Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation
Abstract
:1. Introduction
2. Experimental Section
2.1. Transgenic Lines and Tamoxifen Injection
2.2. Antibodies and Immunofluorescence
2.3. X-gal Staining
2.4. Clonal Analysis
3. Results
3.1. SMA+ Cells in the Early Heart Tube Contribute to the Interventricular Septum
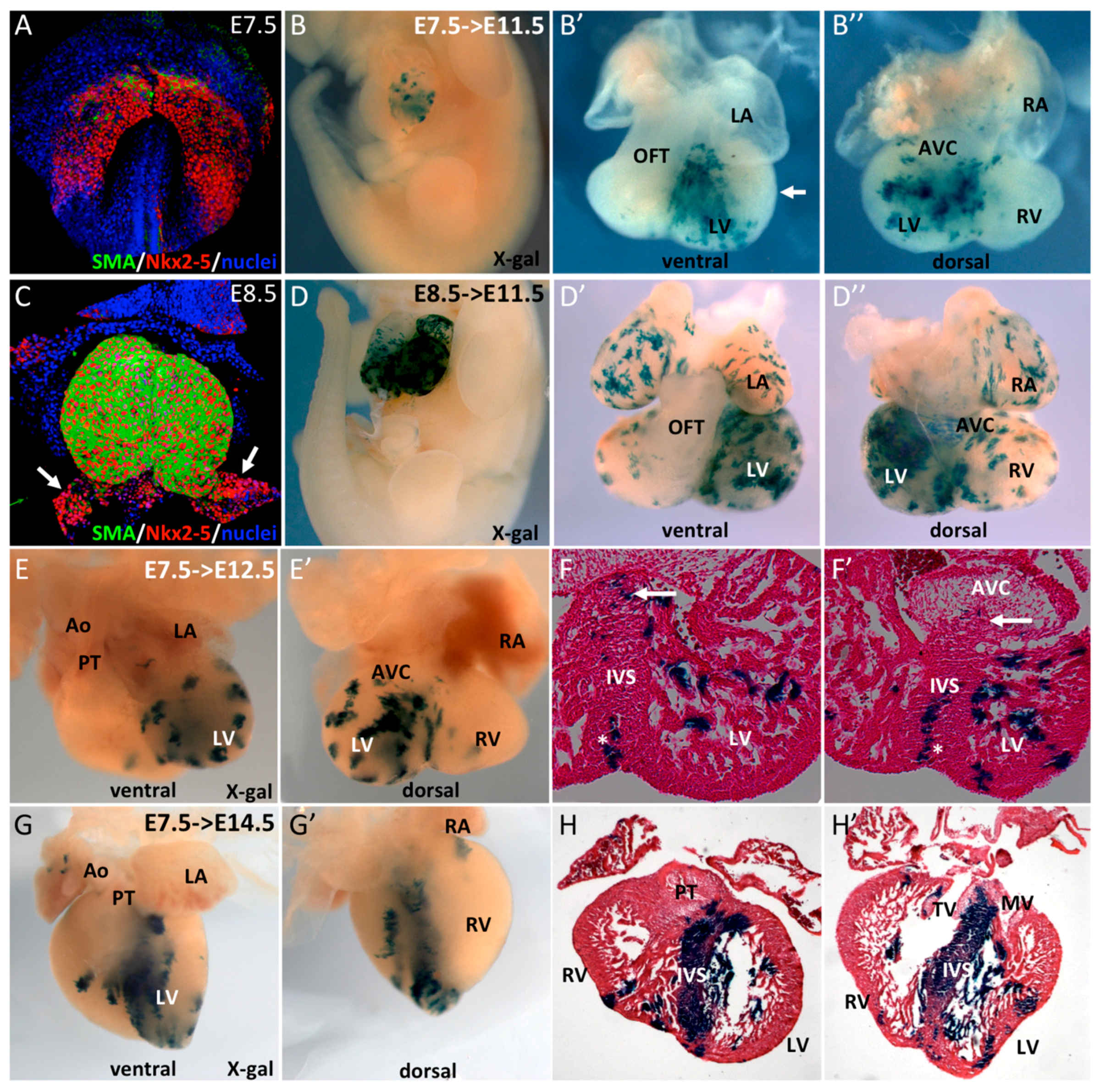
3.2. SMA+ Early Cardiac Cells Contribute to the Ventricular Conduction System
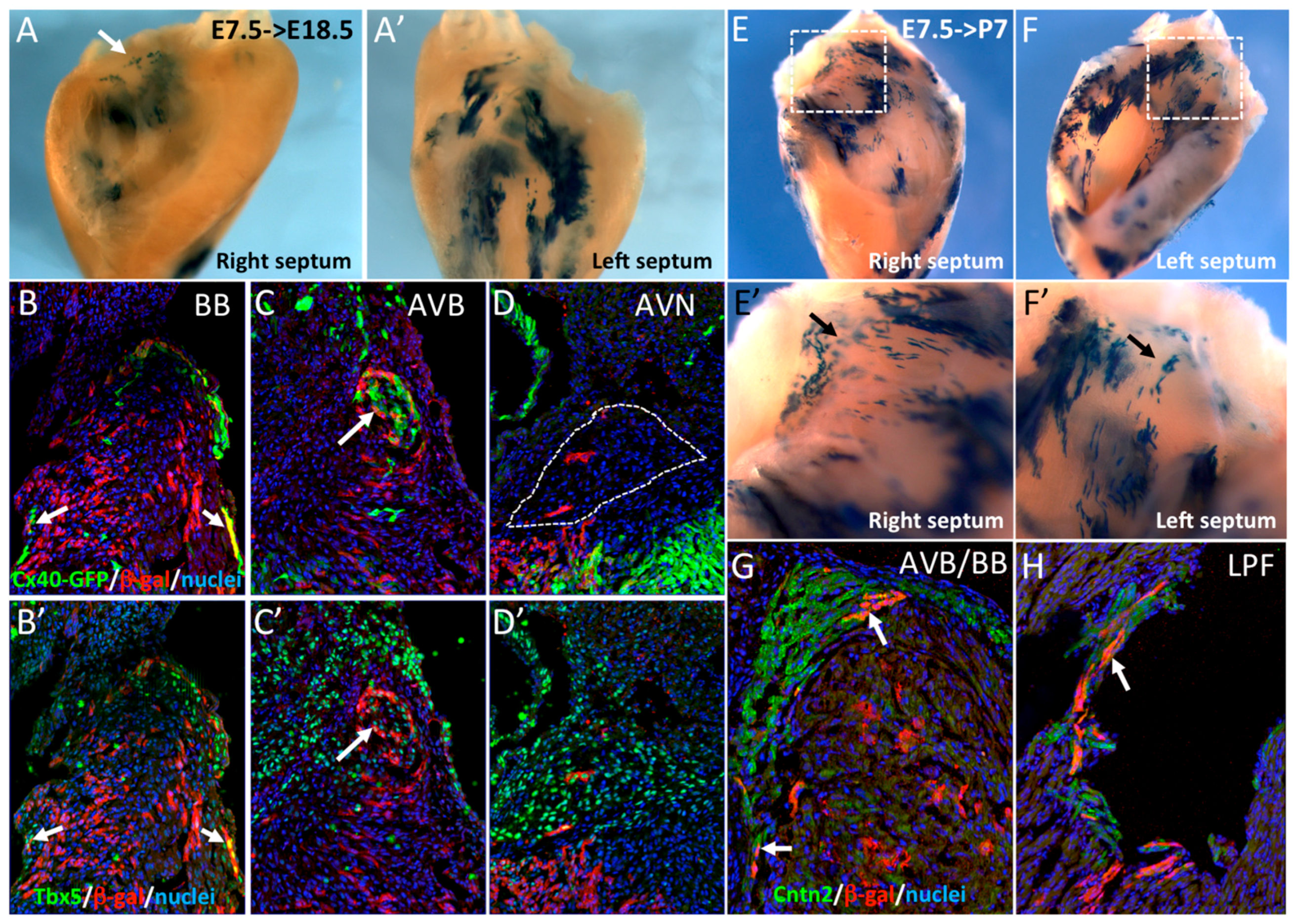
3.3. The Central Ventricular Conduction System Lineage Segregates from SMA+ Early Cardiomyocytes
3.4. SMA-Derived Early Cardiomyocytes form the Primary Heart Tube

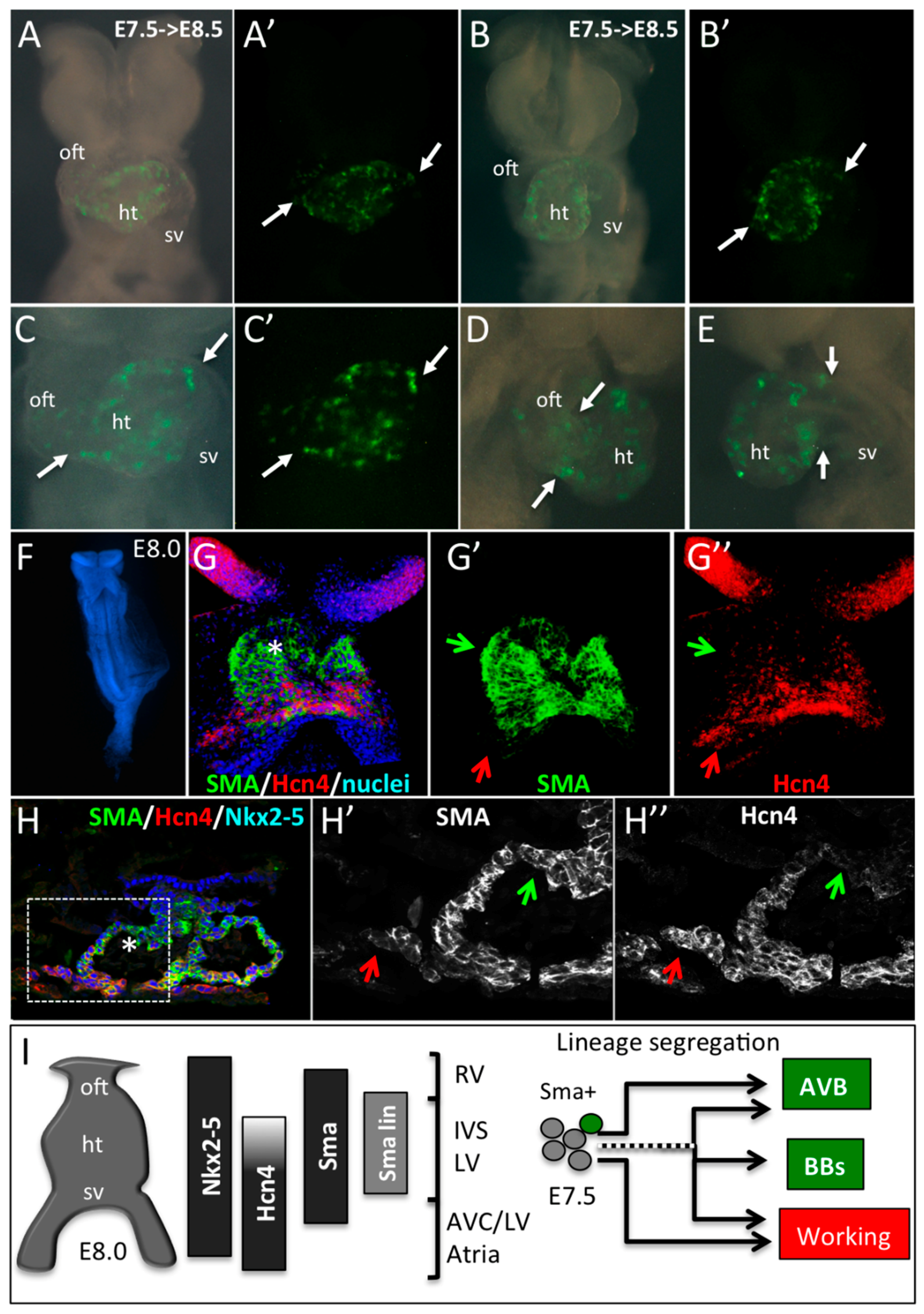
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anderson, R.H.; Ho, S.Y. The morphology of the cardiac conduction system. Novartis Found. Symp. 2003, 250, 6–17; discussion 18–24, 276–279. [Google Scholar] [PubMed]
- Benson, D.W. Genetics of atrioventricular conduction disease in humans. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Moorman, A.F. Development of the cardiac conduction system: Why are some regions of the heart more arrhythmogenic than others? Circ. Arrhythm. Electrophysiol. 2009, 2, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Viragh, S.; Challice, C.E. The development of the conduction system in the mouse embryo heart. Dev. Biol. 1982, 89, 25–40. [Google Scholar] [CrossRef]
- Wessels, A.; Vermeulen, J.L.; Verbeek, F.J.; Viragh, S.; Kalman, F.; Lamers, W.H.; Moorman, A.F. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle. Iii. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat. Rec. 1992, 232, 97–111. [Google Scholar] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Kelly, R.G. Organogenesis of the vertebrate heart. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Meilhac, S.M.; Esner, M.; Kelly, R.G.; Nicolas, J.F.; Buckingham, M.E. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev. Cell. 2004, 6, 685–698. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Liang, X.; Evans, S.M.; Sun, Y. Insights into cardiac conduction system formation provided by HCN4 expression. Trends Cardiovasc. Med. 2015, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Beyer, S.; Kelly, R.G. Establishment of the mouse ventricular conduction system. Cardiovasc. Res. 2011, 91, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Bellon, A.; Moreno, N.; Beyer, S.; Meilhac, S.M.; Buckingham, M.; Franco, D.; Kelly, R.G. Resolving cell lineage contributions to the ventricular conduction system with a Cx40-GFP allele: A dual contribution of the first and second heart fields. Dev. Dyn. 2013, 242, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Spater, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell biol. 2013, 15, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 dynamically marks the first heart field and conduction system precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wendling, O.; Bornert, J.M.; Chambon, P.; Metzger, D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis 2009, 47, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.J.; Larina, I.V.; Dickinson, M.E.; Zimmer, W.E.; Hirschi, K.K. Characterization of bacterial artificial chromosome transgenic mice expressing mcherry fluorescent protein substituted for the murine smooth muscle alpha-actin gene. Genesis 2010, 48, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, B.P.; Van Den Hoff, M.J.; Tesink-Taekema, S.; Moorman, A.F. Recruitment of intra- and extracardiac cells into the myocardial lineage during mouse development. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 271, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, D.L.; Schwartz, R.J. Sequential activation of alpha-actin genes during avian cardiogenesis: Vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J. Cell Biol. 1988, 107, 2575–2586. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Meysen, S.; Mangoni, M.; Bois, P.; van Rijen, H.V.; Abran, P.; Jongsma, H.; Nargeot, J.; Gros, D. Architectural and functional asymmetry of the his-purkinje system of the murine heart. Cardiovasc. Res. 2004, 63, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Snippert, H.J.; van der Flier, L.G.; Sato, T.; van Es, J.H.; van den Born, M.; Kroon-Veenboer, C.; Barker, N.; Klein, A.M.; van Rheenen, J.; Simons, B.D.; et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 2010, 143, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999, 21, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Watanabe, T.; Lin, C.S.; William, C.M.; Tanabe, Y.; Jessell, T.M.; Costantini, F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G. The second heart field. Curr. Top. Dev. Biol. 2012, 100, 33–65. [Google Scholar] [PubMed]
- Aanhaanen, W.T.; Brons, J.F.; Dominguez, J.N.; Rana, M.S.; Norden, J.; Airik, R.; Wakker, V.; de Gier-de Vries, C.; Brown, N.A.; Kispert, A.; et al. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ. Res. 2009, 104, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Moreno-Rascon, N.; Beyer, S.; Dupays, L.; Meilhac, S.M.; Buckingham, M.E.; Franco, D.; Kelly, R.G. Biphasic development of the mammalian ventricular conduction system. Circ. Res. 2010, 107, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Devine, W.P.; Wythe, J.D.; George, M.; Koshiba-Takeuchi, K.; Bruneau, B.G. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. eLIFE 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, M.V.; Castillo, M.M.; Villavicencio, L.; Valencia, A.; Moreno-Rodriguez, R.A. Primitive interventricular septum, its primordium, and its contribution in the definitive interventricular septum: In vivo labelling study in the chick embryo heart. Anat. Rec. 1997, 247, 512–520. [Google Scholar] [CrossRef]
- Van den Berg, G.; Moorman, A.F. Concepts of cardiac development in retrospect. Pediatr. Cardiol. 2009, 30, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.P.; Phoon, C.K.; Aristizabal, O.; McGrath, K.E.; Palis, J.; Turnbull, D.H. Onset of cardiac function during early mouse embryogenesis coincides with entry of primitive erythroblasts into the embryo proper. Circ. Res. 2003, 92, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; de Jong, F.; Denyn, M.M.; Lamers, W.H. Development of the cardiac conduction system. Circ. Res. 1998, 82, 629–644. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choquet, C.; Marcadet, L.; Beyer, S.; Kelly, R.G.; Miquerol, L. Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation. J. Cardiovasc. Dev. Dis. 2016, 3, 2. https://doi.org/10.3390/jcdd3010002
Choquet C, Marcadet L, Beyer S, Kelly RG, Miquerol L. Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation. Journal of Cardiovascular Development and Disease. 2016; 3(1):2. https://doi.org/10.3390/jcdd3010002
Chicago/Turabian StyleChoquet, Caroline, Laetitia Marcadet, Sabrina Beyer, Robert G. Kelly, and Lucile Miquerol. 2016. "Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation" Journal of Cardiovascular Development and Disease 3, no. 1: 2. https://doi.org/10.3390/jcdd3010002
APA StyleChoquet, C., Marcadet, L., Beyer, S., Kelly, R. G., & Miquerol, L. (2016). Segregation of Central Ventricular Conduction System Lineages in Early SMA+ Cardiomyocytes Occurs Prior to Heart Tube Formation. Journal of Cardiovascular Development and Disease, 3(1), 2. https://doi.org/10.3390/jcdd3010002





