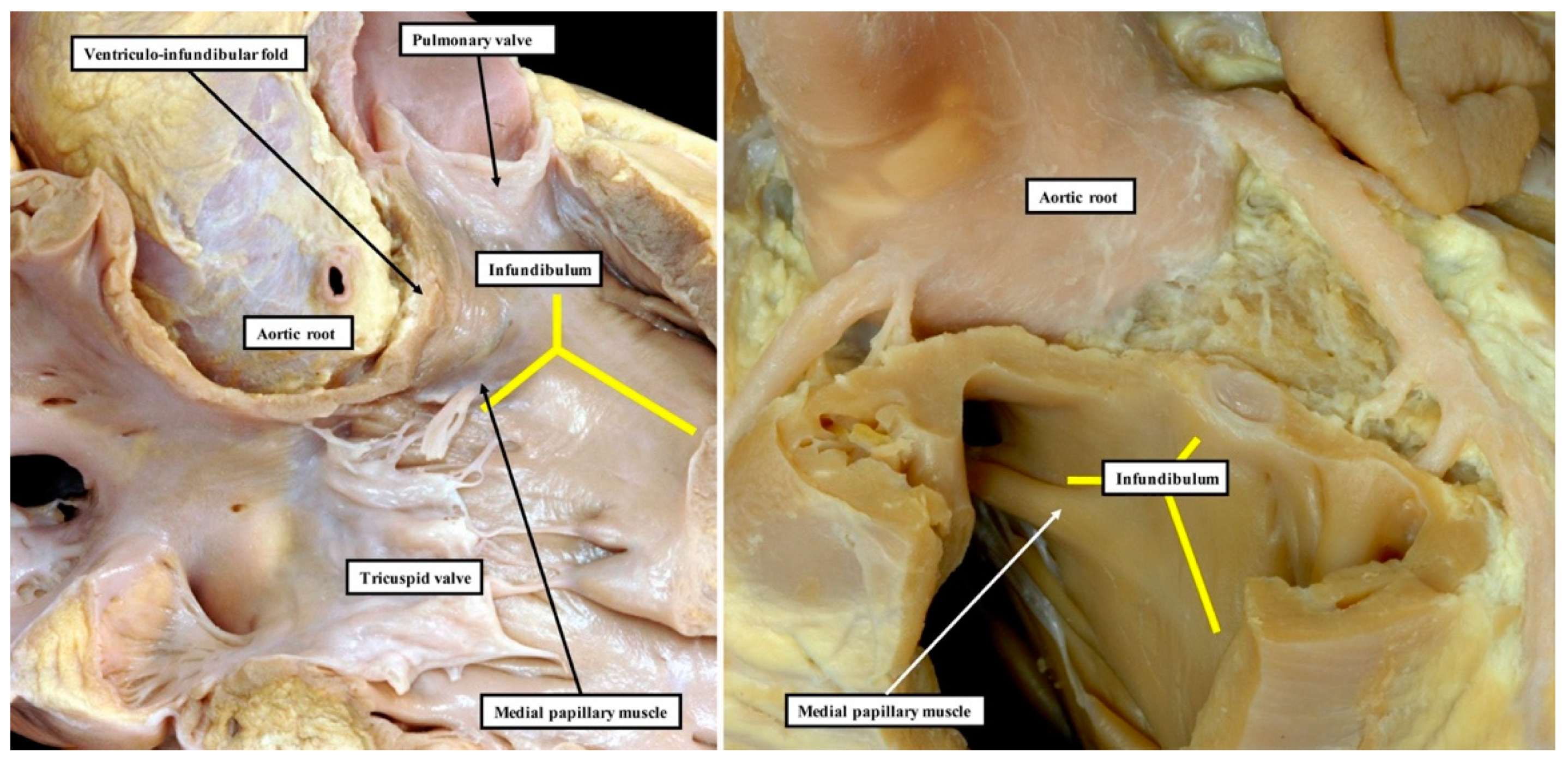
The images confirm that there is no “outlet septum” to be found in the normal heart. Instead, it is a free-standing infundibular sleeve which lifts the leaflets of the pulmonary valve away from the base of the left ventricle (
Figure 4C). It is the presence of this infundibular sleeve that makes possible the surgical procedure known as the Ross operation [
7]. The images also show that there is no “septum of the atrioventricular canal” interposed between the inlets of the right and left ventricles. The inferior component of the muscular ventricular septum, by virtue of the wedged location of the aortic root, interposes between the inlet of the right ventricle and the outlet component of the left ventricle.
It is the lack of any “outlet septum” in the normal heart that is directly relevant to the appropriate change in terminology from “aortopulmonary septal complex” to “outflow tract septal complex” as proposed by Poelmann and Gittenberger-de Groot [
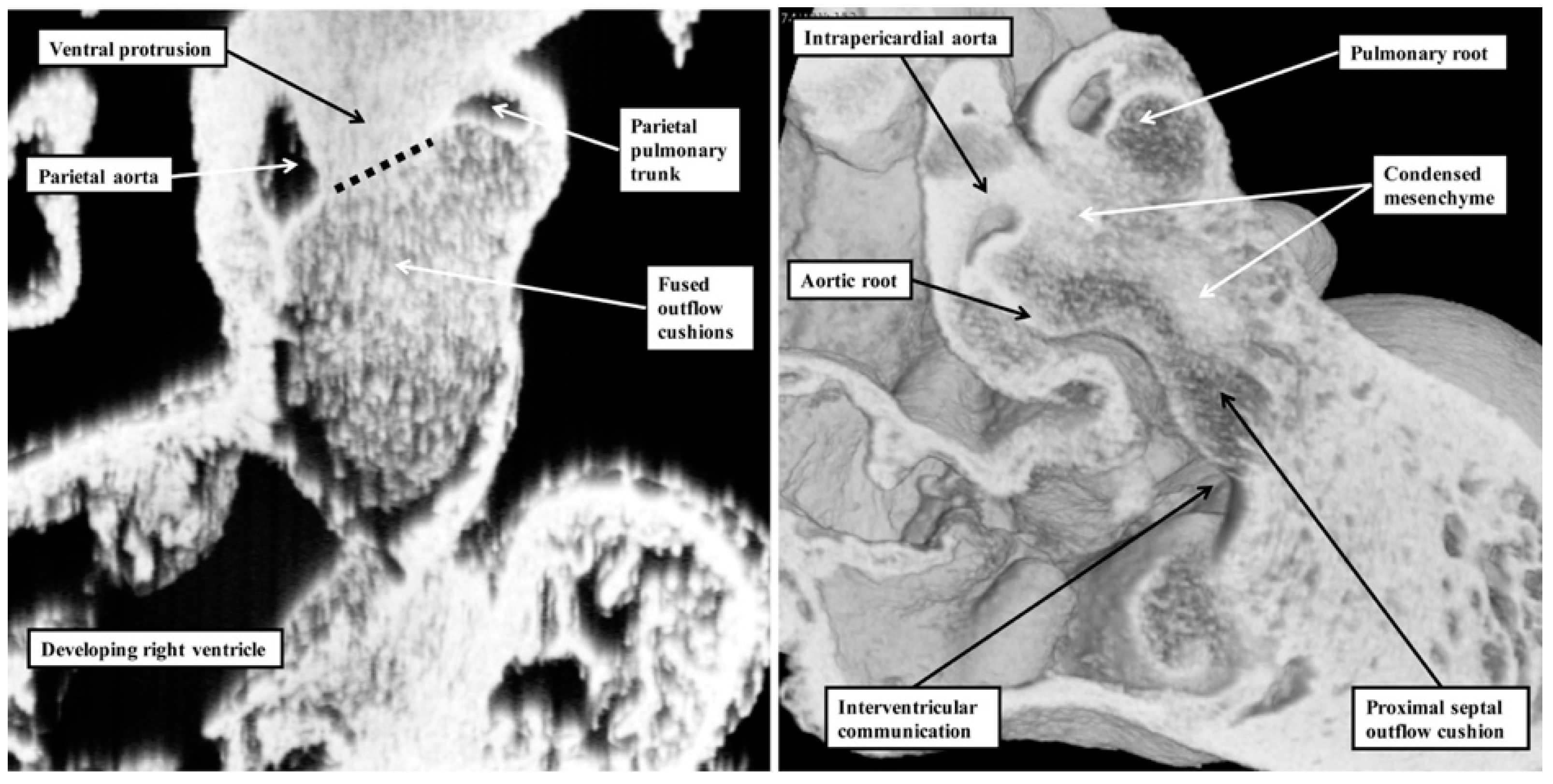
1]. Our investigation of datasets prepared from developing mice reveals that the real aortopulmonary septum separates only the intrapericardial arterial trunks (
Figure 6).
With ongoing development, it is the outflow cushions, rather than the aortopulmonary septum, which fuse to separate the developing aortic and pulmonary roots and the ventricular outflow tracts. Although initially having a septal function, these structures cease to interpose between the outflow channels subsequent to closure of the embryonic interventricular foramen. This is because, once separated, the aortic and pulmonary roots develop their own discrete walls, with no septal structures interposing between them. With the passage of time, the proximal parts of the outflow cushions also fuse and then muscularise. Subsequent to closure of the interventricular communication, the muscularised entities lose their septal function, becoming transformed into the free-standing muscular infundibular sleeve. Thus, by the time of birth, there are no septal entities interposing between the entire lengths of the right and left ventricular outflow channels. This means that although the structures are appropriately described as the “outflow tract septal complex” during the period of intrauterine development [
1], they can no longer accurately be described in this fashion in postnatal life. As we will show, nonetheless, the tissues derived by muscularisation of the proximal outflow can still be recognised as a myocardial outlet septum in the setting of deficient ventricular septation.
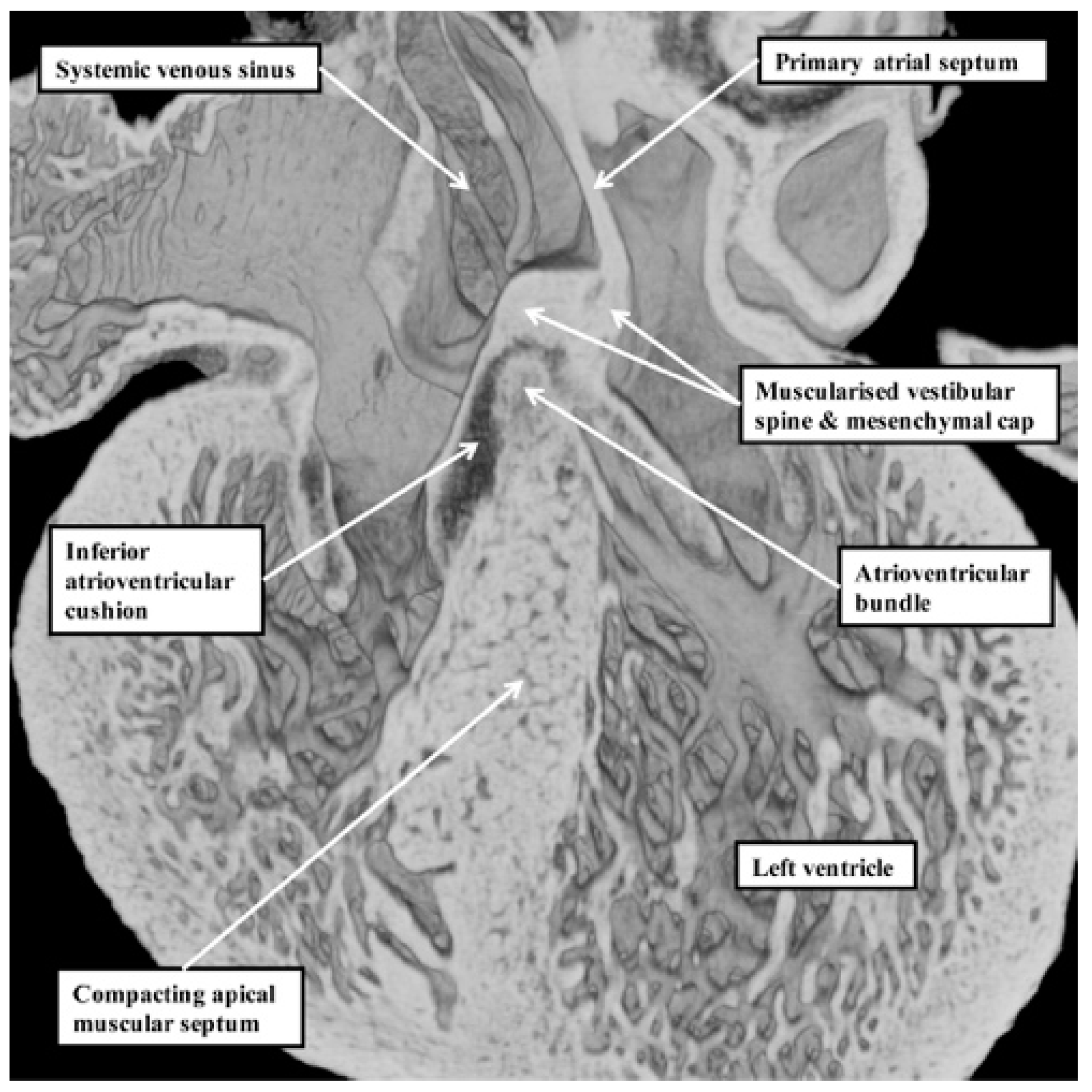
Our developmental findings are also pertinent to the suggestion that there is within the normal muscular ventricular septum a component derived from “the septum of the atrioventricular canal” [
6]. It is now well established that such a septal component is, indeed, key to the separation of the initially common atrioventricular canal into the right and left atrioventricular junctions. This entity is the vestibular spine. Along with the mesenchymal cap carried on the leading edge of the primary atrial septum, the spine is muscularised to form the antero-inferior buttress of the atrial septum [
8]. The spine was initially described in the 19th century [
9], when it was called the “spina vestibuli”. Its importance was re-discovered by Snarr and colleagues, who described the entity as the dorsal mesenchymal protrusion [
10]. Subsequent to the completion of septation, however, the myocardialised entities form part of the atrial, rather than the ventricular, septum. It is the location of the atrioventricular bundle, sandwiched between the crest of the muscular ventricular septum and the insulating tissues of the atrioventricular junctions, which confirms that in the normal heart, there is no ventricular “septum of the atrioventricular canal” (
Figure 7) [
11].
3. Discussion
Poelmann and Gittenberger-de Groot [
1] are correct in asserting that the so-called “aortopulmonary septal complex” is better termed the “outflow tract septal complex”. This fact is of particular importance for the understanding of postnatal anatomy and has significance for the categorisation of ventricular septal defects [
3]. This is because when development proceeds normally, the entities which divide the developing outflow tract, and which initially have a septal location, subsequently lose this septal function. This occurs concomitantly with the development of the discrete walls of the intrapericardial arterial trunks, the separation of the aortic and pulmonary roots, and the formation of the free-standing muscular subpulmonary infundibulum. As suggested above, these findings have implications far beyond the understanding of normal postnatal anatomy. The fact that the proximal outflow cushions initially muscularise to produce a septum between the components of the proximal outflow tract, but that with normal development the muscularised tissues subsequently become the free-standing muscular subpulmonary infundibulum, is key to arbitrating ongoing discussions regarding the optimal means of categorising ventricular septal defects in the clinical setting [
3]. Thus, the findings show that the notion that the normal muscular ventricular septum has a component derived from the “conus” has no developmental foundation. The same goes for the alleged “septum of the atrioventricular canal”. The definitive ventricular septum has only apical muscular and fibrous components, the fibrous part usually being described as the membranous septum. Our initial studies of the anatomy of ventricular septal defects had indicated that all could be categorised, according to the nature of their borders, into those abutting the remnants of the membranous septum, those embedded within the apical muscular septum, or those reflecting the lack of muscularisation of the proximal outflow cushions [
12]. The findings as described by Poelmann and Gittenberger-de Groot [
1] provide further evidence in support of this concept. They can be interpreted to endorse the notion that it is the borders of ventricular septal defects that serve best to define their phenotypic differences [
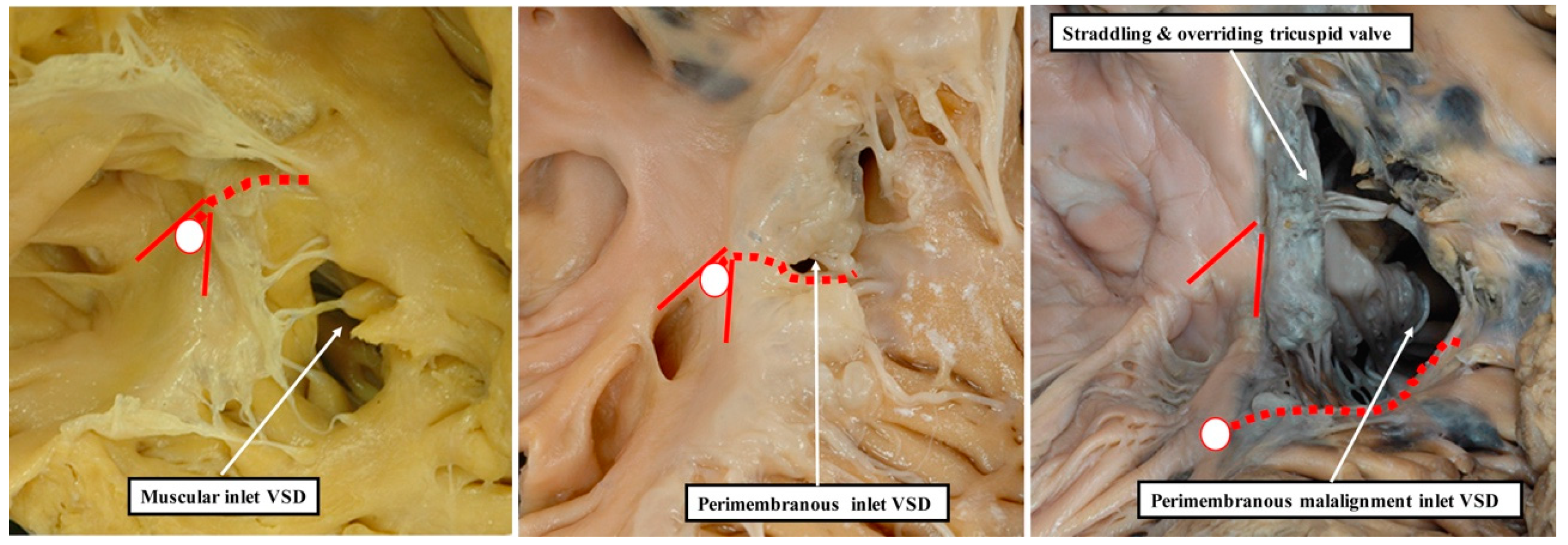
13]. This is because it is not possible, when using geography as the starting point for categorisation, to show these crucial phenotypic differences. This potential deficiency of beginning categorisation on the basis of geography is well demonstrated by considering the defects that open to the inlet of the right ventricle (
Figure 8).
It is knowledge of their borders, rather than their geographical location, which provides the crucial clinical information regarding the disposition of the atrioventricular conduction axis [
14]. As we show in
Figure 9, these borders can now be demonstrated with precision by interrogation during life of computed tomographic datasets.
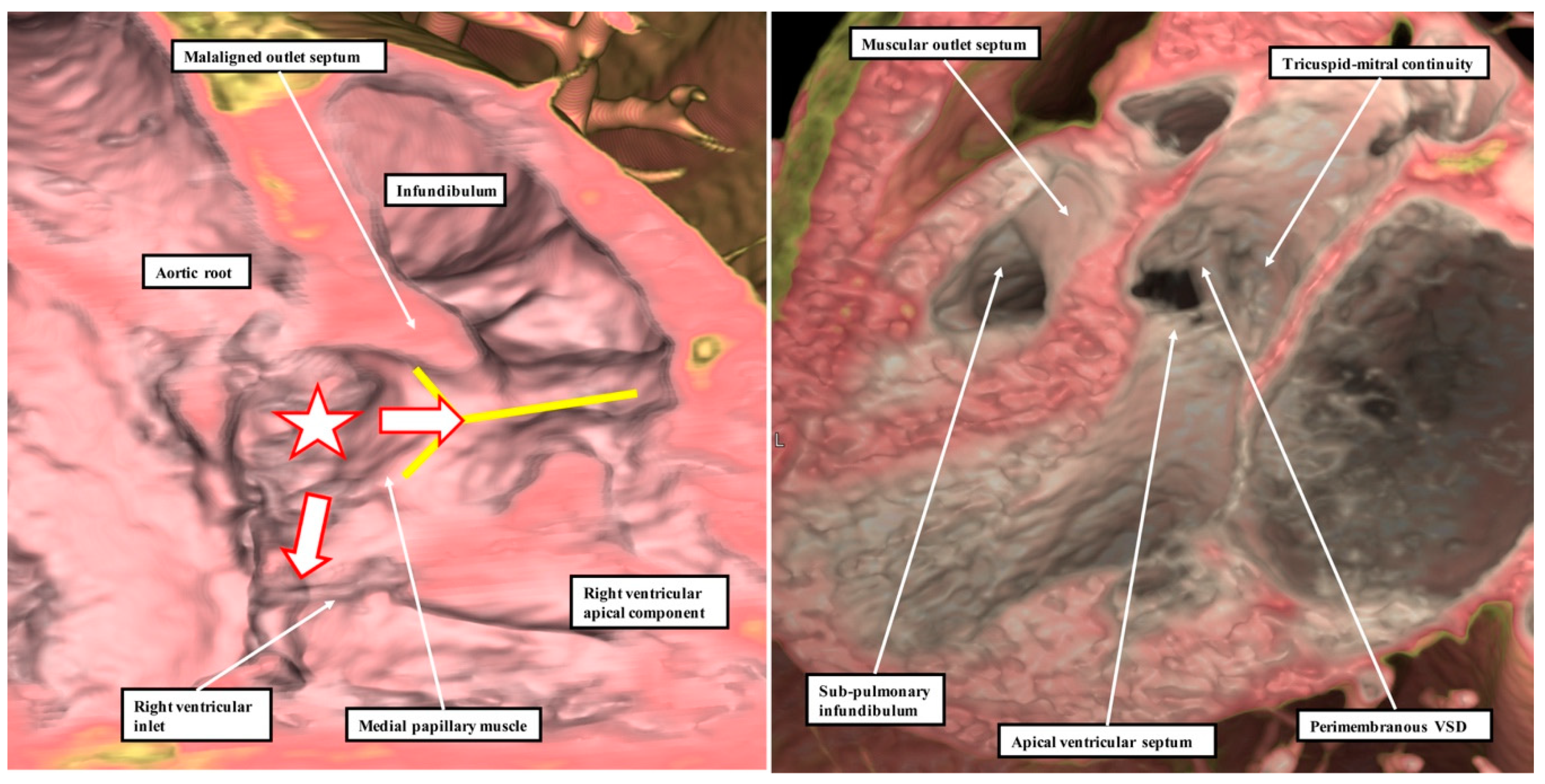
The defect shown in
Figure 9 is then the more important, since it extends so as to open not only to the outlet of the right ventricle in the presence of antero-cephalad malalignment of the muscular outlet septum, but also to the right ventricular inlet. We have previously identified such confluent defects in autopsy archives (
Figure 10).
These confluent defects cannot currently be coded within the definitions submitted by the International Nomenclature Society for inclusion in the 11th iteration of the International Classification of Disease [
3]. It is recognised within the review “Striving for Consensus” [
3], nonetheless, that lesions such as those shown in
Figure 9 and
Figure 10 will likely be included in future revisions of the classification. As is stated in the summary to this document, “it is meant to be an organic and continuously evolving classification system that will likely change with field testing and advances in scientific knowledge”. We submit that the images we provide in our review contribute to the anticipated “advances in scientific knowledge”. We also submit that only when data of this kind are taken into account will it prove possible eventually to determine whether geography or borders, both of importance, is the preferred option for the starting point of categorisation.