Probing the Electrophysiology of the Developing Heart
Abstract
:1. Introduction
1.1. Why Is It Important to Study Electrophysiology of the Developing Heart?
1.2. What Are the Barriers to Studying the Electrophysiology of the Developing Heart?
1.3. Overview of the Development of Pacemaking and Conduction Patterns of the Heart
1.3.1. Earliest Stages of Cardiac Development
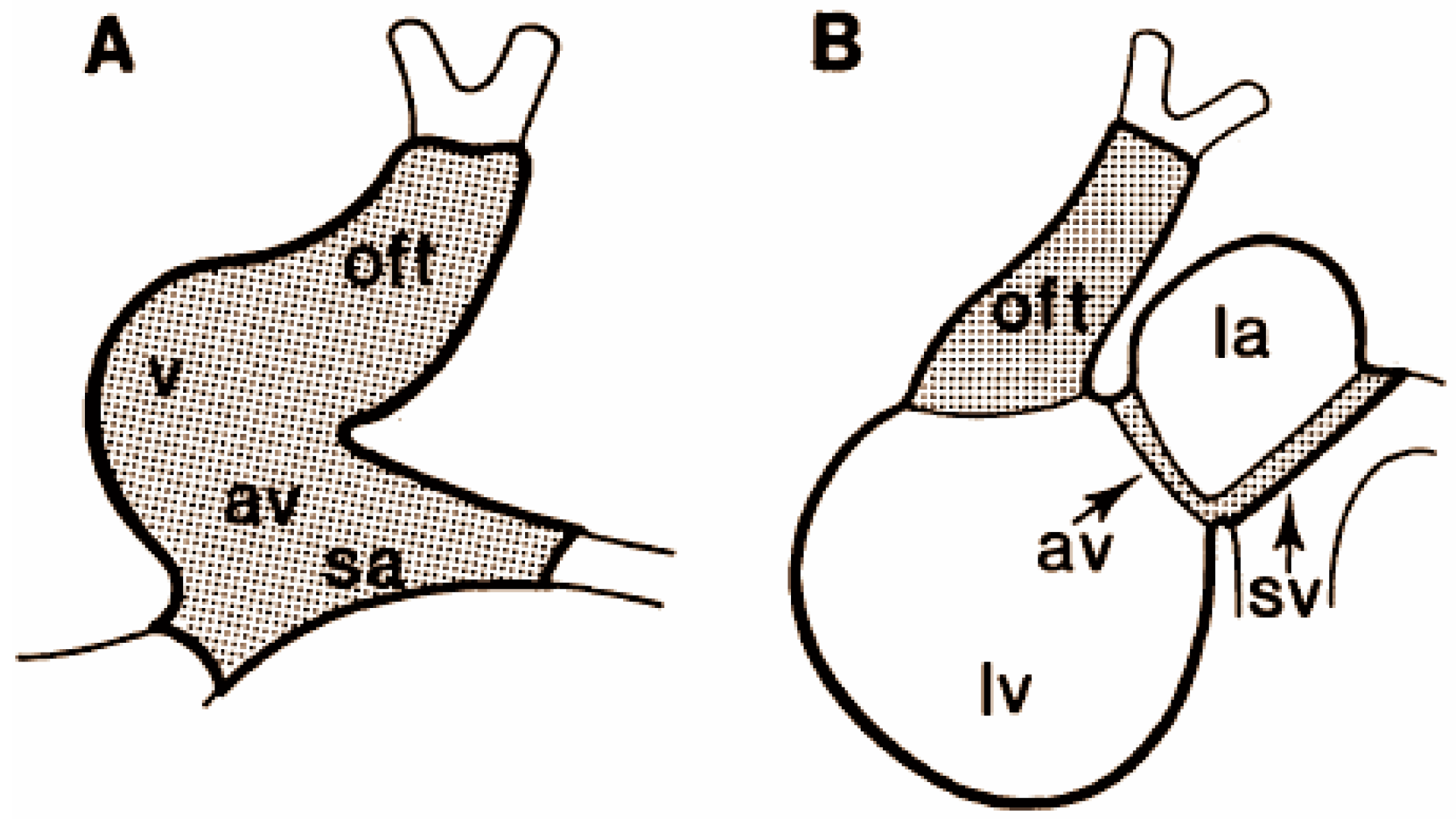
1.3.2. Alternating Regions of Slow and Fast Conduction of the Looping Heart
1.3.3. Transition to the Apex-to-Base Conduction
1.4. Animal Models for the Study of Cardiac Function
1.4.1. The Fruit Fly
1.4.2. Zebrafish
1.4.3. The Birds
1.4.4. Mouse
1.4.5. Rat
1.4.6. Rabbit
1.4.7. Large Animals
2. Electrodes and Application to Study Cardiogenesis
2.1. Electrodes and Technical Considerations
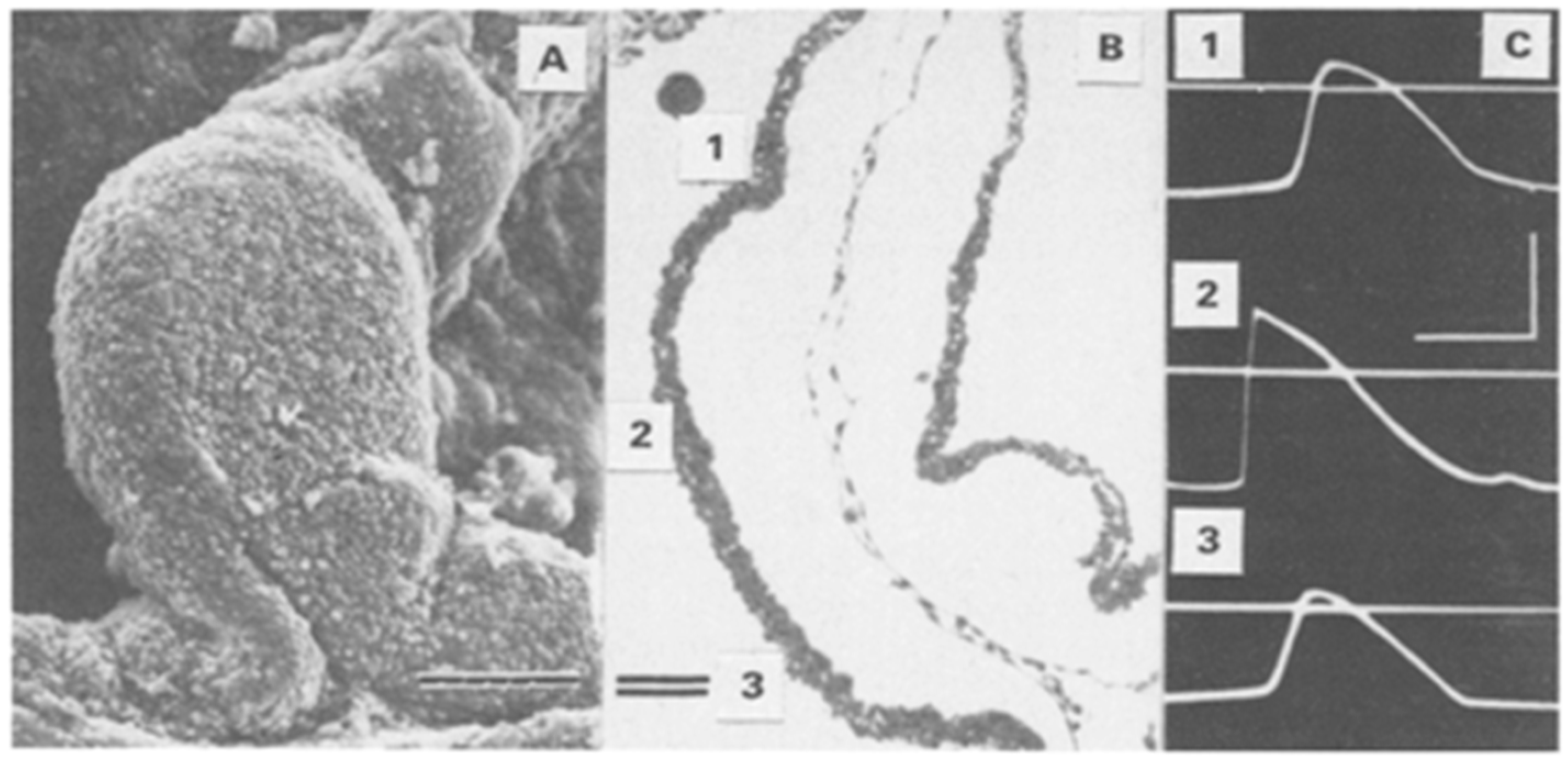
2.2. Probing the Early Stages with Electrodes
2.3. Alternating Regions of Slow and Fast Conduction in the Looping Heart
2.4. Transition during Septation of the Ventricle
2.5. Maturation of the Pacemaking and Cardiac Conduction System
2.6. Electrodes Summary
3. Optical Mapping and Its Applications
3.1. What Is Optical Mapping?
3.2. Application of OM
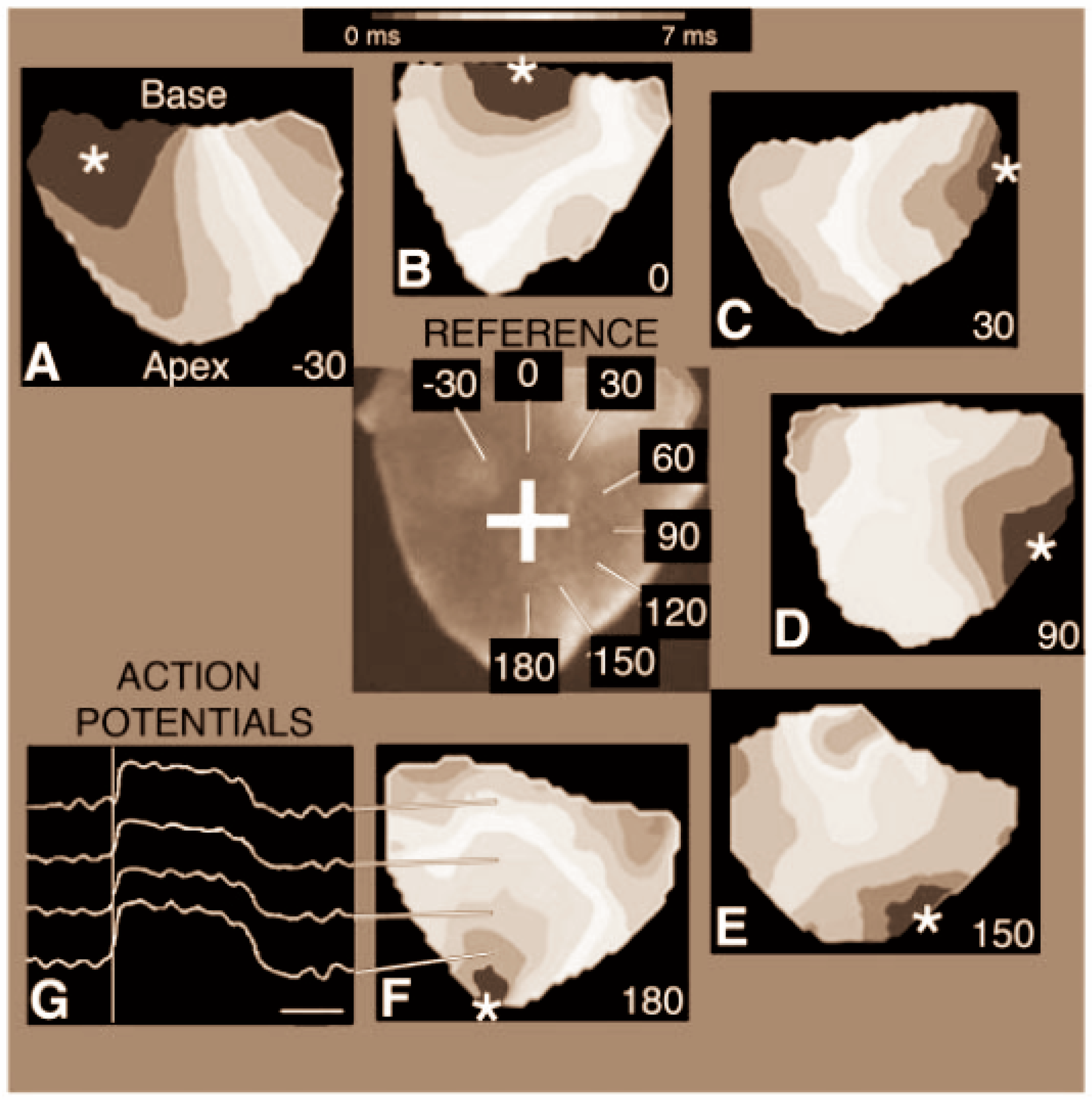
3.2.1. Pioneering OM Studies to Detect Initial Activation Patterns
3.2.2. Finding the Origin of Pacemaker Cells
3.2.3. Gradual Transitions in Conduction Patterns
3.2.4. Heart Field Contributions to Ventricular Maturation
3.2.5. Following the Conduction of the Atrioventricular Junction
3.2.6. Later Maturation of the Conduction System
3.3. Current Limitations of OM and Potential Solutions
3.3.1. OM Is a Terminal Procedure
3.3.2. OM Is a 2D Projection from a 3D Structure
3.3.3. OM Has a Lower Temporal Resolution than Electrodes
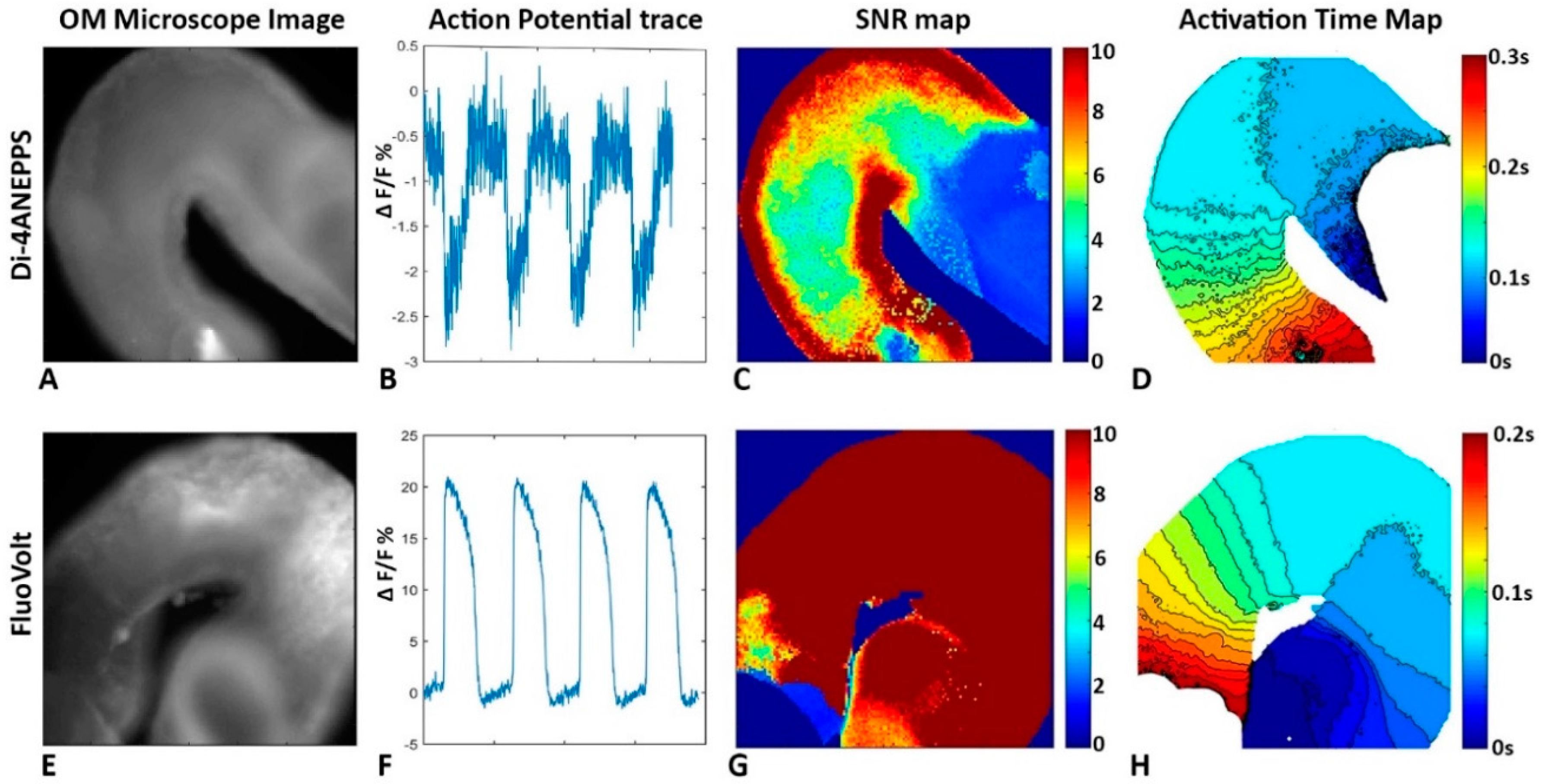
3.3.4. The Embryonic Heart Has Low Signal to Noise Ratios
3.4. Genetic Reporters
4. Controlling the Electrophysiology of the Heart
4.1. The Disadvantages of Electrode Stimulation
4.2. Infrared Pacing and Optogenetics
5. Summary and Future Directions
Acknowledgments
Conflicts of Interest
References
- De Haan, R.L. Organization of the cardiogenic plate in the early chick embryo. Acta Embryol. Morphol. Exp. 1963, 6, 26–38. [Google Scholar]
- Manasek, F.J. Embryonic development of the heart. I. A light and electron microscopic study of early chick embryo. J. Morph. 1978, 125, 333–348. [Google Scholar]
- Patten, B.M. Initiation and early changes in the character of the heart beat in vertebrate embryos. Physiol. Rev. 1949, 29, 31–47. [Google Scholar] [PubMed]
- Koushik, S.V.; Wang, J.; Rogers, R.; Moskophidis, D.; Lambert, N.A.; Creazzo, T.L.; Conway, S.J. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001, 15, 1209–1211. [Google Scholar] [CrossRef] [PubMed]
- Fritz-Six, K.L.; Cox, P.R.; Fischer, R.S.; Xu, B.; Gregorio, C.C.; Zoghbi, H.Y.; Fowler, V.M. Aberrant myofibril assembly in tropomodulin1 null mice leads to aborted heart development and embryonic lethality. J. Cell Biol. 2003, 163, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Drake, C.J.; Schwarz, J.J. The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev. Biol. 1999, 211, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, D.J.; Rentschler, S.; Gourdie, R.G.; Fishman, G.I.; Mikawa, T. Induction and patterning of the cardiac conduction system. Int J. Dev. Biol. 2002, 46, 765–775. [Google Scholar] [PubMed]
- Kowalski, W.J.; Pekkan, K.; Tinney, J.P.; Keller, B.B. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Front. Physiol. 2014, 5, 408. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Garcia-Cardena, G. The vascular origin of hematopoietic cells. Dev. Biol. 2012, 362, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Medvinsky, A.; Rybtsov, S.; Taoudi, S. Embryonic origin of the adult hematopoietic system: Advances and questions. Development 2011, 138, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- North, T.E.; Goessling, W.; Peeters, M.; Li, P.; Ceol, C.; Lord, A.M.; Weber, G.J.; Harris, J.; Cutting, C.C.; Huang, P.; et al. Hematopoietic stem cell development is dependent on blood flow. Cell 2009, 137, 736–748. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; Han, M.; Bravo-Valenzuela, N.J. Changes in vitelline and utero-placental hemodynamics: Implications for cardiovascular development. Front. Physiol. 2014, 5, 390. [Google Scholar] [CrossRef] [PubMed]
- Chuck, E.T.; Freeman, D.M.; Watanabe, M.; Rosenbaum, D.S. Changing activation sequence in the embryonic chick heart. Implications for the development of the his-purkinje system. Circ. Res. 1997, 81, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, S.; Brink, P.R.; Cohen, I.S. Stem cell-based biological pacemakers from proof of principle to therapy: A review. Cytotherapy 2014, 16, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Barbuti, A.; Robinson, R.B. Stem cell-derived nodal-like cardiomyocytes as a novel pharmacologic tool: Insights from sinoatrial node development and function. Pharmacol. Rev. 2015, 67, 368–388. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.F.; Dawkins, J.F.; Cho, H.C.; Marban, E.; Cingolani, E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci. Transl. Med. 2014, 6, 245ra294. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, E.; Ionta, V.; Cheng, K.; Giacomello, A.; Cho, H.C.; Marban, E. Engineered electrical conduction tract restores conduction in complete heart block: From in vitro to in vivo proof of concept. J. Am. Coll. Cardiol. 2014, 64, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Marban, E.; Cingolani, E. Direct reprogramming: Bypassing stem cells for therapeutics. JAMA 2015, 314, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Jay, P.Y.; Rentschler, S. Reprogramming the conduction system: Onward toward a biological pacemaker. Trends Cardiovasc. Med. 2016, 26, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Optical approaches to ontogeny of electrical activity and related functional organization during early heart development. Physiol. Rev. 1991, 71, 53–91. [Google Scholar] [PubMed]
- Chen, F.; De Diego, C.; Chang, M.G.; McHarg, J.L.; John, S.; Klitzner, T.S.; Weiss, J.N. Atrioventricular conduction and arrhythmias at the initiation of beating in embryonic mouse hearts. Dev. Dyn. 2010, 239, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Hurtado, R. Development of the cardiac conduction system. Semin. Cell Dev. Biol. 2007, 18, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Gourdie, R.G.; Harris, B.S.; Bond, J.; Edmondson, A.M.; Cheng, G.; Sedmera, D.; O'Brien, T.X.; Mikawa, T.; Thompson, R.P. His-purkinje lineages and development. Novartis Found. Symp. 2003, 250, 110–122. [Google Scholar] [PubMed]
- Rothenberg, F.; Efimov, I.R.; Watanabe, M. Functional imaging of the embryonic pacemaking and cardiac conduction system over the past 150 years: Technologies to overcome the challenges. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.J.; Stanley, R.L.; Evans, D.J. Developmental stages of the japanese quail. J. Anat. 2010, 216, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Stalsberg, H.; DeHaan, R.L. The precardiac areas and formation of the tubular heart in the chick embryo. Dev. Biol. 1969, 19, 128–159. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Kirby, M.L. Patterning of the heart field in the chick. Dev. Biol. 2008, 319, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Schoenwolf, G.C. Islet-1 marks the early heart rudiments and is asymmetrically expressed during early rotation of the foregut in the chick embryo. Anat. Rec. 2000, 260, 204–207. [Google Scholar] [CrossRef]
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Liu, G.; Mikawa, T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Patten, B.M.; Kramer, T.C. The initiation of contraction in the embryonic chick heart. Am. J. Anat. 1933, 53, 349–375. [Google Scholar] [CrossRef]
- Hirota, A.; Kamino, K.; Komuro, H.; Sakai, T.; Yada, T. Early events in development of electrical activity and contraction in embryonic rat heart assessed by optical recording. J. Physiol. 1985, 369, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Kamino, K.; Komuro, H.; Sakai, T.; Yada, T. Optical studies of excitation-contraction coupling in the early embryonic chick heart. J. Physiol. 1985, 366, 89–106. [Google Scholar] [CrossRef] [PubMed]
- DeHaan, R.L. The potassium-sensitivity of isolated embryonic heart cells increases with development. Dev. Biol. 1970, 23, 226–240. [Google Scholar] [CrossRef]
- de Jong, F.; Opthof, T.; Wilde, A.A.; Janse, M.J.; Charles, R.; Lamers, W.H.; Moorman, A.F. Persisting zones of slow impulse conduction in developing chicken hearts. Circ. Res. 1992, 71, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Arguello, C.; Alanis, J.; Pantoja, O.; Valenzuela, B. Electrophysiological and ultrastructural study of the atrioventricular canal during the development of the chick embryo. J. Mol. Cell Cardiol. 1986, 18, 499–510. [Google Scholar] [CrossRef]
- Arguello, C.; Alanis, J.; Valenzuela, B. The early development of the atrioventricular node and bundle of his in the embryonic chick heart. An electrophysiological and morphological study. Development 1988, 102, 623–637. [Google Scholar] [PubMed]
- Christoffels, V.M.; Burch, J.B.; Moorman, A.F. Architectural plan for the heart: Early patterning and delineation of the chambers and the nodes. Trends Cardiovasc. Med. 2004, 14, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Viragh, S.; Moorman, A.F.; Anderson, R.H.; Lamers, W.H. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ. Res. 2001, 88, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bressan, M.; Yang, P.B.; Louie, J.D.; Navetta, A.M.; Garriock, R.J.; Mikawa, T. Reciprocal myocardial-endocardial interactions pattern the delay in atrioventricular junction conduction. Development 2014, 141, 4149–4157. [Google Scholar] [CrossRef] [PubMed]
- Polo-Parada, L.; Zhang, X.; Modgi, A. Cardiac cushions modulate action potential phenotype during heart development. Dev. Dyn. 2009, 238, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Christoffels, V.M.; Moorman, A.F. The cardiac pacemaker and conduction system develops from embryonic myocardium that retains its primitive phenotype. J. Cardiovasc. Pharmacol. 2010, 56, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Moorman, A.F.; Christoffels, V.M. The atrioventricular node: Origin, development, and genetic program. Trends Cardiovasc. Med. 2010, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Aanhaanen, W.T.; Moorman, A.F.; Christoffels, V.M. Origin and development of the atrioventricular myocardial lineage: Insight into the development of accessory pathways. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Hucker, W.J.; Sharma, V.; Nikolski, V.P.; Efimov, I.R. Atrioventricular conduction with and without AV nodal delay: Two pathways to the bundle of His in the rabbit heart. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Kurian, T.; Ambrosi, C.; Hucker, W.; Fedorov, V.V.; Efimov, I.R. Anatomy and electrophysiology of the human av node. Pacing Clin. Electrophysiol. 2010, 33, 754–762. [Google Scholar] [CrossRef] [PubMed]
- James, T.N. Structure and function of the sinus node, AV node and his bundle of the human heart: Part II--function. Prog. Cardiovasc. Dis. 2003, 45, 327–360. [Google Scholar] [CrossRef] [PubMed]
- James, T.N. Structure and function of the sinus node, AV node and his bundle of the human heart: Part I-structure. Prog. Cardiovasc. Dis. 2002, 45, 235–267. [Google Scholar] [CrossRef] [PubMed]
- Gillers, B.S.; Chiplunkar, A.; Aly, H.; Valenta, T.; Basler, K.; Christoffels, V.M.; Efimov, I.R.; Boukens, B.J.; Rentschler, S. Canonical wnt signaling regulates atrioventricular junction programming and electrophysiological properties. Circ. Res. 2015, 116, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Harris, B.S.; Kuznekoff, L.; Jain, R.; Manderfield, L.; Lu, M.M.; Morley, G.E.; Patel, V.V.; Epstein, J.A. Notch signaling regulates murine atrioventricular conduction and the formation of accessory pathways. J. Clin. Invest. 2011, 121, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, M.M.; Phelps, A.L.; van den Hoff, M.J.; Wessels, A. The epicardium and the development of the atrioventricular junction in the murine heart. J. Dev. Biol. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Markman, M.W.; Vermeulen, J.L.; Anderson, R.H.; Moorman, A.F.; Lamers, W.H. The development of the atrioventricular junction in the human heart. Circ. Res. 1996, 78, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Litchenberg, W.H.; Cole, G.J.; Mikawa, T.; Thompson, R.P.; Gourdie, R.G. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development 1999, 126, 5041–5049. [Google Scholar] [PubMed]
- Gourdie, R.G.; Mima, T.; Thompson, R.P.; Mikawa, T. Terminal diversification of the myocyte lineage generates purkinje fibers of the cardiac conduction system. Development 1995, 121, 1423–1431. [Google Scholar] [PubMed]
- Gourdie, R.G.; Wei, Y.; Kim, D.; Klatt, S.C.; Mikawa, T. Endothelin-induced conversion of embryonic heart muscle cells into impulse-conducting purkinje fibers. Proc. Natl. Acad. Sci. USA 1998, 95, 6815–6818. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.E.; Hurtado, R.; Hewett, K.W.; Shulimovich, M.; Poma, C.P.; Reckova, M.; Justus, C.; Pennisi, D.J.; Tobita, K.; Sedmera, D.; et al. Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting purkinje fibers in the embryonic heart. Development 2004, 131, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Hyer, J.; Johansen, M.; Prasad, A.; Wessels, A.; Kirby, M.L.; Gourdie, R.G.; Mikawa, T. Induction of purkinje fiber differentiation by coronary arterialization. Proc. Natl. Acad. Sci. USA 1999, 96, 13214–13218. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Gourdie, R.G.; Takebayashi-Suzuki, K.; Kanzawa, N.; Hyer, J.; Pennisi, D.J.; Poma, C.P.; Shulimovich, M.; Diaz, K.G.; Layliev, J.; et al. Induction and patterning of the purkinje fibre network. Novartis Found. Symp. 2003, 250, 142–153; discussion 153–156, 276–279. [Google Scholar] [PubMed]
- Takebayashi-Suzuki, K.; Yanagisawa, M.; Gourdie, R.G.; Kanzawa, N.; Mikawa, T. In vivo induction of cardiac purkinje fiber differentiation by coexpression of preproendothelin-1 and endothelin converting enzyme-1. Development 2000, 127, 3523–3532. [Google Scholar] [PubMed]
- Sedmera, D.; Harris, B.S.; Grant, E.; Zhang, N.; Jourdan, J.; Kurkova, D.; Gourdie, R.G. Cardiac expression patterns of endothelin-converting enzyme (ECE): Implications for conduction system development. Dev. Dyn. 2008, 237, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.L.; Vedantham, V.; Barnes, R.M.; Hu, J.; Robinson, A.S.; Bressan, M.; Srivastava, D.; Black, B.L. Specification of the mouse cardiac conduction system in the absence of endothelin signaling. Dev. Biol. 2014, 393, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Zander, J.; Meyers, K.; France, D.; Levine, R.; Porter, G.; Rivkees, S.A.; Morley, G.E.; Fishman, G.I. Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc. Natl. Acad. Sci. USA 2002, 99, 10464–10469. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Schulz, R.A. Heart development in drosophila. Semin. Cell Dev. Biol. 2007, 18, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. The gene tinman is required for specification of the heart and visceral muscles in drosophila. Development 1993, 118, 719–729. [Google Scholar] [PubMed]
- Azpiazu, N.; Frasch, M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor Nkx2–5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Alex, A.; Li, A.; Tanzi, R.E.; Zhou, C. Optogenetic pacing in Drosophila melanogaster. Sci. Adv. 2015, 1, e1500639. [Google Scholar] [CrossRef] [PubMed]
- Lalevee, N.; Monier, B.; Senatore, S.; Perrin, L.; Semeriva, M. Control of cardiac rhythm by ORK1, a drosophila two-pore domain potassium channel. Curr. Biol. 2006, 16, 1502–1508. [Google Scholar] [PubMed]
- Senatore, S.; Rami Reddy, V.; Semeriva, M.; Perrin, L.; Lalevee, N. Response to mechanical stress is mediated by the trpa channel painless in the drosophila heart. PLoS Genet. 2010, 6, e1001088. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Medioni, C.; Senatore, S.; Salmand, P.A.; Lalevee, N.; Perrin, L.; Semeriva, M. The fabulous destiny of the drosophila heart. Curr. Opin. Genet. Dev. 2009, 19, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W.W.; Pinder, A.W. Ontogeny of cardiovascular and respiratory physiology in lower vertebrates. Annu. Rev. Physiol. 1991, 53, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Haffter, P.; Nusslein-Volhard, C. Large scale genetics in a small vertebrate, the zebrafish. Int. J. Dev. Biol. 1996, 40, 221–227. [Google Scholar] [PubMed]
- Stainier, D.Y.; Fouquet, B.; Chen, J.N.; Warren, K.S.; Weinstein, B.M.; Meiler, S.E.; Mohideen, M.A.; Neuhauss, S.C.; Solnica-Krezel, L.; Schier, A.F.; et al. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 1996, 123, 285–292. [Google Scholar] [PubMed]
- Chi, N.C.; Bussen, M.; Brand-Arzamendi, K.; Ding, C.; Olgin, J.E.; Shaw, R.M.; Martin, G.R.; Stainier, D.Y. Cardiac conduction is required to preserve cardiac chamber morphology. Proc. Natl. Acad. Sci. USA 2010, 107, 14662–14667. [Google Scholar] [CrossRef] [PubMed]
- Bartman, T.; Walsh, E.C.; Wen, K.K.; McKane, M.; Ren, J.; Alexander, J.; Rubenstein, P.A.; Stainier, D.Y. Early myocardial function affects endocardial cushion development in zebrafish. PLoS Biol. 2004, 2, E129. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.C.; Shaw, R.M.; Jungblut, B.; Huisken, J.; Ferrer, T.; Arnaout, R.; Scott, I.; Beis, D.; Xiao, T.; Baier, H.; et al. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 2008, 6, e109. [Google Scholar] [CrossRef] [PubMed]
- Bill, B.R.; Petzold, A.M.; Clark, K.J.; Schimmenti, L.A.; Ekker, S.C. A primer for morpholino use in zebrafish. Zebrafish 2009, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Westcot, S.E.; Ekker, S.C. Lessons from morpholino-based screening in zebrafish. Brief. Funct. Genomics 2011, 10, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Stainier, D.Y.; Kontarakis, Z.; Rossi, A. Making sense of anti-sense data. Dev. Cell 2015, 32, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Werdich, A.A.; Brzezinski, A.; Jeyaraj, D.; Khaled Sabeh, M.; Ficker, E.; Wan, X.; McDermott, B.M., Jr.; Macrae, C.A.; Rosenbaum, D.S. The zebrafish as a novel animal model to study the molecular mechanisms of mechano-electrical feedback in the heart. Prog. Biophys Mol. Biol. 2012, 110, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Poynter, G.; Huss, D.; Filla, M.B.; Czirok, A.; Rongish, B.J.; Little, C.D.; Fraser, S.E.; Lansford, R. Dynamic analysis of vascular morphogenesis using transgenic quail embryos. PLoS One 2010, 5, e12674. [Google Scholar] [CrossRef] [PubMed]
- Poynter, G.; Huss, D.; Lansford, R. Japanese quail: An efficient animal model for the production of transgenic avians. Cold Spring Harb. Protoc. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Poynter, G.; Lansford, R. Generating transgenic quail using lentiviruses. Methods Cell Biol. 2008, 87, 281–293. [Google Scholar] [PubMed]
- Seidl, A.H.; Sanchez, J.T.; Schecterson, L.; Tabor, K.M.; Wang, Y.; Kashima, D.T.; Poynter, G.; Huss, D.; Fraser, S.E.; Lansford, R.; et al. Transgenic quail as a model for research in the avian nervous system: A comparative study of the auditory brainstem. J. Comp. Neurol. 2013, 521, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Karunamuni, G.H.; Gu, S.; Ford, M.R.; Peterson, L.M.; Ma, P.; Wang, Y.T.; Rollins, A.M.; Jenkins, M.W.; Watanabe, M. Capturing structure and function in an embryonic heart with biophotonic tools. Front. Physiol. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L. Neural crest, great arteries, and outflow septation. In Cardiac development; Oxford University Press: New York, NY, USA, 2007; pp. 143–160. [Google Scholar]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Bockman, D.E.; Kirby, M.L. Dependence of thymus development on derivatives of the neural crest. Science 1984, 223, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Bockman, D.E. Neural crest and normal development: A new perspective. Anat. Rec. 1984, 209, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Van Mierop, L.H.; Kutsche, L.M. Cardiovascular anomalies in digeorge syndrome and importance of neural crest as a possible pathogenetic factor. Am. J. Cardiol. 1986, 58, 133–137. [Google Scholar] [CrossRef]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hamlin, R.L.; Altschuld, R.A. Extrapolation from mouse to man. Circ. Cardiovasc. Imaging 2011, 4, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Sedmera, D. Developmental anatomy of the heart: A tale of mice and man. Physiol. Genomics 2003, 15, 165–176. [Google Scholar] [CrossRef] [PubMed]
- James, J.F.; Hewett, T.E.; Robbins, J. Cardiac physiology in transgenic mice. Circ. Res. 1998, 82, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ripplinger, C.M. Mouse models of cardiac conduction system markers: Revealing the past, present, and future of pacemaking and conduction. Trends Cardiovasc. Med. 2015, 25, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Evans, S.M.; Sun, Y. Insights into cardiac conduction system formation provided by HCN4 expression. Trends Cardiovasc. Med. 2015, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Vaidya, D.M.; Tamaddon, H.; Degenhardt, K.; Sassoon, D.; Morley, G.E.; Jalife, J.; Fishman, G.I. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001, 128, 1785–1792. [Google Scholar] [PubMed]
- Stroud, D.M.; Darrow, B.J.; Kim, S.D.; Zhang, J.; Jongbloed, M.R.; Rentschler, S.; Moskowitz, I.P.; Seidman, J.; Fishman, G.I. Complex genomic rearrangement in CCS-LacZ transgenic mice. Genesis 2007, 45, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kreuzberg, M.M.; Sohl, G.; Kim, J.S.; Verselis, V.K.; Willecke, K.; Bukauskas, F.F. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ. Res. 2005, 96, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Kruger, O.; Plum, A.; Kim, J.S.; Winterhager, E.; Maxeiner, S.; Hallas, G.; Kirchhoff, S.; Traub, O.; Lamers, W.H.; Willecke, K. Defective vascular development in connexin 45-deficient mice. Development 2000, 127, 4179–4193. [Google Scholar] [PubMed]
- Hoesl, E.; Stieber, J.; Herrmann, S.; Feil, S.; Tybl, E.; Hofmann, F.; Feil, R.; Ludwig, A. Tamoxifen-inducible gene deletion in the cardiac conduction system. J. Mol. Cell Cardiol. 2008, 45, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Spater, D.; Abramczuk, M.K.; Buac, K.; Zangi, L.; Stachel, M.W.; Clarke, J.; Sahara, M.; Ludwig, A.; Chien, K.R. A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nat. Cell Biol 2013, 15, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 dynamically marks the first heart field and conduction system precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, S.; Zhao, Y. Inducible gene deletion in the entire cardiac conduction system using Hcn4-CreERT2 BAC transgenic mice. Genesis 2014, 52, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Guan, Y.; Wang, L.; Qiu, Z.; Liu, M.; Chen, Y.; Wu, L.; Li, Y.; Ma, X.; Liu, M.; et al. CRISPR/Cas-mediated genome editing in the rat via direct injection of one-cell embryos. Nat. Protoc. 2014, 9, 2493–2512. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Shao, Y.; Li, D.; Liu, M. Generation of site-specific mutations in the rat genome via CRISPR/Cas9. Methods Enzymol 2014, 546, 297–317. [Google Scholar] [PubMed]
- Atkinson, A.J.; Logantha, S.J.; Hao, G.; Yanni, J.; Fedorenko, O.; Sinha, A.; Gilbert, S.H.; Benson, A.P.; Buckley, D.L.; Anderson, R.H.; et al. Functional, anatomical, and molecular investigation of the cardiac conduction system and arrhythmogenic atrioventricular ring tissue in the rat heart. J. Am. Heart Assoc. 2013, 2, e000246. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, N.; Tamaki, H.; Kikawada, R.; Yamashina, S. Development of the conduction system in the rat heart as determined by Leu-7 (HNK-1) immunohistochemistry and computer graphics reconstruction. Lab. Invest. 1995, 72, 355–366. [Google Scholar] [PubMed]
- Gorza, L.; Schiaffino, S.; Vitadello, M. Heart conduction system: A neural crest derivative? Brain Res. 1988, 457, 360–366. [Google Scholar] [CrossRef]
- Dobrzynski, H.; Nikolski, V.P.; Sambelashvili, A.T.; Greener, I.D.; Yamamoto, M.; Boyett, M.R.; Efimov, I.R. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ. Res. 2003, 93, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Vettore, S.; Lamar, E.; Chien, K.R.; Gorza, L. Neurofilament m mrna is expressed in conduction system myocytes of the developing and adult rabbit heart. J. Mol. Cell Cardiol. 1996, 28, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, F.; Efimov, I.R. Three-dimensional anatomy of the conduction system of the early embryonic rabbit heart. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006, 288, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Matteoli, M.; Gorza, L. Neurofilament proteins are co-expressed with desmin in heart conduction system myocytes. J. Cell Sci. 1990, 97, 11–21. [Google Scholar] [PubMed]
- Etemadi, M.; Heller, J.A.; Schecter, S.C.; Shue, E.H.; Miniati, D.; Roy, S. Implantable ultra-low pulmonary pressure monitoring system for fetal surgery. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Mummery, C.L.; Lee, R.T. Model systems for cardiovascular regenerative biology. Cold Spring Harb. Perspect. Med. 2013, 3, a014019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, E.Y.; Adzick, N.S. Fetoscopy. Semin. Laparosc. Surg. 1998, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.; Strumper, D.; Witteler, R.; Merschhoff, G.; Alexiene, R.; Callenbeck, C.; Asfour, B.; Reckers, J.; Aryee, S.; Vahlhaus, C.; et al. Fetoscopic direct fetal cardiac access in sheep: An important experimental milestone along the route to human fetal cardiac intervention. Circulation 2000, 102, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.; Szabo, Z.; Suda, K.; Petrossian, E.; Ko, E.; Kececioglu, D.; Moore, P.; Silverman, N.H.; Harrison, M.R.; Chou, T.M.; et al. Fetoscopic and open transumbilical fetal cardiac catheterization in sheep. Potential approaches for human fetal cardiac intervention. Circulation 1997, 95, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Kohl, T.; Witteler, R.; Strumper, D.; Gogarten, W.; Asfour, B.; Reckers, J.; Merschhoff, G.; Marcus, A.E.; Weyand, M.; Van Aken, H.; et al. Operative techniques and strategies for minimally invasive fetoscopic fetal cardiac interventions in sheep. Surg. Endosc. 2000, 14, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Jedeikin, L.A. Regional distribution of glycogen and phosphorylase in the ventricles of the heart. Circ. Res. 1964, 14, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Thornell, L.E. Distinction of glycogen and ribosome particles in cow purkinje fibers by enzymatic digestion en bloc and in sections. J. Ultrastruct. Res. 1974, 47, 153–168. [Google Scholar] [CrossRef]
- Thornell, L.E. An ultrahistochemical study on glycogen in cow purkinje fibres. J. Mol. Cell Cardiol. 1974, 6, 439–448. [Google Scholar] [CrossRef]
- Van Mierop, L.H.S. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am. J. Physiol. 1967, 212, 407–415. [Google Scholar] [PubMed]
- Brown, K.T.; Flaming, D.G. Advanced micropipette techniques for cell Physiology. In Ibro handbook series: Methods in the neurosciences; Brown, K.T., Ed.; Wiley: Hoboken, NJ., USA, 1986. [Google Scholar]
- Kornblum, A.; Pillekamp, F.; Matzkies, M.; Fleischmann, B.; Bonnemeier, H.; Schunkert, H.; Brockmeier, K.; Hescheler, J.; Reppel, M. A new model to perform electrophysiological studies in the early embryonic mouse heart. Cell Physiol. Biochem. 2013, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Tzeng, T.H.; Huang, Y.C.; Chen, Y.H.; Chang, Y.C.; Ho, Y.L.; Wu, J.T.; Lee, H.H.; Lai, P.J.; Liu, K.Y.; et al. Non-invasive drosophila ecg recording by using eutectic gallium-indium alloy electrode: A feasible tool for future research on the molecular mechanisms involved in cardiac arrhythmia. PLoS One 2014, 9, e104543. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, P.; Ma, Q.D.; Bartsch, R.P. Maternal-fetal heartbeat phase synchronization. Proc. Natl. Acad. Sci. USA 2009, 106, 13641–13642. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, P.; Geue, D.; Thiel, M.; Cysarz, D.; Lange, S.; Romano, M.C.; Wessel, N.; Kurths, J.; Gronemeyer, D.H. Influence of paced maternal breathing on fetal-maternal heart rate coordination. Proc. Natl. Acad. Sci. USA 2009, 106, 13661–13666. [Google Scholar] [CrossRef] [PubMed]
- Komamura, K.; Adachi, Y.; Miyamoto, M.; Kawai, J.; Haruta, Y.; Uehara, G. Micro-magnetocardiography system with a single-chip squid magnetometer array for qt analysis and diagnosis of myocardial injury in small animals. IEEE Trans. Biomed. Circuits Syst. 2008, 2, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Brisinda, D.; Sorbo, A.R.; Venuti, A.; Fenici, R. Percutaneous method for single-catheter multiple monophasic action potential recordings during magnetocardiographic mapping in spontaneously breathing rodents. Physiol. Meas. 2012, 33, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Gutbrod, S.R.; Sulkin, M.S.; Rogers, J.A.; Efimov, I.R. Patient-specific flexible and stretchable devices for cardiac diagnostics and therapy. Prog. Biophys. Mol. Biol. 2014, 115, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Sissman, N.J. Developmental landmarks in cardiac morphogenesis: Comparative chronology. Am. J. Cardiol. 1970, 25, 141–148. [Google Scholar] [CrossRef]
- Martinsen, B.J. Reference guide to the stages of chick heart embryology. Dev. Dyn. 2005, 233, 1217–1237. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K. Regulation of heart morphology: Current molecular and cellular Perspect.ives on the coordinated emergence of cardiac form and function. Birth Defects Res. C Embryo Today 2003, 69, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Linask, K.K.; Yu, X.; Chen, Y.; Han, M.D. Directionality of heart looping: Effects of Pitx2c misexpression on flectin asymmetry and midline structures. Dev. Biol. 2002, 246, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hirota, A.; Kamino, K. Optical signals from early embryonic chick heart stained with potential sensitive dyes: Evidence for electrical activity. J. Physiol. 1980, 304, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hirota, A.; Kamino, K. Optical indications of pace-maker potential and rhythm generation in early embryonic chick heart. J. Physiol. 1981, 312, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hirota, A.; Kamino, K. Optical recording of development of electrical activity in embryonic chick heart during early phases of cardiogenesis. J. Physiol. 1981, 311, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Hirota, A.; Kamino, K. Action potential synchrony in embryonic precontractile chick heart: Optical monitoring with potentiometric dyes. J. Physiol. 1981, 319, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Salzberg, B.M.; Grinvald, A.; Cohen, L.B.; Kamino, K.; Lesher, S.; Boyle, M.B.; Waggoner, A.S.; Wang, C.H. Improvements in optical methods for measuring rapid changes in membrane potential. J. Membr. Biol. 1981, 58, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Sakai, T.; Fujii, S.; Kamino, K. Initial development of conduction pattern of spontaneous action potential in early embryonic precontractile chick heart. Dev. Biol. 1983, 99, 517–523. [Google Scholar] [CrossRef]
- Sakai, T.; Yada, T.; Hirota, A.; Komuro, H.; Kamino, K. A regional gradient of cardiac intrinsic rhythmicity depicted in embryonic cultured multiple hearts. Pflugers Arch. 1998, 437, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Sawanobori, T.; Hirota, A.; Fujii, S.; Kamino, K. Optical recording of conducted action potential in heart muscle using a voltage-sensitive dye. Jpn. J. Physiol. 1981, 31, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Sperelakis, N.; Shigenobu, K. Changes in membrane properties of chick embryonic hearts during development. J. Gen. Physiol. 1972, 60, 430–453. [Google Scholar] [CrossRef] [PubMed]
- Sperelakis, N.; Pappano, A.J. Physiology and pharmacology of developing heart cells. Pharmacol. Ther. 1983, 22, 1–39. [Google Scholar] [CrossRef]
- Renaud, J.F.; Romey, G.; Lombet, A.; Lazdunski, M. Differentiation of the fast Na+ channel in embryonic heart cells: Interaction of the channel with neurotoxins. Proc. Natl. Acad. Sci. USA 1981, 78, 5348–5352. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, L.M.; Markwald, R.R. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ. Res. 1995, 77, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ypey, D.L.; Clapham, D.E.; DeHaan, R.L. Development of electrical coupling and action potential synchrony between paired aggregates of embryonic heart cells. J. Membr. Biol. 1979, 51, 75–96. [Google Scholar] [CrossRef] [PubMed]
- De Haan, R.L. Development of pacemaker tissue in the embryonic heart. Ann. N. Y. Acad. Sci. 1965, 127, 7–18. [Google Scholar] [CrossRef]
- Moorman, A.F.; Christoffels, V.M. Cardiac chamber formation: Development, genes, and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, F.; Watanabe, M.; Eloff, B.; Rosenbaum, D. Emerging patterns of cardiac conduction in the chick embryo: Waveform analysis with photodiode array-based optical imaging. Dev. Dyn. 2005, 233, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Reckova, M.; Bigelow, M.R.; Dealmeida, A.; Stanley, C.P.; Mikawa, T.; Gourdie, R.G.; Thompson, R.P. Developmental transitions in electrical activation patterns in chick embryonic heart. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Gurjarpadhye, A.; Hewett, K.W.; Justus, C.; Wen, X.; Stadt, H.; Kirby, M.L.; Sedmera, D.; Gourdie, R.G. Cardiac neural crest ablation inhibits compaction and electrical function of conduction system bundles. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1291–H1300. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Wenink, A.C.G.; Becker, A.E.; Janse, M.J. Development of the specialized tissues. In Conduction System of the Heart: Structure, Function and Clinical Implications; Wellens, H.J.J., Lie, K.I., Janse, M.J., Eds.; Stein: Leiden, The Netherlands, 1976. [Google Scholar]
- Tawara, S. The Conduction System of the Mammalian Heart: An. Anatomico-Histological Study of the Atrioventricular Bundle and the Purkinje Fibers; Imperial College Press: London, UK, 2000. [Google Scholar]
- Vicente-Steijn, R.; Kolditz, D.P.; Mahtab, E.A.; Askar, S.F.; Bax, N.A.; LM, V.D.G.; Wisse, L.J.; Passier, R.; Pijnappels, D.A.; Schalij, M.J.; et al. Electrical activation of sinus venosus myocardium and expression patterns of RhoA and Isl-1 in the chick embryo. J. Cardiovasc. Electrophysiol. 2010, 21, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Eralp, I.; Lie-Venema, H.; Bax, N.A.; Wijffels, M.C.; Van Der Laarse, A.; Deruiter, M.C.; Bogers, A.J.; Van Den Akker, N.M.; Gourdie, R.G.; Schalij, M.J.; et al. Epicardium-derived cells are important for correct development of the purkinje fibers in the avian heart. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006, 288, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Poelmann, R.E.; Jongbloed, M.R.; Molin, D.G.; Fekkes, M.L.; Wang, Z.; Fishman, G.I.; Doetschman, T.; Azhar, M.; Gittenberger-de Groot, A.C. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: A role in induction? Anat. Embryol. 2004, 208, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Blom, N.M.; Aoyama, N.; Sucov, H.; Wenink, A.C.; Poelmann, R.E. The role of neural crest and epicardium-derived cells in conduction system formation. Novartis Found. Symp. 2003, 250, 125–134. [Google Scholar] [PubMed]
- James, T.N. Normal and abnormal consequences of apoptosis in the human heart. Annu. Rev. Physiol. 1998, 60, 309–325. [Google Scholar] [CrossRef] [PubMed]
- James, T.N. Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation 1994, 90, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, B.F.; De Carvalho, A.P.; De Mello, W.C. Transmembrane potentials of single fibres of the atrio-ventricular node. Nature 1958, 181, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Alanis, J.; Benitez, D. Action potential from a. V. Node transitional cells. Arch. Int. Physiol. Biochim. 1964, 72, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Alanis, J.; Benitez, D. Regions of the rabbit's heart atrio-ventricular node at which impulse propagation becomes critical. Arch. Int. Physiol. Biochim. 1964, 72, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.A. The atrioventricular node and bundle in the ferret heart: A light and quantitative electron microscopic study. Am. J. Anat. 1979, 154, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.; et al. Formation of the venous pole of the heart from an Nkx2–5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, T. Recherches sur la differentiation du tissue nodal et connecteur du coeur de mammiferes. Archs. Biol. Belgium 1936, 47, 1–70. [Google Scholar]
- Walls, E.W. The development of the specialized conducting tissue of the human heart. J. Anat. 1947, 81, 93–110. [Google Scholar] [PubMed]
- Challice, C.E.; Viragh, S. The architectural development of the early mammalian heart. Tissue Cell 1974, 6, 447–462. [Google Scholar] [CrossRef]
- Viragh, S.; Challice, C.E. The development of the conduction system in the mouse embryo heart. I. The first embryonic AV conduction pathway. Dev. Biol. 1977, 56, 382–396. [Google Scholar] [CrossRef]
- Viragh, S.; Challice, C.E. The development of the conduction system in the mouse embryo heart. II. Histogenesis of the atrioventricular node and bundle. Dev. Biol. 1977, 56, 397–411. [Google Scholar] [CrossRef]
- Viragh, S.; Challice, C.E. The development of the conduction system in the mouse embryo heart. III. The development of sinus muscle and sinoatrial node. Dev. Biol. 1982, 89, 25–40. [Google Scholar] [CrossRef]
- Benninghoff, A. Uber die beziehungen des reizleitungsystems und der papillarmuskeln zu den konturfasern gesellschaft. Verh. Anat. Ges. 1923, 32, 185–208. [Google Scholar]
- Wenink, A.C. Development of the human cardiac conducting system. J. Anat. 1976, 121, 617–631. [Google Scholar] [PubMed]
- Marino, T.A.; Truex, R.C.; Marino, D.R. The development of the atrioventricular node and bundle in the ferret heart. Am. J. Anat. 1979, 154, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.A. Probit analysis of the atrioventricular (AV) junctional tissues of the ferret heart. Cell Tissue Res. 1979, 199, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Retzer, R. The anatomy of the conductive system in the mammalian heart. Johns Hopkins Med. J. 1908, 19, 208–215. [Google Scholar]
- Tandler, J. The development of the heart. In Manual of Human Embryology; Keibel, F., Mall, F.P., Eds.; J.B. Lippincott: Philadelphia, PA, USA, 1912; pp. 534–570. [Google Scholar]
- Shaner, R.F. The development of the atrioventricular node, bundle of his, and sinoatrial node in the calf, with a description of a third embryonic node-like structure. Anat. Rec. 1929, 44, 85–99. [Google Scholar] [CrossRef]
- Truex, R.C.; Marino, T.A.; Marino, D.R. Observations on the development of the human atrioventricular node and bundle. Anat. Rec. 1978, 192, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.; de Jong, F.; Denyn, M.M.; Lamers, W.H. Development of the cardiac conduction system. Circ. Res. 1998, 82, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Alanis, J.; Benitez, D. The electrical activity of the transitional cells of the auricle, the AV node and the bundle of His changes produced by digitalis drugs. Arch. Inst. Cardiol. Mex. 1964, 34, 68–81. [Google Scholar] [PubMed]
- Alanis, J.; Benitez, D. Two preferential conducting pathways within the bundle of his of the dog heart. Jpn. J. Physiol. 1975, 25, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.S.; Jalife, J. Optical Mapping of Cardiac Excitation and Arrhythmias; Futura Publishing Company Inc.: Armonk, NY, USA, 2001. [Google Scholar]
- Salama, G.; Morad, M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science 1976, 191, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Morad, M.; Salama, G. Optical probes of membrane potential in heart muscle. J. Physiol. 1979, 292, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Knisley, S.B.; Justice, R.K.; Kong, W.; Johnson, P.L. Ratiometry of transmembrane voltage-sensitive fluorescent dye emission in hearts. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1421–H1433. [Google Scholar] [PubMed]
- Efimov, I.R.; Nikolski, V.P.; Salama, G. Optical imaging of the heart. Circ. Res. 2004, 95, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Reckova, M.; Rosengarten, C.; Torres, M.I.; Gourdie, R.G.; Thompson, R.P. Optical mapping of electrical activation in the developing heart. Microsc. Microanal. 2005, 11, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ideker, R.E.; Smith, W.M.; Blanchard, S.M.; Reiser, S.L.; Simpson, E.V.; Wolf, P.D.; Danieley, N.D. The assumptions of isochronal cardiac mapping. Pacing Clin. Electrophysiol. 1989, 12, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Haws, C.W.; Lux, R.L. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 1990, 81, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Grinvald, A. Real-time optical mapping of neuronal activity: From single growth cones to the intact mammalian brain. Annu. Rev. Neurosci. 1985, 8, 263–305. [Google Scholar] [CrossRef] [PubMed]
- Morley, G.E.; Vaidya, D.; Samie, F.H.; Lo, C.; Delmar, M.; Jalife, J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J. Cardiovasc. Electrophysiol. 1999, 10, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Eloff, B.C.; Lerner, D.L.; Yamada, K.A.; Schuessler, R.B.; Saffitz, J.E.; Rosenbaum, D.S. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc. Res. 2001, 51, 681–690. [Google Scholar] [CrossRef]
- Kamino, K.; Hirota, A.; Fujii, S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature 1981, 290, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Hirota, A.; Kamino, K.; Komuro, H.; Sakai, T. Mapping of early development of electrical activity in the embryonic chick heart using multiple-site optical recording. J. Physiol. 1987, 383, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Optical studies of early developing cardiac and neural activities using voltage-sensitive dyes. Jpn. J. Physiol. 1990, 40, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Gourdie, R.G.; Kubalak, S.; Mikawa, T. Conducting the embryonic heart: Orchestrating development of specialized cardiac tissues. Trends Cardiovasc. Med. 1999, 9, 18–26. [Google Scholar] [CrossRef]
- Rothenberg, F.; Nikolski, V.P.; Watanabe, M.; Efimov, I.R. Electrophysiology and anatomy of embryonic rabbit hearts before and after septation. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H344–H351. [Google Scholar] [CrossRef] [PubMed]
- Chuck, E.T.; Meyers, K.; France, D.; Creazzo, T.L.; Morley, G.E. Transitions in ventricular activation revealed by two-dimensional optical mapping. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 280, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Mosimann, C.; Panakova, D.; Werdich, A.A.; Musso, G.; Burger, A.; Lawson, K.L.; Carr, L.A.; Nevis, K.R.; Sabeh, M.K.; Zhou, Y.; et al. Chamber identity programs drive early functional partitioning of the heart. Nat. Commun. 2015, 6, 8146. [Google Scholar] [CrossRef] [PubMed]
- Panakova, D.; Werdich, A.A.; Macrae, C.A. Wnt11 patterns a myocardial electrical gradient through regulation of the l-type Ca2+ channel. Nature 2010, 466, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Swift, L.M.; Asfour, H.; Posnack, N.G.; Arutunyan, A.; Kay, M.W.; Sarvazyan, N. Properties of blebbistatin for cardiac optical mapping and other imaging applications. Pflugers Arch. 2012, 464, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Brack, K.E.; Narang, R.; Winter, J.; Ng, G.A. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Exp. Physiol. 2013, 98, 1009–1027. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Morley, G.E.; Fishman, G.I. Molecular and functional maturation of the murine cardiac conduction system. Cold Spring Harb. Symp Quant. Biol 2002, 67, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Rohde, G.K.; Dawant, B.M.; Lin, S.F. Correction of motion artifact in cardiac optical mapping using image registration. IEEE Trans. Biomed. Eng. 2005, 52, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Tai, D.C.; Caldwell, B.J.; LeGrice, I.J.; Hooks, D.A.; Pullan, A.J.; Smaill, B.H. Correction of motion artifact in transmembrane voltage-sensitive fluorescent dye emission in hearts. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H985–H993. [Google Scholar] [CrossRef] [PubMed]
- Salama, G.; Lombardi, R.; Elson, J. Maps of optical action potentials and nadh fluorescence in intact working hearts. Am. J. Physiol. 1987, 252, H384–H394. [Google Scholar] [PubMed]
- Lin, S.F.; Wikswo, J.P. Panoramic optical imaging of electrical propagation in isolated heart. J. Biomed. Opt. 1999, 4, 200–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, S.F.; Roth, B.J.; Wikswo, J.P., Jr. Quatrefoil reentry in myocardium: An optical imaging study of the induction mechanism. J. Cardiovasc. Electrophysiol. 1999, 10, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.W.; Watanabe, M.; Rollins, A.M. Longitudinal imaging of heart development with optical coherence tomography. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Di Diego, J.M.; Sicouri, S.; Myles, R.C.; Burton, F.L.; Smith, G.L.; Antzelevitch, C. Optical and electrical recordings from isolated coronary-perfused ventricular wedge preparations. J. Mol. Cell Cardiol. 2013, 54, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Knisley, S.B.; Neuman, M.R. Simultaneous electrical and optical mapping in rabbit hearts. Ann. Biomed. Eng. 2003, 31, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Li, W.; Efimov, I.R. Multiparametric optical mapping of the langendorff-perfused rabbit heart. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Maevsky, E.; Ivanitsky, G.; Bogdanova, L.; Axenova, O.; Karmen, N.; Zhiburt, E.; Senina, R.; Pushkin, S.; Maslennikov, I.; Orlov, A.; et al. Clinical results of perftoran application: Present and future. Artif. Cells Blood Substit. Immobil. Biotechnol. 2005, 33, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Wang, Y.T.; Gu, S.; Watanabe, M.; Rollins, A.M.; Jenkins, M.W. Three-dimensional correction of conduction velocity in the embryonic heart using integrated optical mapping and optical coherence tomography. J. Biomed. Opt. 2014. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, J.H.; Kralj, J.M.; Douglass, A.D.; Engert, F.; Cohen, A.E. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol. 2014, 5, 344. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, Y.T.; Ma, P.; Werdich, A.A.; Rollins, A.M.; Jenkins, M.W. Mapping conduction velocity of early embryonic hearts with a robust fitting algorithm. Biomed. Opt. Express 2015, 6, 2138–2157. [Google Scholar] [CrossRef] [PubMed]
- Maynard, E.M.; Nordhausen, C.T.; Normann, R.A. The Utah intracortical electrode array: A recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 228–239. [Google Scholar] [CrossRef]
- Sedmera, D.; Wessels, A.; Trusk, T.C.; Thompson, R.P.; Hewett, K.W.; Gourdie, R.G. Changes in activation sequence of embryonic chick atria correlate with developing myocardial architecture. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Hayashi, S.; Ojima, A.; Taniguchi, T.; Miyamoto, N.; Nakamori, C.; Nagasawa, C.; Kitamura, T.; Osada, T.; Honda, Y.; et al. Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 2015, 75, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Akemann, W.; Song, C.; Mutoh, H.; Knopfel, T. Route to genetically targeted optical electrophysiology: Development and applications of voltage-sensitive fluorescent proteins. Neurophotonics 2015, 2, 021008. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chen, T.W.; Hu, A.; Shields, B.C.; Guo, C.; Looger, L.L.; Kim, D.S.; Svoboda, K. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS One 2014, 9, e108697. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.M.; Hochbaum, D.R.; Douglass, A.D.; Cohen, A.E. Electrical spiking in escherichia coli probed with a fluorescent voltage-indicating protein. Science 2011, 333, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, D.R.; Zhao, Y.; Farhi, S.L.; Klapoetke, N.; Werley, C.A.; Kapoor, V.; Zou, P.; Kralj, J.M.; Maclaurin, D.; Smedemark-Margulies, N.; et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods 2014, 11, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, C.; Li, J.Z.; Grewe, B.F.; Zhang, Y.; Eismann, S.; Schnitzer, M.J. High-speed recording of neural spikes in awake mice and flies with a fluorescent voltage sensor. Science 2015, 350, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Butson, C.R.; McIntyre, C.C. Role of electrode design on the volume of tissue activated during deep brain stimulation. J. Neural. Eng. 2006, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, S. The electrical constants of Purkinje fibres. J. Physiol. 1952, 118, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, S. Electrical constants of trabecular muscle from mammalian heart. J. Physiol. 1970, 210, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Roth, B.J.; Rosenbaum, D.S. Optical measurement of cell-to-cell coupling in intact heart using subthreshold electrical stimulation. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, 533–542. [Google Scholar]
- McCreery, D.B.; Agnew, W.F.; Yuen, T.G.; Bullara, L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 1990, 37, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.W.; Duke, A.R.; Gu, S.; Chiel, H.J.; Fujioka, H.; Watanabe, M.; Jansen, E.D.; Rollins, A.M. Optical pacing of the embryonic heart. Nat. Photon. 2010, 4, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Bruegmann, T.; Malan, D.; Hesse, M.; Beiert, T.; Fuegemann, C.J.; Fleischmann, B.K.; Sasse, P. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 2010, 11, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Arrenberg, A.B.; Stainier, D.Y.; Baier, H.; Huisken, J. Optogenetic control of cardiac function. Science 2010, 330, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Dittami, G.M.; Rajguru, S.M.; Lasher, R.A.; Hitchcock, R.W.; Rabbitt, R.D. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J. Physiol. 2011, 589, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Gu, S.; Ma, P.; Watanabe, M.; Rollins, A.M.; Jenkins, M.W. Optical stimulation enables paced electrophysiological studies in embryonic hearts. Biomed. Opt. Express 2014, 5, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.W.; Wang, Y.T.; Doughman, Y.Q.; Watanabe, M.; Cheng, Y.; Rollins, A.M. Optical pacing of the adult rabbit heart. Biomed. Opt. Express 2013, 4, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Abilez, O.J.; Wong, J.; Prakash, R.; Deisseroth, K.; Zarins, C.K.; Kuhl, E. Multiscale computational models for optogenetic control of cardiac function. Biophys. J. 2011, 101, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Valiunas, V.; Lu, Z.; Bien, H.; Liu, H.; Wang, H.Z.; Rosati, B.; Brink, P.R.; Cohen, I.S.; Entcheva, E. Stimulating cardiac muscle by light: Cardiac optogenetics by cell delivery. Circ. Arrhythm. Electrophysiol. 2011, 4, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Bingen, B.O.; Engels, M.C.; Schalij, M.J.; Jangsangthong, W.; Neshati, Z.; Feola, I.; Ypey, D.L.; Askar, S.F.; Panfilov, A.V.; Pijnappels, D.A.; et al. Light-induced termination of spiral wave arrhythmias by optogenetic engineering of atrial cardiomyocytes. Cardiovasc. Res. 2014, 104, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, U.; Gepstein, L. Optogenetics for in vivo cardiac pacing and resynchronization therapies. Nat. Biotechnol. 2015, 33, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.B.; Klimas, A.; Ambrosi, C.M.; Tomek, J.; Corbett, A.; Entcheva, A.; Bub, G. Optical control of excitation waves in cardiac tissue. Nat. Photon. 2015. [Google Scholar] [CrossRef]
- Williams, J.C.; Xu, J.; Lu, Z.; Klimas, A.; Chen, X.; Ambrosi, C.M.; Cohen, I.S.; Entcheva, E. Computational optogenetics: Empirically-derived voltage- and light-sensitive channelrhodopsin-2 model. PLoS Comput. Biol. 2013, 9, e1003220. [Google Scholar] [CrossRef] [PubMed]
- Lothet, E.H.; Kilgore, K.L.; Bhadra, N.; Bhadra, N.; Vrabec, T.; Wang, Y.T.; Jansen, E.D.; Jenkins, M.W.; Chiel, H.J. Alternating current and infrared produce an onset-free reversible nerve block. Neurophotonics 2014, 1, 011010. [Google Scholar] [CrossRef] [PubMed]
- Duke, A.R.; Jenkins, M.W.; Lu, H.; McManus, J.M.; Chiel, H.J.; Jansen, E.D. Transient and selective suppression of neural activity with infrared light. Sci. Rep. 2013, 3, 2600. [Google Scholar] [CrossRef] [PubMed]


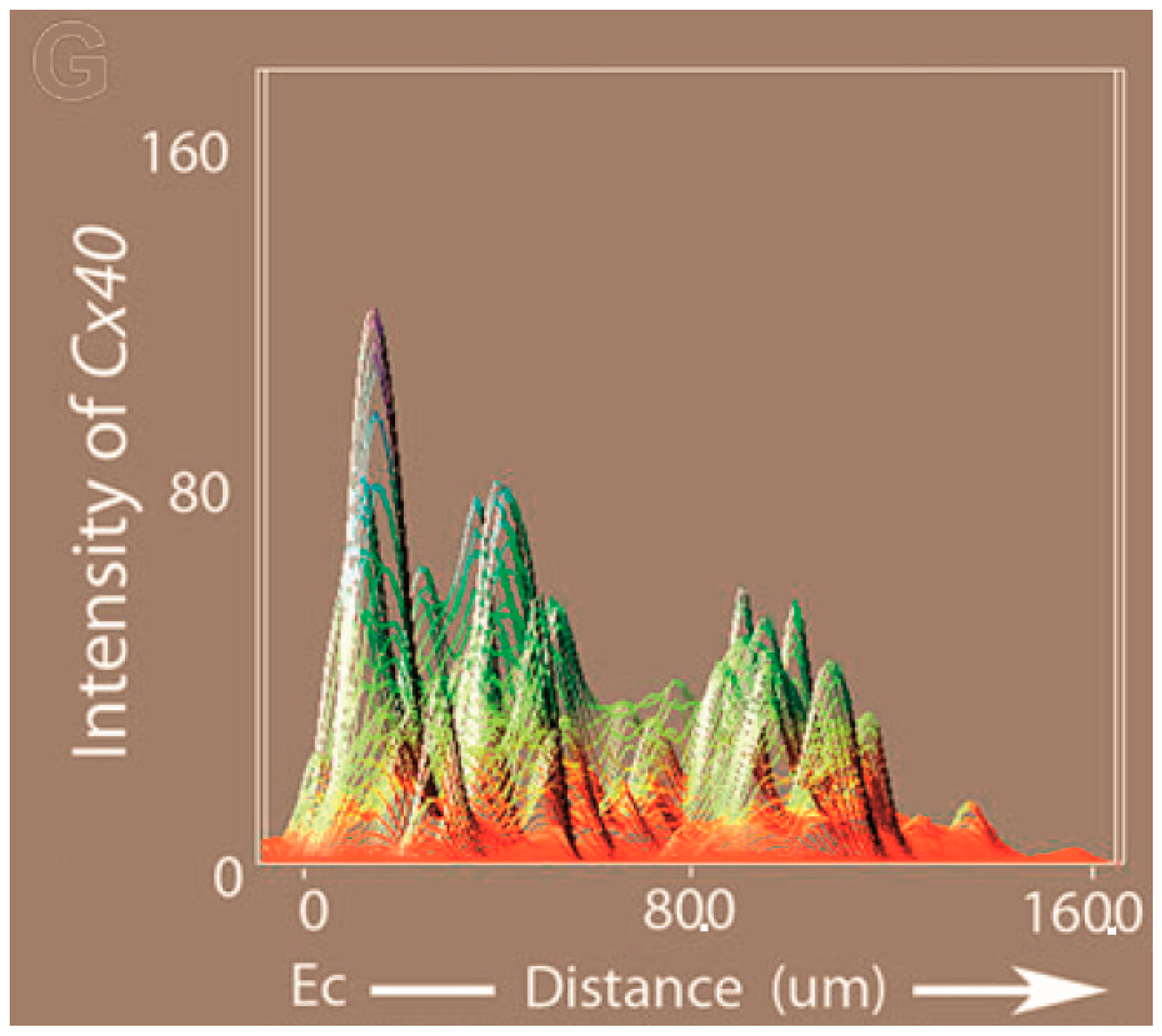
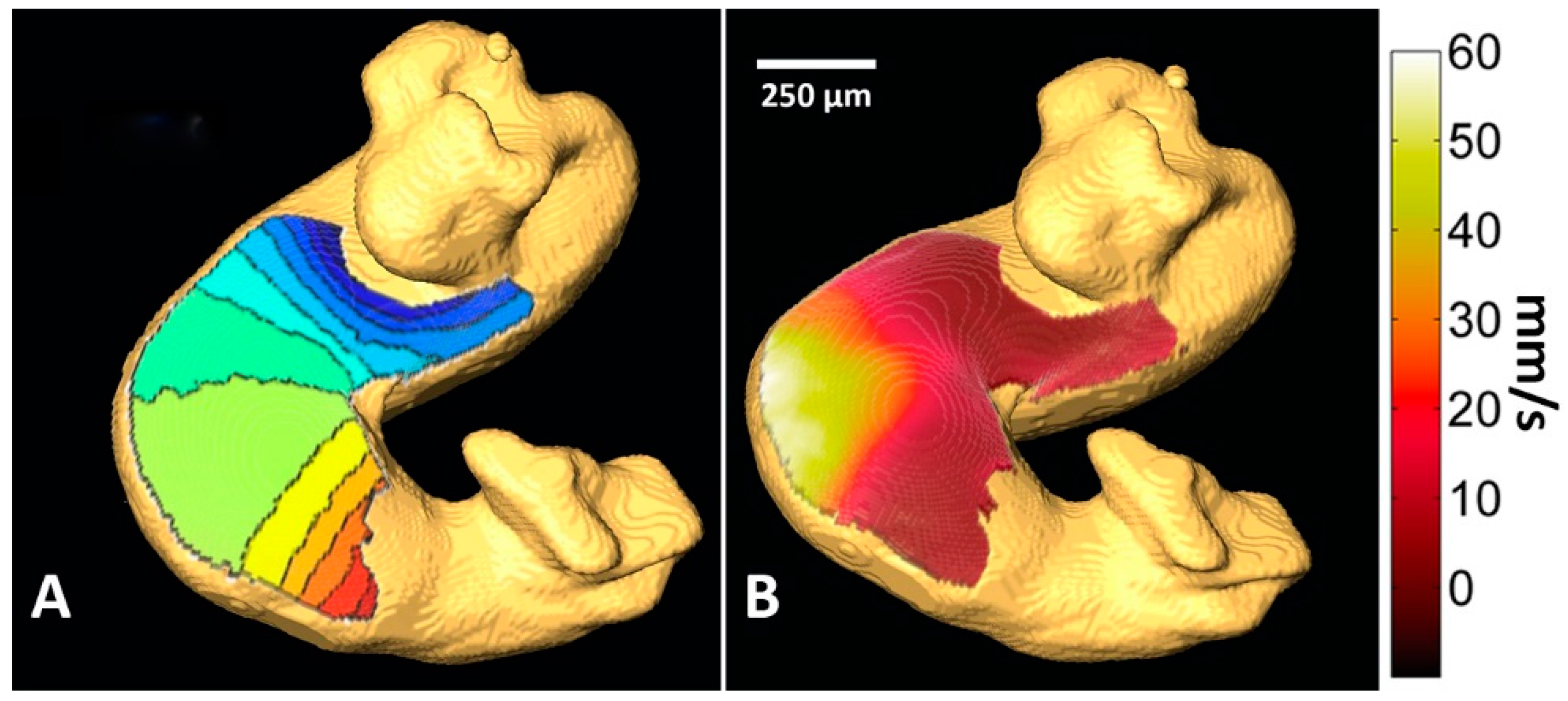

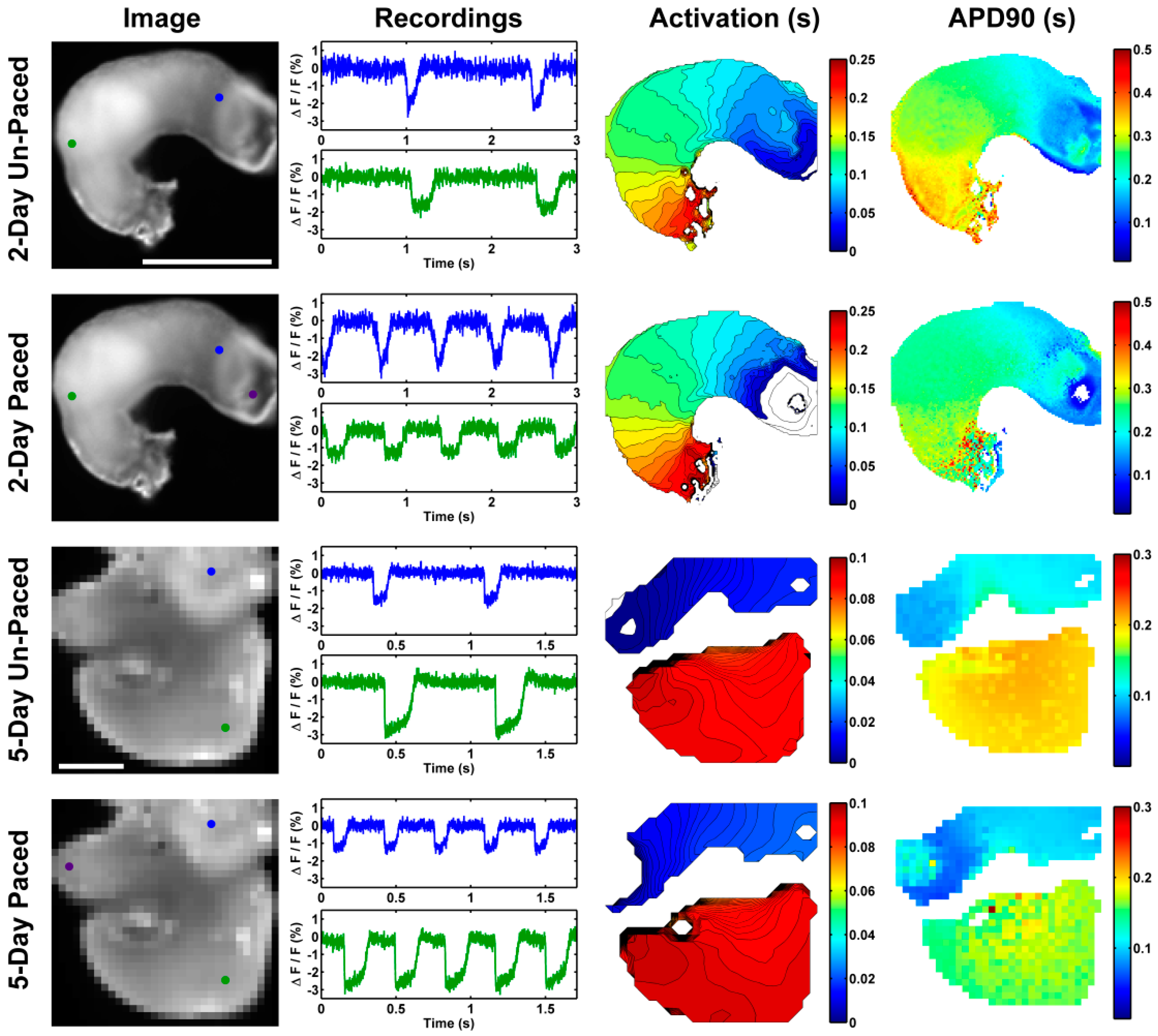
| Mouse Line | Other Names (See MGI) | References |
|---|---|---|
| CCS-lacZ | Tg(En2-lacZ)1Alj, MC4 | Rentschler et al., 2001 [102] |
| Cx30.2-LacZ | Gjd3tm1.2Kwi, Cx30.2LacZ | Kreuzberge et al., 2005 [104] |
| Cx45-LacZ | Gjc1tm1Kwi | Kreuzberg et al., 2005 [104] and Kruger et al., 2000 [105] |
| HCN4-CreERT2 | Hcn4tm1(cre-/ERTs)Anlu or Hcn4tm1(cre/ESR1)Anlu, Hcn4tm2.1(cre/ERT2)Sev | Hoesl et al., 2008 [106], Spater et al., 2013 [107] derived from Liang et al., 2013 [108] |
| Hcn4-CreERT2 | Tg(Hcn4-cre/ERT2)1Yzhao | Wu et al., 2014 [109] |
| HCN4-nEGFP | Hcn4tm1Sev, HCN4-H2B-EGFP | Liang et al., 2013 [108], Sun et al., 2007 [110] |
| HCN4-nLacZ | HCN4H2BGFP HCN4nlacZ (both markers in one) | Liang et al., 2013 [108] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Rollins, A.M.; Polo-Parada, L.; Ma, P.; Gu, S.; Jenkins, M.W. Probing the Electrophysiology of the Developing Heart. J. Cardiovasc. Dev. Dis. 2016, 3, 10. https://doi.org/10.3390/jcdd3010010
Watanabe M, Rollins AM, Polo-Parada L, Ma P, Gu S, Jenkins MW. Probing the Electrophysiology of the Developing Heart. Journal of Cardiovascular Development and Disease. 2016; 3(1):10. https://doi.org/10.3390/jcdd3010010
Chicago/Turabian StyleWatanabe, Michiko, Andrew M. Rollins, Luis Polo-Parada, Pei Ma, Shi Gu, and Michael W. Jenkins. 2016. "Probing the Electrophysiology of the Developing Heart" Journal of Cardiovascular Development and Disease 3, no. 1: 10. https://doi.org/10.3390/jcdd3010010
APA StyleWatanabe, M., Rollins, A. M., Polo-Parada, L., Ma, P., Gu, S., & Jenkins, M. W. (2016). Probing the Electrophysiology of the Developing Heart. Journal of Cardiovascular Development and Disease, 3(1), 10. https://doi.org/10.3390/jcdd3010010






