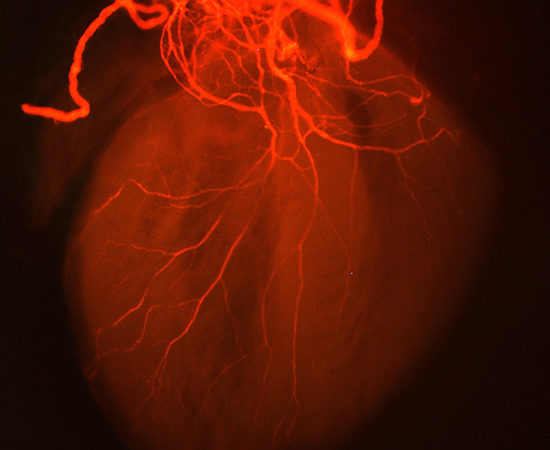
Defective Vagal Innervation in Murine Tbx1 Mutant Hearts
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains
2.2. In Situ Hybridization and Immunolabeling
2.3. Electrocardiogram Recordings and Pharmacological Studies
2.4. Isolation and Culture of NCCs
2.5. Statistical Analysis
3. Results
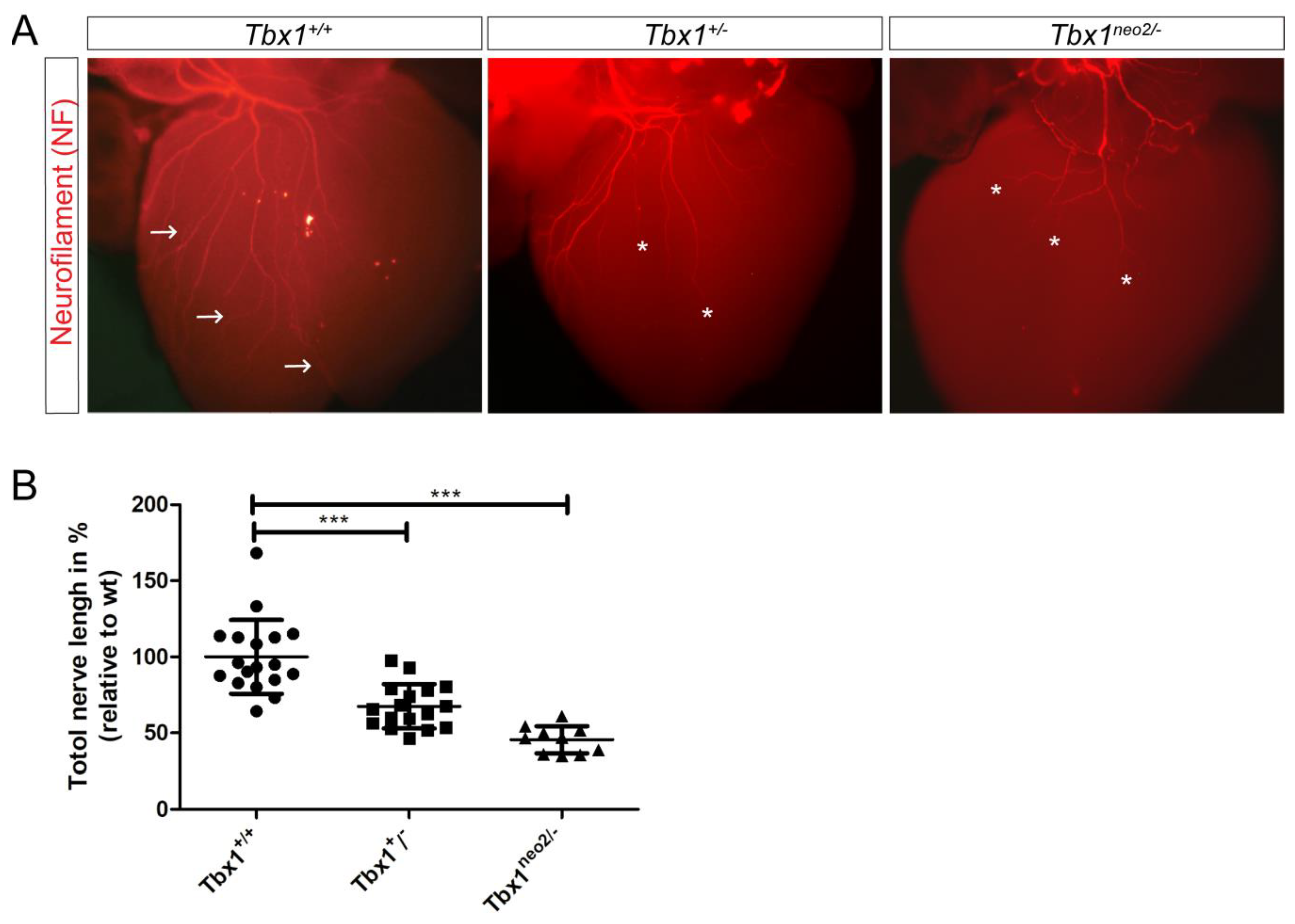
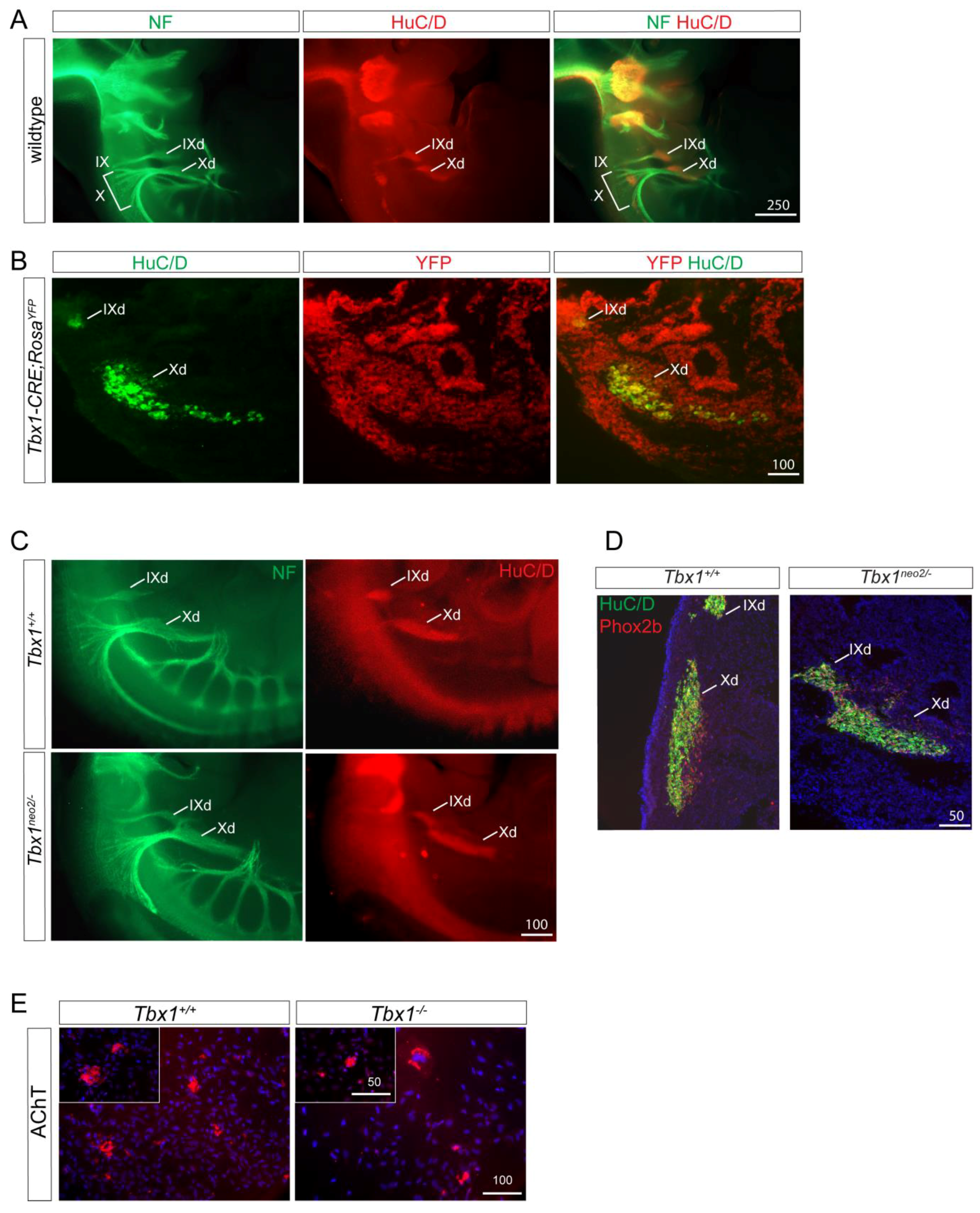
3.1. Defective Cardiac Innervation in Tbx1 Mutants
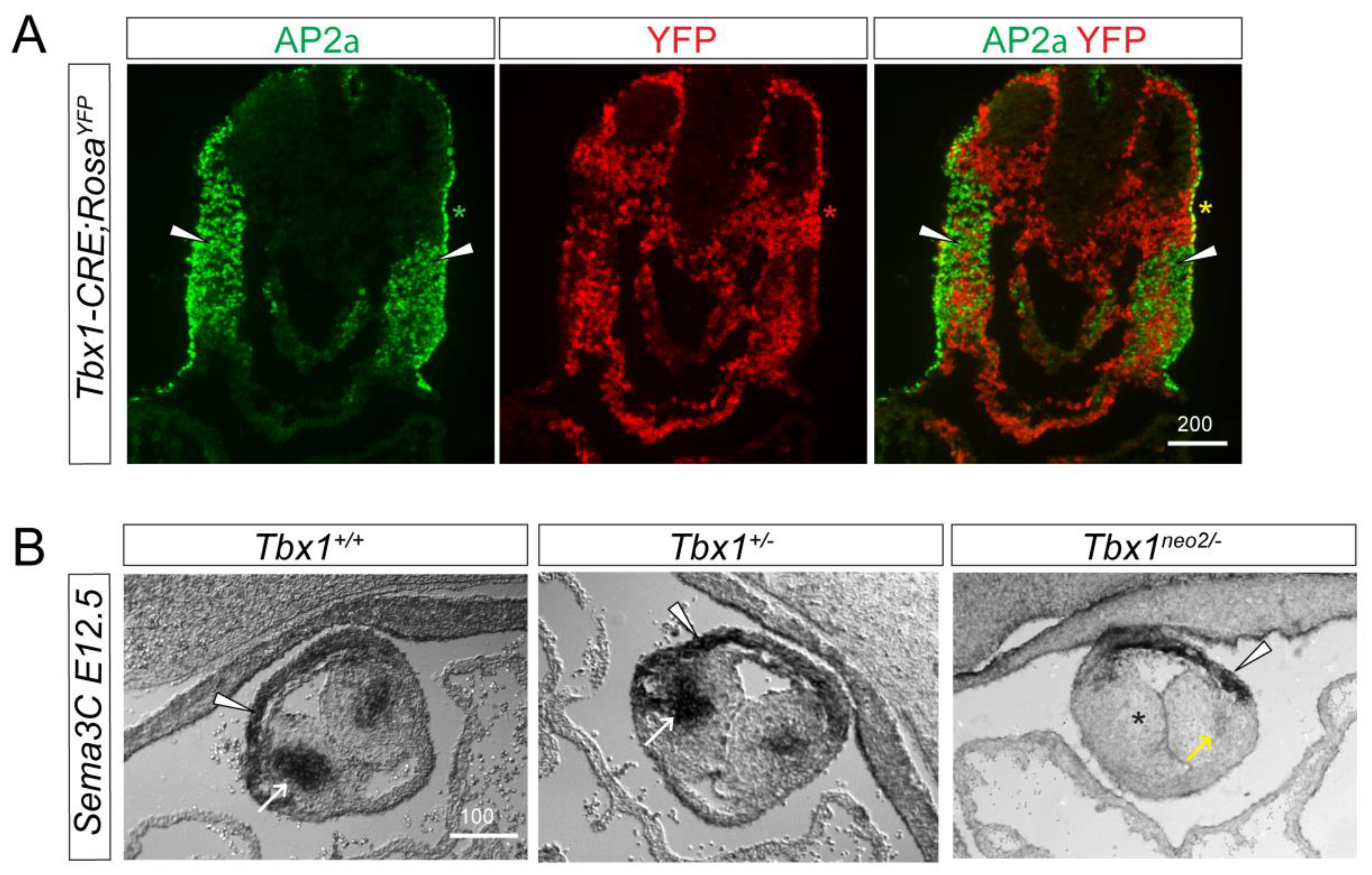
3.2. TBX1 Cell and Non-Cell Autonomous Role in Cardiac Innervation Defects
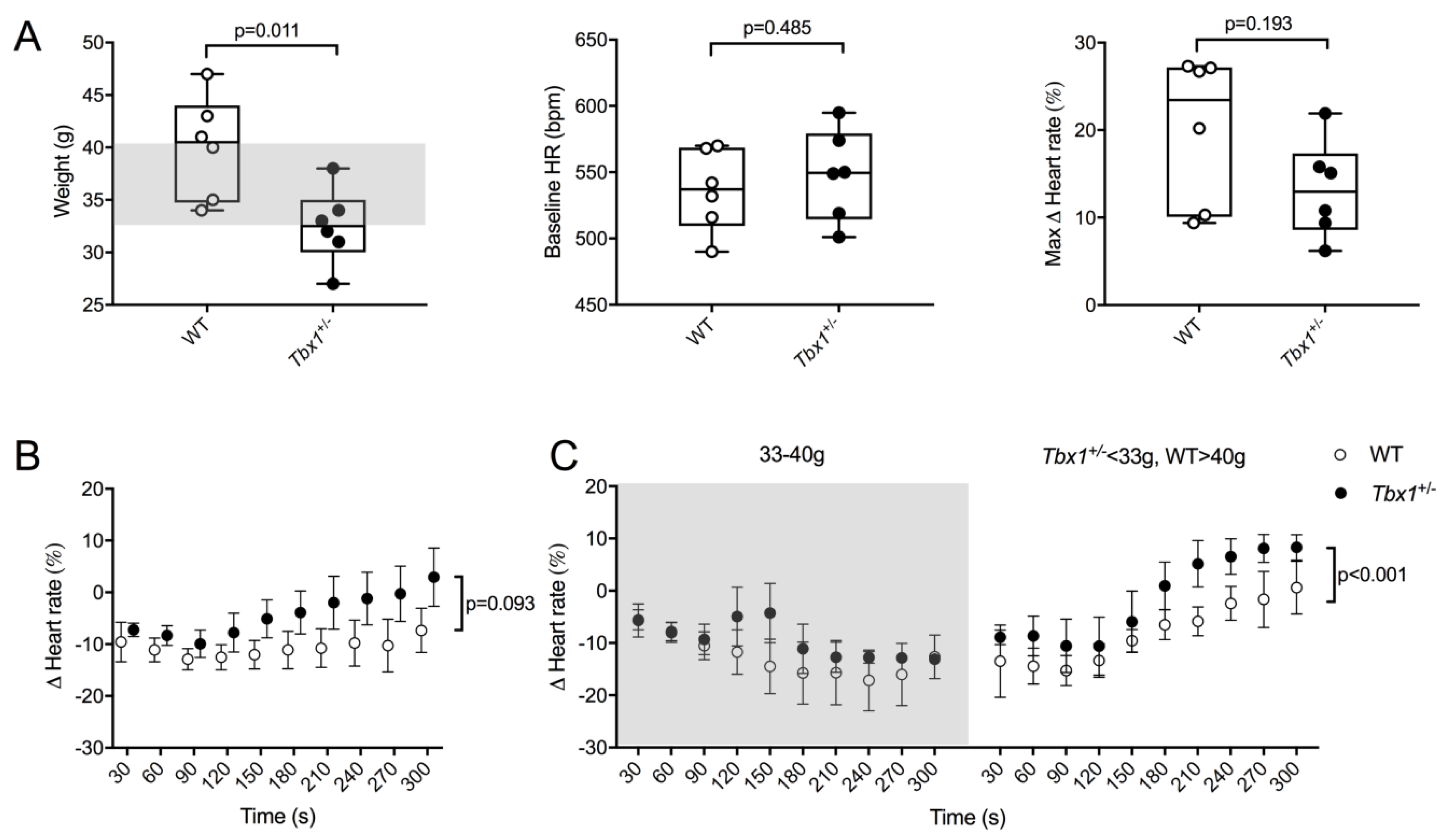
3.3. Functional Relevance of Cardiac Innervation Deficiency in Tbx1 Mutant Animals
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindsay, E.A. Chromosomal microdeletions: Dissecting del22q11 syndrome. Nat. Rev. Genet. 2001, 2, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Scambler, P.J. The 22q11 deletion syndromes. Hum. Mol. Genet. 2000, 9, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.K.; Goodship, J.A.; Wilson, D.I.; Philip, N.; Levy, A.; Seidel, H.; Schuffenhauer, S.; Oechsler, H.; Belohradsky, B.; Prieur, M.; et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: A european collaborative study. J. Med. Genet. 1997, 34, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Jerome, L.A.; Papaioannou, V.E. Digeorge syndrome phenotype in mice mutant for the t-box gene, tbx1. Nat. Genet. 2001, 27, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.A.; Vitelli, F.; Su, H.; Morishima, M.; Huynh, T.; Pramparo, T.; Jurecic, V.; Ogunrinu, G.; Sutherland, H.F.; Scambler, P.J.; et al. Tbx1 haploinsufficiency in the digeorge syndrome region causes aortic arch defects in mice. Nature 2001, 410, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Merscher, S.; Funke, B.; Epstein, J.A.; Heyer, J.; Puech, A.; Lu, M.M.; Xavier, R.J.; Demay, M.B.; Russell, R.G.; Factor, S.; et al. Tbx1 is responsible for cardiovascular defects in velo-cardio-facial/digeorge syndrome. Cell 2001, 104, 619–629. [Google Scholar] [CrossRef]
- Yagi, H.; Furutani, Y.; Hamada, H.; Sasaki, T.; Asakawa, S.; Minoshima, S.; Ichida, F.; Joo, K.; Kimura, M.; Imamura, S.; et al. Role of tbx1 in human del22q11.2 syndrome. Lancet 2003, 362, 1366–1373. [Google Scholar] [CrossRef]
- Calmont, A.; Ivins, S.; Van Bueren, K.L.; Papangeli, I.; Kyriakopoulou, V.; Andrews, W.D.; Martin, J.F.; Moon, A.M.; Illingworth, E.A.; Basson, M.A.; et al. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating gbx2 expression in the pharyngeal ectoderm. Development 2009, 136, 3173–3183. [Google Scholar] [CrossRef] [PubMed]
- Vitelli, F.; Morishima, M.; Taddei, I.; Lindsay, E.A.; Baldini, A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Hum. Mol. Genet. 2002, 11, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, B.A.; Maynard, T.M.; Fralish, M.S.; Nuwayhid, S.; Zohn, I.E.; Moody, S.A.; LaMantia, A.S. Dysphagia and disrupted cranial nerve development in a mouse model of digeorge (22q11) deletion syndrome. Dis. Model. Mech. 2014, 7, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Calmont, A.; Thapar, N.; Scambler, P.J.; Burns, A.J. Absence of the vagus nerve in the stomach of Tbx1−/− mutant mice. Neurogastroenterol. Motil. 2011, 23, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Begbie, J.; Graham, A. Integration between the epibranchial placodes and the hindbrain. Science 2001, 294, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Nassenstein, C.; Taylor-Clark, T.E.; Myers, A.; Ru, F.; Nandigama, R.; Bettner, W.; Undem, B. Phenotypic distinctions between neural crest and placodal derived vagal c-fibers in mouse lungs. J. Physiol. 2010, 588, 4769–4783. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Stewart, D.E. Neural crest origin of cardiac ganglion cells in the chick embryo: Identification and extirpation. Dev. Biol. 1983, 97, 433–443. [Google Scholar] [CrossRef]
- Huynh, T.; Chen, L.; Terrell, P.; Baldini, A. A fate map of tbx1 expressing cells reveals heterogeneity in the second cardiac field. Genesis 2007, 45, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Watanabe, T.; Lin, C.S.; William, C.M.; Tanabe, Y.; Jessell, T.M.; Costantini, F. Cre reporter strains produced by targeted insertion of eyfp and ecfp into the rosa26 locus. BMC Dev. Biol. 2001, 1, 4. [Google Scholar] [CrossRef]
- Zhang, Z.; Huynh, T.; Baldini, A. Mesodermal expression of tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development 2006, 133, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Plein, A.; Calmont, A.; Fantin, A.; Denti, L.; Anderson, N.A.; Scambler, P.J.; Ruhrberg, C. Neural crest-derived sema3c activates endothelial nrp1 for cardiac outflow tract septation. J. Clin. Investig. 2015, 125, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.F. Expression and interactions of the two closely related homeobox genes phox2a and phox2b during neurogenesis. Development 1997, 124, 4065–4075. [Google Scholar] [PubMed]
- Pfaltzgraff, E.R.; Mundell, N.A.; Labosky, P.A. Isolation and culture of neural crest cells from embryonic murine neural tube. J. Vis. Exp. 2012, e4134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Baldini, A. In vivo response to high-resolution variation of tbx1 mrna dosage. Hum. Mol. Genet. 2008, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; McKenzie, J.W.; Weidman, T.A. Developing innervation of the chick heart: A histofluorescence and light microscopic study of sympthetic innervation. Anat. Rec. 1980, 196, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Weidman, T.A.; McKenzie, J.W. An ultrastructural study of the cardia ganglia in the bulbar plexus of the developing chick heart. Dev. Neurosci. 1980, 3, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Pappano, A.J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol. Rev. 1977, 29, 3–33. [Google Scholar] [PubMed]
- Garg, V.; Yamagishi, C.; Hu, T.; Kathiriya, I.S.; Yamagishi, H.; Srivastava, D. Tbx1, a digeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev. Biol. 2001, 235, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bockman, D.E.; Redmond, M.E.; Waldo, K.; Davis, H.; Kirby, M.L. Effect of neural crest ablation on development of the heart and arch arteries in the chick. Am. J. Anat. 1987, 180, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar] [PubMed]
- Raft, S.; Nowotschin, S.; Liao, J.; Morrow, B.E. Suppression of neural fate and control of inner ear morphogenesis by tbx1. Development 2004, 131, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, T.; Good, D.J.; Haigney, M.; Delgado-Romero, P.; Eckhaus, M.A.; Koch, W.J.; Kirsch, I.R. Predisposition to arrhythmia and autonomic dysfunction in nhlh1-deficient mice. Mol. Cell. Biol. 2002, 22, 4977–4983. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Mark, A.L.; Heistad, D.D.; Schmid, P.G. Influence of cardiopulmonary vagal afferent activity on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ. Res. 1975, 37, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Kanazawa, H.; Kimura, K.; Hattori, F.; Ieda, Y.; Taniguchi, M.; Lee, J.K.; Matsumura, K.; Tomita, Y.; Miyoshi, S.; et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat. Med. 2007, 13, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, V.; Anderson, R.H.; Henderson, D.J. Autonomic innervation of the developing heart: Origins and function. Clin. Anat. 2009, 22, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Coppola, E.; Rallu, M.; Richard, J.; Dufour, S.; Riethmacher, D.; Guillemot, F.; Goridis, C.; Brunet, J.F. Epibranchial ganglia orchestrate the development of the cranial neurogenic crest. Proc. Natl. Acad. Sci. USA 2010, 107, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Takano-Maruyama, M.; Chen, Y.; Gaufo, G.O. Placodal sensory ganglia coordinate the formation of the cranial visceral motor pathway. Dev. Dyn. 2010, 239, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Onitsuka, I.; Hatch, J.; Uchida, Y.; Ray, S.; Huang, S.; Li, W.; Zang, H.; Ruiz-Lozano, P.; Mukouyama, Y.S. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 2013, 140, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.S.; Chow, E.W.; Husted, J.; Hodgkinson, K.A.; Oechslin, E.; Harris, L.; Silversides, C. Premature death in adults with 22q11.2 deletion syndrome. J. Med. Genet. 2009, 46, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Fung, W.L.; Butcher, N.J.; Costain, G.; Andrade, D.M.; Boot, E.; Chow, E.W.; Chung, B.; Cytrynbaum, C.; Faghfoury, H.; Fishman, L.; et al. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet. Med. 2015, 17, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Balint, H.; Silversides, C.; Bassett, A.S. 417 Sudden death risk in patients with tetralogy of fallot and 22q11.2 deletion syndrome. Can. J. Cardiol. 2011, 27, S213. [Google Scholar] [CrossRef]
- Verschure, D.O.; Boot, E.; van Amelsvoort, T.A.; Booij, J.; van Eck-Smit, B.L.; Somsen, G.A.; Verberne, H.J. Cardiac sympathetic activity in 22q11.2 deletion syndrome. Int. J. Cardiol. 2016, 212, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Corr, P.; Yamaha, K.; Witkowski, F. Mechanisms controlling cardiac autonomic functions and their relation to arrhythmogenesis. In The Heart and the Cardiovascular System; Raven Press: New York, NY, USA, 1986; pp. 1343–1404. [Google Scholar]
- Finlay, M.; Harmer, S.C.; Tinker, A. The control of cardiac ventricular excitability by autonomic pathways. Pharmacol. Ther. 2017, 174, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.; Stramba-Badiale, M. Parasympathetic nervous system and malignant arrhythmias. In Neurocardiology; Kulbertus, H., Frank, M., Eds.; Futura Publishing Company: New York, NY, USA, 1987; pp. 79–102. [Google Scholar]
- Randall, V.; McCue, K.; Roberts, C.; Kyriakopoulou, V.; Beddow, S.; Barrett, A.N.; Vitelli, F.; Prescott, K.; Shaw-Smith, C.; Devriendt, K.; et al. Great vessel development requires biallelic expression of chd7 and tbx1 in pharyngeal ectoderm in mice. J. Clin. Investig. 2009, 119, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, Q.; Vieira, J.M.; Howard, B.; Eickholt, B.J.; Ruhrberg, C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development 2008, 135, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Ivins, S.; Lammerts van Beuren, K.; Roberts, C.; James, C.; Lindsay, E.; Baldini, A.; Ataliotis, P.; Scambler, P.J. Microarray analysis detects differentially expressed genes in the pharyngeal region of mice lacking tbx1. Dev. Biol. 2005, 285, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Theveniau-Ruissy, M.; Dandonneau, M.; Mesbah, K.; Ghez, O.; Mattei, M.G.; Miquerol, L.; Kelly, R.G. The del22q11.2 candidate gene tbx1 controls regional outflow tract identity and coronary artery patterning. Circ. Res. 2008, 103, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Shibata, S.; Miyagawa-Tomita, S.; Ong, S.G.; Takahashi, H.; Kume, T.; Okano, H.; Matsuoka, R.; Yamagishi, H. Regulation of sema3c and the interaction between cardiac neural crest and second heart field during outflow tract development. Sci. Rep. 2017, 7, 6771. [Google Scholar] [CrossRef] [PubMed]
- Feiner, L.; Webber, A.L.; Brown, C.B.; Lu, M.M.; Jia, L.; Feinstein, P.; Mombaerts, P.; Epstein, J.A.; Raper, J.A. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 2001, 128, 3061–3070. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calmont, A.; Anderson, N.; Suntharalingham, J.P.; Ang, R.; Tinker, A.; Scambler, P.J. Defective Vagal Innervation in Murine Tbx1 Mutant Hearts. J. Cardiovasc. Dev. Dis. 2018, 5, 49. https://doi.org/10.3390/jcdd5040049
Calmont A, Anderson N, Suntharalingham JP, Ang R, Tinker A, Scambler PJ. Defective Vagal Innervation in Murine Tbx1 Mutant Hearts. Journal of Cardiovascular Development and Disease. 2018; 5(4):49. https://doi.org/10.3390/jcdd5040049
Chicago/Turabian StyleCalmont, Amélie, Naomi Anderson, Jenifer P. Suntharalingham, Richard Ang, Andrew Tinker, and Peter J. Scambler. 2018. "Defective Vagal Innervation in Murine Tbx1 Mutant Hearts" Journal of Cardiovascular Development and Disease 5, no. 4: 49. https://doi.org/10.3390/jcdd5040049
APA StyleCalmont, A., Anderson, N., Suntharalingham, J. P., Ang, R., Tinker, A., & Scambler, P. J. (2018). Defective Vagal Innervation in Murine Tbx1 Mutant Hearts. Journal of Cardiovascular Development and Disease, 5(4), 49. https://doi.org/10.3390/jcdd5040049