Postnatal Cardiac Autonomic Nervous Control in Pediatric Congenital Heart Disease
Abstract
:1. Introduction
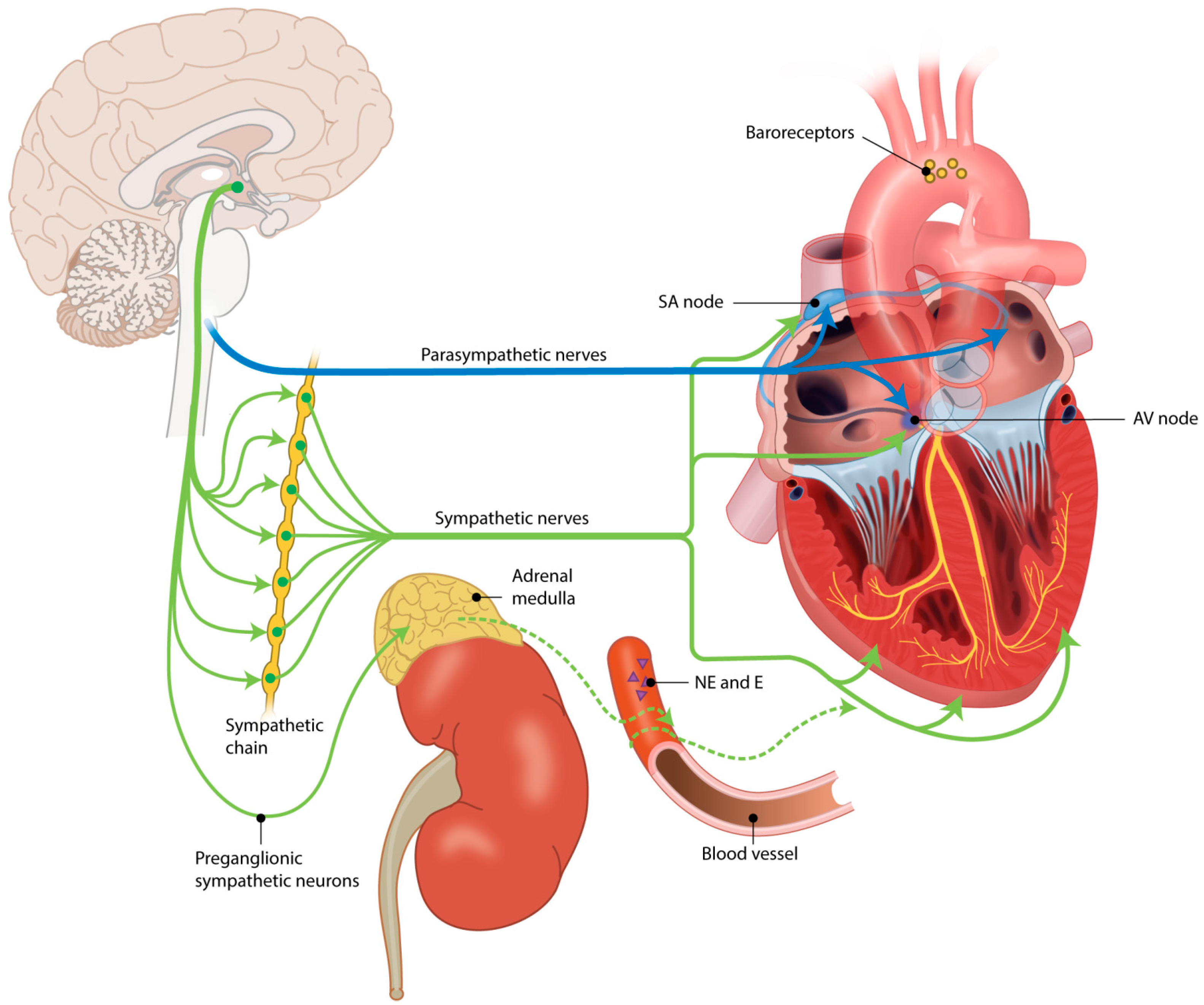
2. Autonomic Nervous System
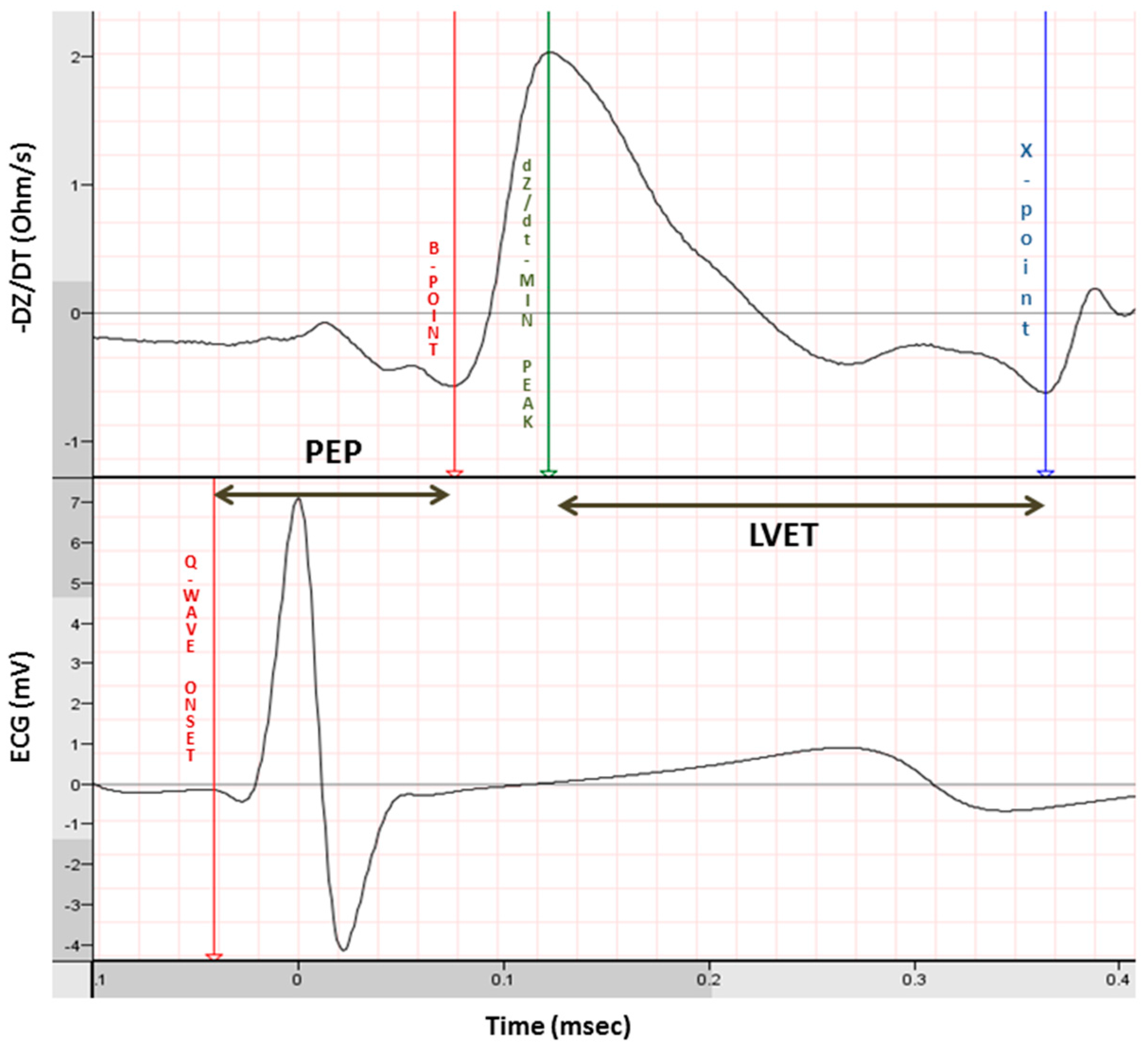
3. Postnatal Measurement of Cardiac Autonomic Control
4. Cardiac Autonomic Nervous System in Healthy Maturation
5. Cardiac Autonomic Control in Pediatric Congenital Heart Disease
5.1. Ventricular and Atrial Septal Defect
5.2. Transposition of the Great Arteries
5.3. Univentricular Heart
5.4. Coarctation of the Aorta
5.5. Tetralogy of Fallot
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| ANS | autonomic nervous system |
| ASD | atrial septal defect |
| ASO | arterial switch operation |
| BDG | bidirectional Glenn shunt |
| BP | blood pressure |
| BPV | blood pressure variability |
| BRS | baroreceptor sensitivity |
| CHD | congenital heart disease |
| CoA | coarctation of the aorta |
| CPL | complex cyanotic heart disease |
| DFA | detrended fluctuation analysis |
| E | epinephrine HRV—heart rate variability |
| LBNT | low body negative pressure test |
| LRS | left to right shunt |
| MIGB | metaiodobenzylguanidine |
| NE | norepinephrine |
| NN interval | normal-to-normal interbeat interval |
| NYHA | New York Heart Assosiation |
| PEP | pre ejection period |
| PNS | parasympathetic nervous system |
| PST | pulmonary stenosis |
| Qp/Qs | pulmonary to systemic flow ratio |
| SD | standard deviation |
| SNS | sympathetic nervous system |
| TGA | transposition of the great arteries |
| TOF | tetralogy of Fallot |
| VA | ventricular arrhythmia |
| VSD | ventricle septal defect |
References
- Khoshnood, B.; Lelong, N.; Houyel, L.; Thieulin, A.C.; Jouannic, J.M.; Magnier, S.; Delezoide, A.L.; Magny, J.F.; Rambaud, C.; Bonnet, D.; et al. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: A population-based study. Heart 2012, 98, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circ 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Engelfriet, P.M.; Duffels, M.G.; Moller, T.; Boersma, E.; Tijssen, J.G.; Thaulow, E.; Gatzoulis, M.A.; Mulder, B.J. Pulmonary arterial hypertension in adults born with a heart septal defect: The Euro Heart Survey on adult congenital heart disease. Heart 2007, 93, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Zomer, A.C.; Vaartjes, I.; Uiterwaal, C.S.; van der Velde, E.T.; van den Merkhof, L.F.; Baur, L.H.; Ansink, T.J.; Cozijnsen, L.; Pieper, P.G.; Meijboom, F.J.; et al. Circumstances of death in adult congenital heart disease. Int. J. Cardiol. 2012, 154, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Verheugt, C.L.; Uiterwaal, C.S.; van der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.P.; Vliegen, H.W.; Grobbee, D.E.; Mulder, B.J. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Brateanu, A. Heart rate variability after myocardial infarction: What we know and what we still need to find out. Curr. Med. Res. Opin. 2015, 31, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Lakusic, N.; Mahovic, D.; Kruzliak, P.; Cerkez, H.J.; Novak, M.; Cerovec, D. Changes in Heart Rate Variability after Coronary Artery Bypass Grafting and Clinical Importance of These Findings. Biomed. Res. Int. 2015, 2015, 680515. [Google Scholar] [CrossRef] [PubMed]
- Lammers, A.; Kaemmerer, H.; Hollweck, R.; Schneider, R.; Barthel, P.; Braun, S.; Wacker, A.; Brodherr-Heberlein, S.; Hauser, M.; Eicken, A.; et al. Impaired cardiac autonomic nervous activity predicts sudden cardiac death in patients with operated and unoperated congenital cardiac disease. J. Thorac. Cardiovasc. Surg. 2006, 132, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Huikuri, H.V.; Stein, P.K. Heart rate variability in risk stratification of cardiac patients. Prog. Cardiovasc. Dis. 2013, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, S.; Gast, K.B.; de, M.R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace 2013, 15, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Wilpers, A.; Myers, M.; Nugent, J.D.; Fifer, W.P.; Williams, I.A. Autonomic regulation in fetuses with congenital heart disease. Early Hum. Dev. 2015, 91, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Heinicke, E.; Jaekel, S.; Tetschke, F.; Di Pietro, P.D.; Haueisen, J.; Schleusner, E.; Schneider, U. Indices of fetal development derived from heart rate patterns. Early Hum. Dev. 2009, 85, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Al, N.B.; van Amerom, J.F.; Forsey, J.; Jaeggi, E.; Grosse-Wortmann, L.; Yoo, S.J.; Macgowan, C.K.; Seed, M. Fetal circulation in left-sided congenital heart disease measured by cardiovascular magnetic resonance: A case-control study. J. Cardiovasc. Magn. Reson. 2013, 15, 65. [Google Scholar] [CrossRef]
- Clouchoux, C.; du Plessis, A.J.; Bouyssi-Kobar, M.; Tworetzky, W.; McElhinney, D.B.; Brown, D.W.; Gholipour, A.; Kudelski, D.; Warfield, S.K.; McCarter, R.J.; et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex 2013, 23, 2932–2943. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhou, Q.C.; Zhou, J.W.; Li, M.; Long, C.; Peng, Q.H. Volume of intracranial structures on three-dimensional ultrasound in fetuses with congenital heart disease. Ultrasound Obstet. Gynecol. 2014, 46, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Manzar, S.; Nair, A.K.; Pai, M.G.; Al-Khusaiby, S.M. Head size at birth in neonates with transposition of great arteries and hypoplastic left heart syndrome. Saudi. Med. J. 2005, 26, 453–456. [Google Scholar] [PubMed]
- Kaltman, J.R.; Di, H.; Tian, Z.; Rychik, J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet. Gynecol. 2005, 25, 32–36. [Google Scholar] [CrossRef] [PubMed]
- May, L.E.; Glaros, A.; Yeh, H.W.; Clapp, J.F., III; Gustafson, K.M. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum. Dev. 2010, 86, 213–217. [Google Scholar] [CrossRef] [PubMed]
- May, L.E.; Scholtz, S.A.; Suminski, R.; Gustafson, K.M. Aerobic ex ercise during pregnancy influences infant heart rate variability at one month of age. Early Hum. Dev. 2014, 90, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Grassi, G.; Giannattasio, C.; Seravalle, G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999, 34, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Bonet, S.; Agusti, A.; Arnau, J.M.; Vidal, X.; Diogene, E.; Galve, E.; Laporte, J.R. Beta-adrenergic blocking agents in heart failure: Benefits of vasodilating and non-vasodilating agents according to patients’ characteristics: A meta-analysis of clinical trials. Arch. Intern. Med. 2000, 160, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Coote, J.H. Myths and realities of the cardiac vagus. J. Physiol. 2013, 591, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Dae, M.W.; O’Connell, J.W.; Botvinick, E.H.; Ahearn, T.; Yee, E.; Huberty, J.P.; Mori, H.; Chin, M.C.; Hattner, R.S.; Herre, J.M. Scintigraphic assessment of regional cardiac adrenergic innervation. Circ 1989, 79, 634–644. [Google Scholar] [CrossRef]
- Geus, E.J.; Lien, R.; Neijts, M. Willemsen Genetics of autonomic nervous system activity. In The Oxford Handbook of Molecular Psychology; Oxford University Press: Oxford, UK, 2015; pp. 357–390. [Google Scholar]
- Billman, G.E. Heart rate variability—A historical perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Hon, E.H.; Lee, S.T. Electronic evaluation of the fetal heart rate. VIII. Paterns preceding fetal death, further observations. Am. J. Obstet. Gynecol. 1963, 87, 814–826. [Google Scholar] [PubMed]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381.
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L. The human respiratory gate. J. Physiol. 2003, 548, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.; Allen, M.T.; Fahrenberg, J.; Kelsey, R.M.; Lovallo, W.R.; Van Doornen, L.J. Methodological guidelines for impedance cardiography. Psychophysiology 1990, 27, 1–23. [Google Scholar] [PubMed]
- Van, L.R.; Neijts, M.; Willemsen, G.; de Geus, E.J. Ambulatory measurement of the ECG T-wave amplitude. Psychophysiology 2015, 52, 225–237. [Google Scholar]
- Eyre, E.L.; Duncan, M.J.; Birch, S.L.; Fisher, J.P. The influence of age and weight status on cardiac autonomic control in healthy children: A review. Auton. Neurosci. 2014, 186, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Heragu, N.P.; Scott, W.A. Heart rate variability in healthy children and in those with congenital heart disease both before and after operation. Am. J. Cardiol. 1999, 83, 1654–1657. [Google Scholar] [CrossRef]
- Seppala, S.; Laitinen, T.; Tarvainen, M.P.; Tompuri, T.; Veijalainen, A.; Savonen, K.; Lakka, T. Normal values for heart rate variability parameters in children 6–8 years of age: The PANIC Study. Clin. Physiol. Funct. Imaging 2014, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Clays, E.; De, B.M.; Huybrechts, I.; Marild, S.; Vanaelst, B.; De, H.S.; Sioen, I. Determinants and reference values of short-term heart rate variability in children. Eur. J. Appl. Physiol. 2013, 113, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, M.S.; Hathaway, D.; Tolley, B. Cardiovascular autonomic function in healthy adolescents. Heart Lung 2003, 32, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, A.E.; van, L.R.; van, E.M.; Gemke, R.J.; Vrijkotte, T.G.; de Geus, E.J. Measuring cardiac autonomic nervous system (ANS) activity in children. J. Vis. Exp. 2013, 74, e50073. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. The neurohormonal hypothesis: A theory to explain the mechanism of disease progression in heart failure. J. Am. Coll. Cardiol. 1992, 20, 248–254. [Google Scholar] [CrossRef]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Hasking, G.J.; Esler, M.D.; Jennings, G.L.; Burton, D.; Johns, J.A.; Korner, P.I. Norepinephrine spillover to plasma in patients with congestive heart failure: Evidence of increased overall and cardiorenal sympathetic nervous activity. Circ 1986, 73, 615–621. [Google Scholar] [CrossRef]
- Reiken, S.; Gaburjakova, M.; Gaburjakova, J.; He Kl, K.L.; Prieto, A.; Becker, E.; Yi Gh, G.H.; Wang, J.; Burkhoff, D.; Marks, A.R. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circ 2001, 104, 2843–2848. [Google Scholar] [CrossRef]
- Lown, B.; Verrier, R.L. Neural activity and ventricular fibrillation. N. Engl. J. Med. 1976, 294, 1165–1170. [Google Scholar] [PubMed]
- Billman, G.E. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: Implications for future anti-arrhythmic drug development. Pharmacol. Ther. 2006, 111, 808–835. [Google Scholar] [CrossRef] [PubMed]
- Schomer, A.C.; Nearing, B.D.; Schachter, S.C.; Verrier, R.L. Vagus nerve stimulation reduces cardiac electrical instability assessed by quantitative T-wave alternans analysis in patients with drug-resistant focal epilepsy. Epilepsia 2014, 55, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, I.M.; Belevych, A.E.; Sridhar, A.; Nishijima, Y.; Ho, H.T.; He, Q.; Kukielka, M.; Terentyev, D.; Terentyeva, R.; Liu, B.; et al. Endurance exercise training normalizes repolarization and calcium-handling abnormalities, preventing ventricular fibrillation in a model of sudden cardiac death. J. Appl. Physiol. 2012, 113, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Vanoli, E.; Stramba-Badiale, M.; De Ferrari, G.M.; Billman, G.E.; Foreman, R.D. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circ 1988, 78, 969–979. [Google Scholar] [CrossRef]
- Vanoli, E.; de Ferrari, G.M.; Stramba-Badiale, M.; Hull, S.S., Jr.; Foreman, R.D.; Schwartz, P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 1991, 68, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Rouhi-Boroujeni, H.; Rouhi-Boroujeni, H.; Rouhi-Boroujeni, P.; Sedehi, M. Long-term pulmonary functional status following coronary artery bypass grafting surgery. ARYA. Atheroscler. 2015, 11, 163–166. [Google Scholar] [PubMed]
- Reller, M.D.; Strickland, M.J.; Riehle-Colarusso, T.; Mahle, W.T.; Correa, A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J. Pediatr. 2008, 153, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Horner, S.M.; Murphy, C.F.; Coen, B.; Dick, D.J.; Harrison, F.G.; Vespalcova, Z.; Lab, M.J. Contribution to heart rate variability by mechanoelectric feedback. Stretch of the sinoatrial node reduces heart rate variability. Circ 1996, 94, 1762–1767. [Google Scholar] [CrossRef]
- Hata, T.; Mano, S.; Kusuki, M.; Matsuura, H.; Miyata, M.; Yamazaki, T.; Nagaoka, S. Difference in autonomic nervous control between ventricular septal defect and atrial septal defect based on heart rate variability. Pac. Clin. Electrophysiol. 2007, 30 (Suppl. S1), S212–S214. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.P.; Nugent, S.T.; Hellenbrand, W.; Craig, M.; Gillis, D.A. Sinus arrhythmia in children with atrial septal defect: An analysis of heart rate variability before and after surgical repair. Br. Heart J. 1989, 61, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Bakari, S.; Koca, B.; Oztunc, F.; Abuhandan, M. Heart rate variability in patients with atrial septal defect and healthy children. J. Cardiol. 2013, 61, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Massin, M.M.; Derkenne, B.; von, B.G. Heart rate behavior in children with atrial septal defect. Cardiology 1998, 90, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, J.; Karwot, B.; Szkutnik, M.; Sredniawa, B.; Chodor, B.; Zeifert, B.; Skiba, A.; Zyla-Frycz, M.; Kalarus, Z. Comparison of heart rate variability between surgical and interventional closure of atrial septal defect in children. Am. J. Cardiol. 2003, 92, 356–358. [Google Scholar] [CrossRef]
- Kul, Y.M.; Su, K.N. Change of complex and periodic heart rate dynamics with change of pulmonary artery pressure in infants with left-to-right shunt lesion. Int. J. Cardiol. 1997, 60, 143–150. [Google Scholar] [CrossRef]
- Ohuchi, H.; Suzuki, H.; Toyohara, K.; Tatsumi, K.; Ono, Y.; Arakaki, Y.; Echigo, S. Abnormal cardiac autonomic nervous activity after right ventricular outflow tract reconstruction. Circ 2000, 102, 2732–2738. [Google Scholar] [CrossRef]
- Massin, M.; von, B.G. Clinical and haemodynamic correlates of heart rate variability in children with congenital heart disease. Eur. J. Pediatr. 1998, 157, 967–971. [Google Scholar] [CrossRef] [PubMed]
- McGlone, L.; Patel, N.; Young, D.; Danton, M.D. Impaired cardiac autonomic nervous control after cardiac bypass surgery for congenital heart disease. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Currie, K.D.; Martin, A.A.; Millar, P.J.; Stone, N.D.; Timmons, B.W.; Dillenburg, R.F.; MacDonald, M.J. Vascular and autonomic function in preschool-aged children with congenital heart disease. Congenit. Heart Dis. 2012, 7, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Aletti, F.; Ferrario, M.; de Jesus, T.B.; Stirbulov, R.; Silva, A.B.; Cerutti, S.; Sampaio, L.M. Heart rate variability in children with cyanotic and acyanotic congenital heart disease: Analysis by spectral and non linear indices. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 4189–4192. [Google Scholar] [PubMed]
- Buchhorn, R.; Hulpke-Wette, M.; Nothroff, J.; Paul, T. Heart rate variability in infants with heart failure due to congenital heart disease: Reversal of depressed heart rate variability by propranolol. Med. Sci. Monit. 2002, 8, CR661–CR666. [Google Scholar] [PubMed]
- Dzimiri, N.; Galal, O.; Moorji, A.; Bakr, S.; Abbag, F.; Fadley, F.; Almotrefi, A.A. Regulation of sympathetic activity in children with various congenital heart diseases. Pediatr. Res. 1995, 38, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Goudjil, S.; Imestouren, F.; Chazal, C.; Ghostine, G.; Wallois, F.; Leke, A.; Kongolo, G. Patent ductus arteriosus in preterm infants is associated with cardiac autonomic alteration and predominant parasympathetic stimulation. Early Hum. Dev. 2013, 89, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Kaltman, J.R.; Hanna, B.D.; Gallagher, P.R.; Gaynor, J.W.; Godinez, R.I.; Tanel, R.E.; Shah, M.J.; Vetter, V.L.; Rhodes, L.A. Heart rate variability following neonatal heart surgery for complex congenital heart disease. Pac. Clin. Electrophysiol. 2006, 29, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kondo, C.; Nakazawa, M.; Momma, K.; Kusakabe, K. Sympathetic denervation and reinnervation after arterial switch operation for complete transposition. Circ 1998, 97, 2414–2419. [Google Scholar] [CrossRef]
- Harrison, T.M.; Brown, R.L. Autonomic nervous system function in infants with transposition of the great arteries. Biol. Res. Nurs. 2012, 14, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.M. Autonomic nervous system function, child behavior, and maternal sensitivity in three-year-old children with surgically corrected transposition. Heart Lung 2013, 46, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.M.; Ferree, A. Maternal-infant interaction and autonomic function in healthy infants and infants with transposition of the great arteries. Res. Nurs. Health 2014, 37, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Doksoz, O.; Oner, T.; Guven, B.; Karaarslan, U.; Ozdemir, R.; Yozgat, Y.; Mese, T.; Tavli, V.; Okur, F.F.; Alayunt, E.A. Mid-term assessment of cardiac autonomic functions in children with transposition of the great arteries after arterial switch operation. Anadol. Kardiyol. Derg. 2014, 14, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, C.; Ostman-Smith, I.; Gilljam, T.; Lambert, G.; Friberg, P. Cardiac autonomic function in adolescents operated by arterial switch surgery. Int. J. Cardiol. 2013, 168, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Madan, K.; Garg, P.; Deepak, K.K.; Talwar, S.; Airan, B.; Choudhary, S.K. Heart rate variability in patients undergoing univentricular heart repair. Asian Cardiovasc. Thorac. Ann. 2014, 22, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rydberg, A.; Karlsson, M.; Hornsten, R.; Wiklund, U. Can analysis of heart rate variability predict arrhythmia in children with Fontan circulation? Pediatr. Cardiol. 2008, 29, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Hasegawa, S.; Yasuda, K.; Yamada, O.; Ono, Y.; Echigo, S. Severely impaired cardiac autonomic nervous activity after the Fontan operation. Circ 2001, 104, 1513–1518. [Google Scholar] [CrossRef]
- Butera, G.; Bonnet, D.; Iserin, L.; Sidi, D.; Kachaner, J.; Villain, E. Total cavopulmonary and atriopulmonary connections are associated with reduced heart rate variability. Heart 1999, 82, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Polson, J.W.; McCallion, N.; Waki, H.; Thorne, G.; Tooley, M.A.; Paton, J.F.; Wolf, A.R. Evidence for cardiovascular autonomic dysfunction in neonates with coarctation of the aorta. Circ 2006, 113, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Polson, J.W.; Martin, R.P.; Paton, J.F.; Wolf, A.R. Normalization of autonomic function in children with coarctation of the aorta after surgical correction in infancy. Hypertension 2009, 54, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.; Polson, J.W.; Martin, R.P.; Caputo, M.; Wilson, D.G.; Cockcroft, J.R.; Paton, J.F.; Wolf, A.R. Relationship of aortic pulse wave velocity and baroreceptor reflex sensitivity to blood pressure control in patients with repaired coarctation of the aorta. Am. Heart J. 2011, 162, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Beekman, R.H.; Katz, B.P.; Moorehead-Steffens, C.; Rocchini, A.P. Altered baroreceptor function in children with systolic hypertension after coarctation repair. Am. J. Cardiol. 1983, 52, 112–117. [Google Scholar] [CrossRef]
- Choy, M.; Rocchini, A.P.; Beekman, R.H.; Rosenthal, A.; Dick, M.; Crowley, D.; Behrendt, D.; Snider, A.R. Paradoxical hypertension after repair of coarctation of the aorta in children: Balloon angioplasty versus surgical repair. Circ 1987, 75, 1186–1191. [Google Scholar] [CrossRef]
- Silvilairat, S.; Wongsathikun, J.; Sittiwangkul, R.; Pongprot, Y.; Chattipakorn, N. Heart rate variability and exercise capacity of patients with repaired tetralogy of Fallot. Pediatr. Cardiol. 2011, 32, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Butera, G.; Bonnet, D.; Sidi, D.; Kachaner, J.; Chessa, M.; Bossone, E.; Carminati, M.; Villain, E. Patients operated for tetralogy of fallot and with non-sustained ventricular tachycardia have reduced heart rate variability. Herz 2004, 29, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Wyller, V.B.; Saul, J.P.; Barbieri, R.; de, L.C.; Hopp, E.; Norum, I.B.; Thaulow, E. Autonomic heart rate control at rest and during unloading of the right ventricle in repaired tetralogy of Fallot in adolescents. Am. J. Cardiol. 2008, 102, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Bossers, S.S.; Duppen, N.; Kapusta, L.; Maan, A.C.; Duim, A.R.; Bogers, A.J.; Hazekamp, M.G.; van, I.G.; Helbing, W.A.; Blom, N.A. Comprehensive rhythm evaluation in a large contemporary Fontan populationdagger. Eur. J. Cardiothorac. Surg. 2015, 48, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Baumgartner, D.; Baumgartner, C.; Horer, J.; Schreiber, C.; Hess, J.; Vogt, M. Impaired elastic properties of the ascending aorta persist within the first 3 years after neonatal coarctation repair. Pediatr. Cardiol. 2009, 30, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Frydrychowicz, A.; Markl, M.; Hirtler, D.; Harloff, A.; Schlensak, C.; Geiger, J.; Stiller, B.; Arnold, R. Aortic hemodynamics in patients with and without repair of aortic coarctation: In vivo analysis by 4D flow-sensitive magnetic resonance imaging. Investig. Radiol. 2011, 46, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Canniffe, C.; Ou, P.; Walsh, K.; Bonnet, D.; Celermajer, D. Hypertension after repair of aortic coarctation--a systematic review. Int. J. Cardiol. 2013, 167, 2456–2461. [Google Scholar] [CrossRef] [PubMed]
- Buchhorn, R.; Ziemer, G.; Jetter, H.M.; Borst, M.; Selbach, J. Renal sympathetic denervation for resistant hypertension in a child after aortic coarctation surgery. Int. J. Cardiol. 2014, 172, e447–e448. [Google Scholar] [CrossRef] [PubMed]
| HRV Variable (Measure) | Principle |
|---|---|
| Time domain measures | |
| Mean RR | Average of all NN intervals |
| SDNN (ms) | Standard deviation of all NN intervals (inter beat interval of two successive sinus beats) |
| SDANN (ms) | Standard deviation of the average NN intervals over a short period (typically 5 min) over the entire recording |
| RMSSD (ms) | Root mean square of successive differences between adjacent NN intervals |
| Coefficient of variation | Ratio of standard deviation of NN intervals and the mean of RR intervals |
| NN50 | Number of pairs of successive NN intervals that differ >50 ms |
| pNN50 | Proportion of NN50/total number of measured NN intervals |
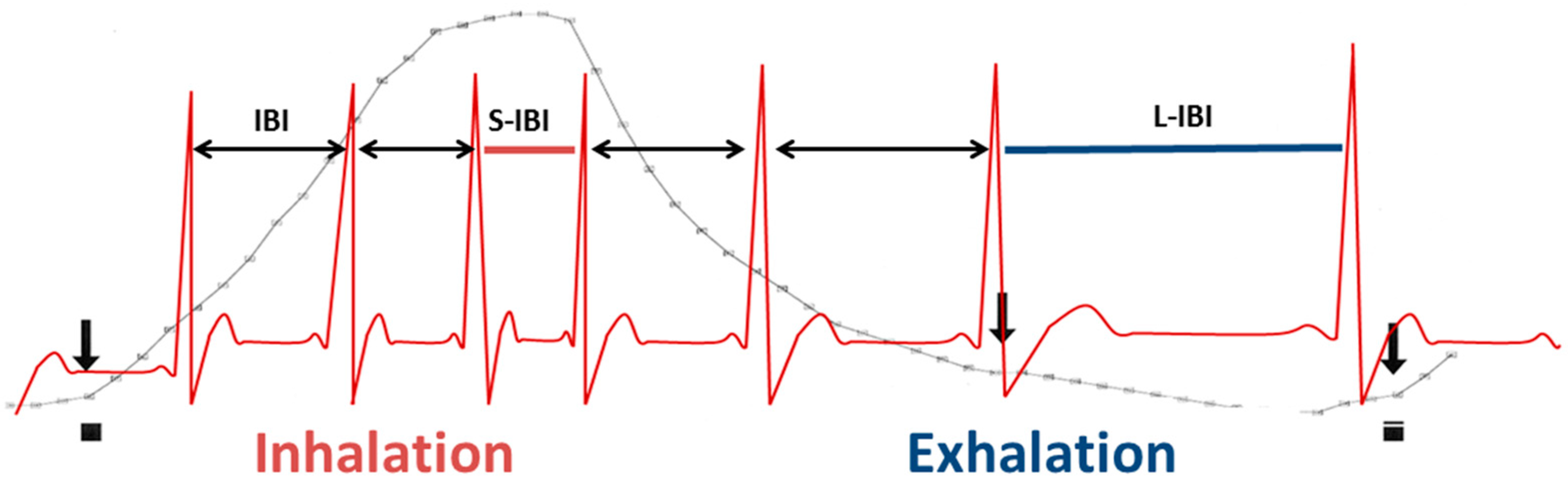
| pvRSA (ms) | Respiratory sinus arrhythmia determined by peak-valley method. The shortest NN interval during inspiration is subtracted from the longest NN interval during exhalation (see Figure 2) |
| Frequency domain measures | |
| HF (ms2) | High frequency power. Power in the respiratory frequency range, typically from 0.15–0.4 Hz |
| HFnu | HF/(LF + HF) |
| LF (ms2) | Low frequency power. Power in the low frequency range, typically from 0.04–0.15 Hz |
| LFnu | LF/(LF + HF) |
| LF/HF | Ratio of low frequency power to high frequency power |
| VLF (ms2) | Very low frequency power. Power in the very low frequency range, typically from 0.003–0.04 Hz |
| ULF (ms2) | Ultra-low frequency power. Power in the ultra-low frequency range, typically <0.003 HZ |
| TP (ms2) | ULF + VLF + LF + HF |
| Preop | Immediate Postop | Remote Postop | |||
|---|---|---|---|---|---|
| ASD | HRV ↓ #,*,1 | Surgery | HRV ↓ (vs. preop) | Surgery | HRV ↑ (vs. preop) |
| Cath | HRV ↑ (vs. preop) | Cath | HRV ↑ (vs. preop) | ||
| VSD | RSA ↑ (vs. ASD) | Cath | HRV ↓ in PH vs. NPP | No studies available | |
| TGA | SNS innervation intact | Surgery | SNS denervation | Surgery | Reïnnervation SNS in 32% *,2 |
| HRV ↓ # | No difference or lower HRV #,*,3 | PNS normalized; SNS ↓ # | |||
| Uni ventricular heart | No studies available | Surgery | No studies available | Surgery | PNS ↓; sympathetic denervation; SNS ↑ # |
| CoA | BRS & HRV ↓ # | Surgery | SNS ↑ (vs. cath) | Surgery | HRV and BRS normalized |
| Cath | No studies available | Cath | No studies available | ||
| TOF | No studies available | Surgery | No studies available | Surgery | HRV ↓ # |
| Author | Patient Group (N, Type CHD) | Age Patients | Pre or Postop | Control Group (N, Age) | Measure | Outcome Preoperatively | Outcome Postoperatively |
|---|---|---|---|---|---|---|---|
| Heragu et al. [34] | N = 36. Type of CHD not specified. | 2 w–15 y | Preop & ± 1 w postop | N = 45. 3 w–16 y | 24 h HRV | P vs. C: no difference in mean RR, SDNN, SDNN/RR, SDANN, LF and LF/HF. TP and HF ↓ | All measures except for LF/HF ↓ compared to preop. LF/HF ↑ postop. |
| Massin et al. [59] | N = 258. Various types CHD. | 2 d–14 y | Both | N = 210. 3 d–14 y | 24 h HRV | SDNN, SDNNi, SDANNi, rMSSD, pNN50, LF, HF, LF/HF ↓ in pts in NYHA class II–IV. | SDNN, SDNNi, SDANNi, rMSSD, pNN50, LF, HF, LF/HF ↓ in pts in NYHA class II–IV. |
| Ohuchi et al. [58] | N = 143. ASD, VSD, RVOTR. | Mean 14.6 y | Preop & >1 m postop | N = 47. 15.6 ± 4 | 5 min HRV. Plasma NE. BRS, scintigraphy, blockade study. | - | P vs C: BRS, Log HF & Log LF, H/M↓. Plasma NE and =. |
| McGlone et al. [60] | N = 20. Various CHD | 0–16 y | Preop & 1 d postop | No | 1 h HRV preop, 24 h HVR postop | - | sNN50, SDNN, SDANN, RMSSD, SDNNi ↓ after surgery. |
| Currie et al. [61] | N = 12. TOF, CoA. | 4 ± 1 y | Postop | N = 12. 5 ± 1 y | 5 min HRV | - | P vs. C: SDRR, RMSSD, pNN50, log LF, LF, log HF, HF, LF/HF, DFA =. |
| Aletti et al. [62] | N = 15. Various CHD. | Mean 24 m | Preop | N = 10. 15 ± 10 m | 10 min HRV | P vs. C: TP and LF ↑. Mean RR, VLF, HF =. | - |
| Buchorn et al. [63] | N = 14. Various CHD. | 2.6 ± 1.9 m | Preop | N = 70. 2.1 ± 2.7 m | 24 h HRV. Plasma NE and E. | P (standard treatment) vs. C: SDNN, SDANN, rMSSD, VLF, HF, LF ↓. Mean RR, pNN50, LF/HF, TP =. P (propanolol) vs. C: Mean RR ↑, VLF ↓. All other =. | - |
| Dzimir et al. [64] | N = 112. TOF, VSD, PST, COA, CPL. | Median 36 m | During catherization | N = 14. median 48 m | Plasma E&NE. α- and β adrenoreceptor activity | PST vs. C: plasma E and NE ↑. All groups except CoA increased α-adrenoreceptor densities. LRS & PST vs C: Β-adrenoreceptors ↓. | - |
| Goudjil et al. [65] | N = 22. PDA. | 28 w | Preop | N = 22. 28 w | 4 min HRV | P vs C: TP, LF, HF nu, HF/LF, SNDD ↓. HF, mean RR, RMSSD, SDSD =. | - |
| Kaltman et al. [66] | N = 60. Various CHD. | 4.9 ± 3.3 y | Preop & 0–6 m postop | No | 24 h HRV | Pts with biventricular vs. univentricular heart: mean HR, LF, LF, LF/HF =. | Pts with biventr vs. univentr heart: Mean HR ↓ LF ↑ at discharge. 3–6 m postop: Mean HR, LF, LF, LF/HF =. |
| Bakari et al. [54] | N = 28. ASD. | 6.6 ± 2.1 y | Preop | N = 32. 6.4 ± 2.2 y | 24 h HRV | P vs C: SDNN, SDANN, rMSSD, SD, SDNN index, PNN50, mean RR, TP, HF, LF/HF ↓. | - |
| Massin et al. [55] | N = 20. ASD. | Range 3–14 y | Preop | N = 210. 3 d–14 y | 24 h HRV | P vs. C: mean RR, SDNNi, SDANNi, pNN50, LF, HF ↓. | - |
| Finley et al. [53] | N = 10. ASD. | Range 4–16 y | Preop & ± 5 m postop | N = 10. mean 6.1 y | 10–15 min HRV and respiration standing and supine | P vs. C: Supine: mean RR, LF, resp rate =, SDNN, HF ↓. LF/HF ↑. Upright: mean RR, LF, HF, LF/H, resp rate =. SDNN ↓ | P preop vs. P postop: Supine: mean RR, LF, HF, HF/LF =. SDNN ↑. Upright: mean RR, SDNN, LF, HF, HF/LF =. |
| Bialkowski et al. [56] | N = 19. ASD | 2.5–14 y | Preop & 1 & 3 m postop | From earlier non-english publication | 24 h HRV | P vs. C: SDNN, SDANN, SDNN index, rMSSD, pNN50 ↓. | P (preop) vs. P (after transcutaneous intervention): SDNN and SDANN ↑ 1m after intervention. SDNN, SDANN, SDNN index, rMSSD, pNN50 ↑ 3m after intervention. P (preop) vs. P (after surgical repair): SDNN, SDANN, SDNN index, rMSSD, pNN50 ↓ 1m postop. SDNN, SDANN index ↑ 3m postop. |
| Hata et al. [52] | N = 43. ASD, VSD. | ASD 4.6 ± 3.6 y; VSD 4.1 ± 6.4 y | Preop | No | HRV and respiration (time of recording not specified). | P (ASD) vs. P (VSD): HF, RSA ↓. Respiratory rate, LF, TP, LF/HF = . | - |
| Kul Yum et al. [57] | N = 32. VSD. | <12 months | Preop | No | 5 min HRV during catherization | P (hypertemsive) vs. P (nonhypertensive) HR, SDNN =. LF and HF ↓. | - |
| Kondo et al. [67] | N = 51. TGA. | Mean 4.8 y | Preop & 1 m–10 y postop | N = 51. 4.2–6 m | Scintigraphy | All pts and controls showed positive MIBG uptake. | <1 m after ASO, all pts showed negative MIBG uptake. 15/47 pts negative MIBG uptake late postop. |
| Harrison et al. [68] | N = 15. TGA. | 0–8 w | Preop & 2–8 w postop | N = 16. Age-matched. | 2–4 h HRV | P vs. C: HF, LF ↓. | P vs. C: HF, LF =. Pts showed delayed recovery of HF after feeding. |
| Harrison et al. [69] | N = 15. TGA. | 0–3 y | 2 w and 3 y postop | N = 12. Age matched | 15 m HRV | - | P vs. C: HF =. HF reactivity ↓. |
| Harrison et al. [70] | N = 15. TGA. | 2–8 w | 2–8 w postop | N = 16. Age matched | 15 m HRV | - | P vs. C 2w postop: baseline HF, recovery HF ↓. P vs C 8w postop; baseline HF, recovery HF =. |
| Doksoz et al. [71] | N = 22. TGA. | Mean 59.5 ± 38.7 m | Postop | N = 22. Age 65.1 ± 39.4 m | 24 h HRV | - | P vs. C: mean HR, max HR, min HR = . SDANN, VLF ↑. When awake, SDNN, rMSSD, pNN50, TP, VLF ↑. When asleep SDNN, rMSSD, pNN50, TP, VLF =. |
| Falkenberg et al. [72] | N = 8. TGA. | 15.8 ± 1.5 y | 15 y postop | N = 15. Age 19.7 ± 1 y | BRS and NE whole body and cardiac spillover | - | P vs. C: mean HR, BRS =. Total body NE spillover, regional spillover ↓. |
| Madan et al. [73] | N = 46. Univentricular heart. | BDG 5.2 ± 5.3 y Fontan 9.3 ± 5.9 y | Preop & 2 and 9 m postop | No | 900 s HRV | P (BDG) vs. P (Fontan): coefficient of variance, SDNN, RNSSD, NN50, pNN50, VLF, HF, TP, LF/HF =. | P (BDG) vs. P (Fontan) 2 m postop: RMSSD ↑. P (BDG) 9m postop: coefficient of variance, TP, LF, VLF ↑. P (BDG) 9m postop: RMSSD, HF, LF, VLF ↑. P (BDG) vs. P (Fontan) 9 m postop: Coefficient of variation ↑. |
| Rydberg et al. [74] | N = 15. Univentricular heart. | Age not specified | Mean 7.2 y postop | No | 24 h HRV. Poincaré plots. | - | P (with VA) vs. P (without VA): TP, VLF, LF, HF, HF/LF =. SD poincaré plots ↑. |
| Ohuchi et al. [75] | N = 63. Univentricular heart. | TCPC 12.8 ± 5.0 y, APC 14.2 ± 3.9 y | Postop | N = 44. 14.7 ± 3.9 y | Blockade study, scintigraphy, plasma NE | - | P vs. C: Log HF, log LF, BRS, H/M ↓. NE ↑. |
| Butera et al. [76] | N = 39. Univentricular heart. | Mean 12.2 ± 4.1 y | Mean 5.8 y postop | N = 18. 11.1 ± 2.5 y | 24 h HRV | - | P vs. C: SDNN, RMSSD, pNN50, TP, LF, HF ↓. |
| Polson et al. [77] | N = 8. CoA. | Term neonates | Preop | N = 13. term neonates. | Spontaneous BRS, 15 min HRV, BPV | P vs. C: HR =. BRS ↓. SDNN, RMSSD, TP, LF, HF ↓. BPV HF ↑. | - |
| Kenny et al. [78] | N = 6. CoA. | 5 y | Postop | N = 7. Age & sex matched | Spontaneous BRS, 15 min HRV, BPV | P vs. C: BRS, SDNN, TP, LF, HF, BPV =. | |
| Kenny et al. [79] | N = 29. CoA. | 14-18 y | <1 y posop | N = 20. 15.7 ± 0.3 y | BRS, HRV, BPV | P vs. C: HR, HF, LF, BPV =. | |
| Beekman et al. [80] | N = 6. CoA. | Mean 16.8 ± 2.0 y | 1–11 y postop | N = 7. 15.4 ± 4 y | BP, HR response to exercise, BRS | - | P vs. C: BRS ↓. BP response to exercise ↑. Resting HR, max HR =. |
| Choy et al. [81] | N = 15. CoA. | Mean 8.5 y | Preop & <1 y postop | No | BP, Plasma renin and catecholamine | P (surgical intervention) vs. P (cathetherization): plasma renin, plasma catecholamines ↑. | |
| Silvilairat et al. [82] | N = 30. TOF. | Median 14 y | 2–16 y postop | No | 24 h HRV | - | Positive correlation between LF and VO2peak and HF and VO2peak. |
| Butera et al. [83] | N = 23. TOF. | 14 ± 6.6 y | Postop | N = 18. 11.2 ± 4.9 y | 24 h HRV | - | P vs. C: SDNN, RMSSD, pNN50, TP, LF, HF, LF/HF ↓. |
| Wyller et al. [84] | N = 17. TOF. | Median 16 y | 10–16 y postop | N = 56. Age range 13–18 y | 24 h HRV, LBNP | - | P vs. C during rest: LF, HF, LF/HF, SDNN, pNN50, RMSSD =. P vs. C during LBNP: HR response ↓. TP, HF, SDNN, pNN50, RMSSD ↓ in controls and ↑ in pts. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nederend, I.; Jongbloed, M.R.M.; De Geus, E.J.C.; Blom, N.A.; Ten Harkel, A.D.J. Postnatal Cardiac Autonomic Nervous Control in Pediatric Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 16. https://doi.org/10.3390/jcdd3020016
Nederend I, Jongbloed MRM, De Geus EJC, Blom NA, Ten Harkel ADJ. Postnatal Cardiac Autonomic Nervous Control in Pediatric Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2016; 3(2):16. https://doi.org/10.3390/jcdd3020016
Chicago/Turabian StyleNederend, Ineke, Monique R. M. Jongbloed, Eco J. C. De Geus, Nico A. Blom, and Arend D. J. Ten Harkel. 2016. "Postnatal Cardiac Autonomic Nervous Control in Pediatric Congenital Heart Disease" Journal of Cardiovascular Development and Disease 3, no. 2: 16. https://doi.org/10.3390/jcdd3020016
APA StyleNederend, I., Jongbloed, M. R. M., De Geus, E. J. C., Blom, N. A., & Ten Harkel, A. D. J. (2016). Postnatal Cardiac Autonomic Nervous Control in Pediatric Congenital Heart Disease. Journal of Cardiovascular Development and Disease, 3(2), 16. https://doi.org/10.3390/jcdd3020016







