Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole
Abstract
:1. Introduction
1.1. The SAN Can Be Divided into Subdomains by Anatomical and Genetic Criteria
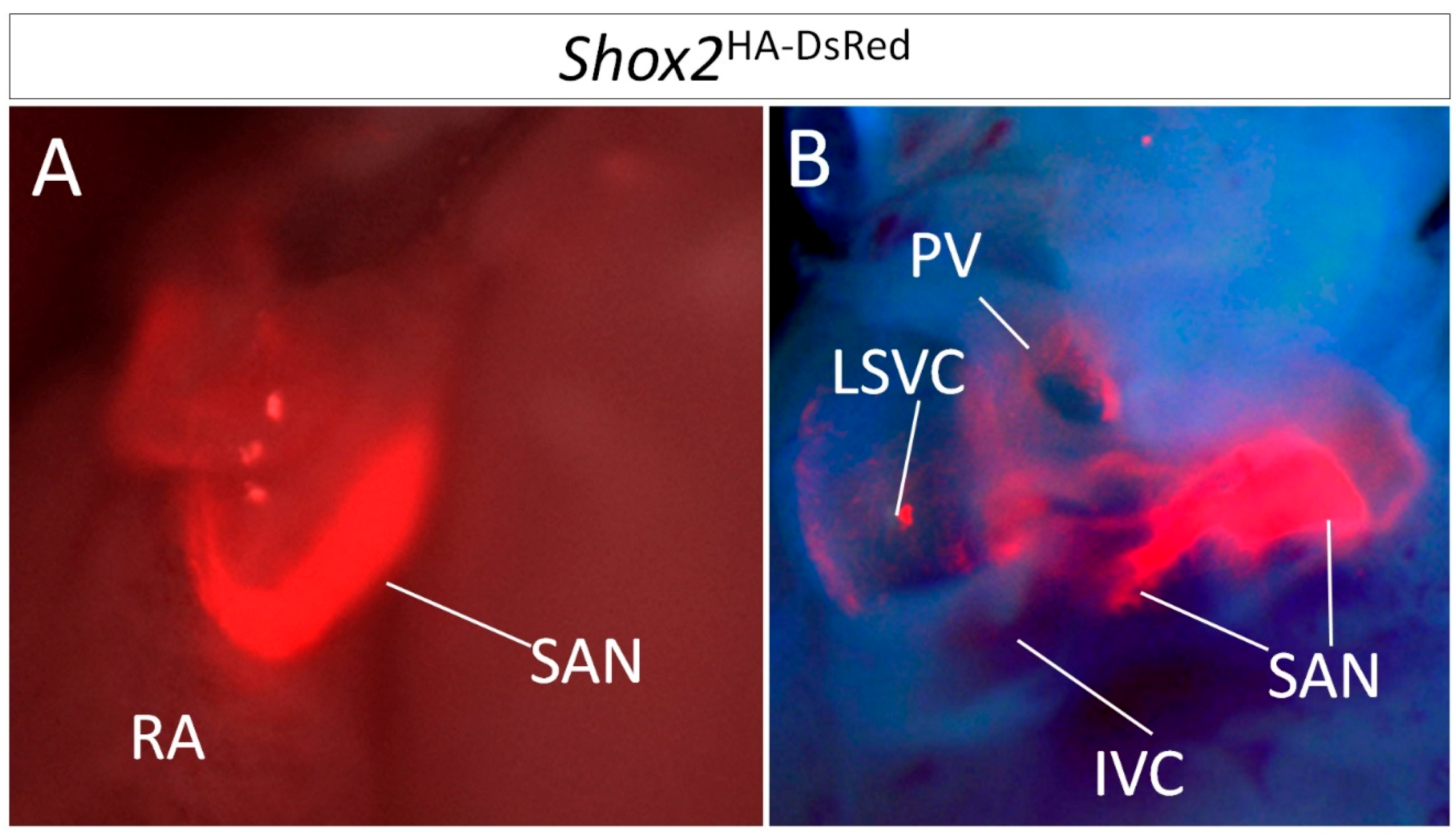
1.2. Lineage Development of the SAN in Relation to Other Venous Pole Structures
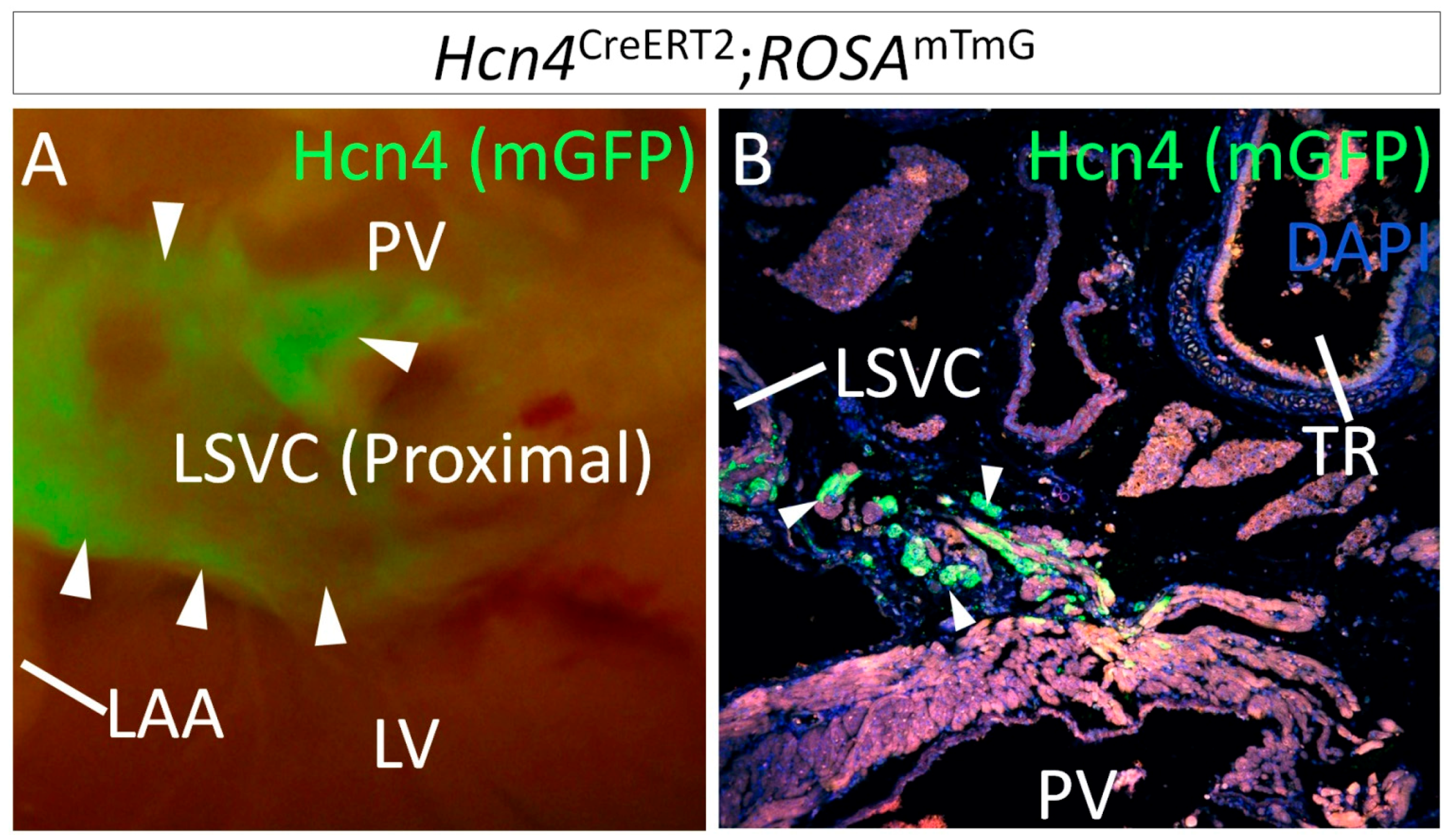
2. Genetic Models for SAN Development
2.1. Genes and Genetic Models for SAN Dysgenesis without Complications in the SV and PV Myocardium

2.2. Gene and Genetic Models Affecting Both the SAN and Other Venous Pole Components
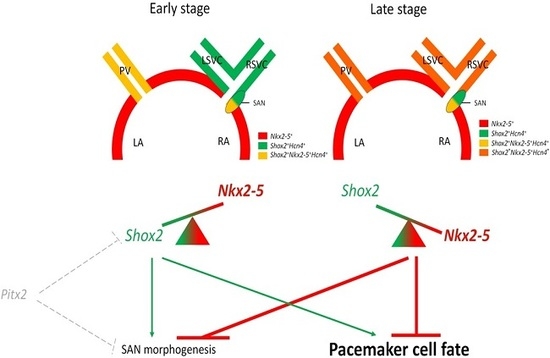
3. Shox2-Nkx2-5 Antagonistic Mechanism in the Regulation of Pacemaker Properties
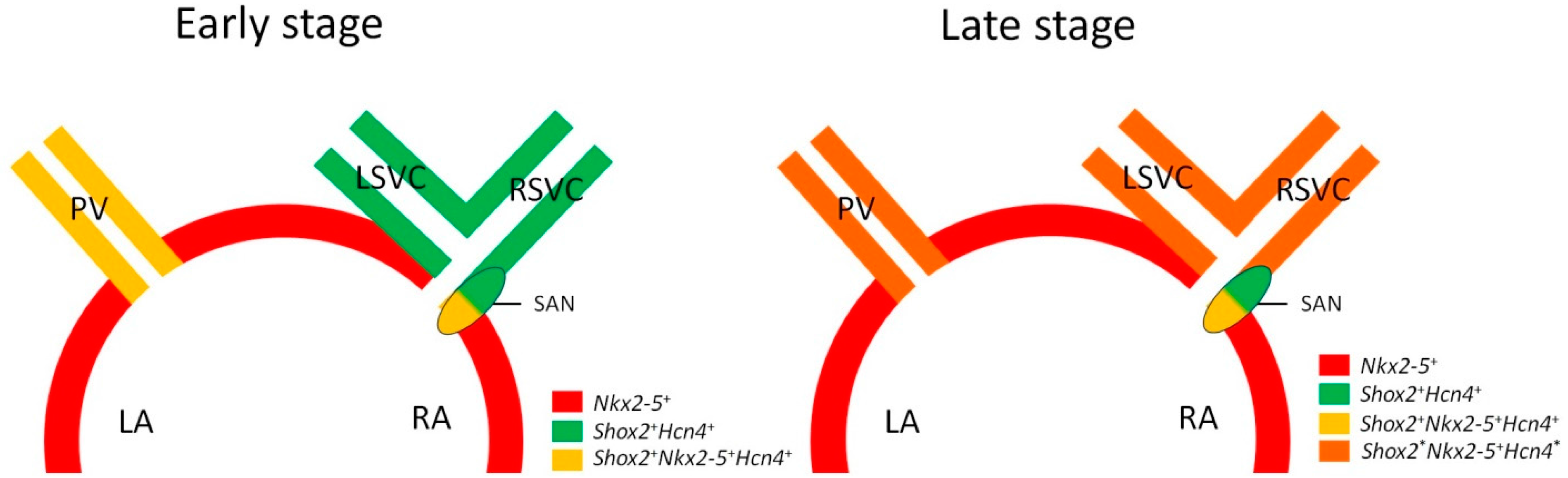
3.1. Shox2-Nkx2-5 Antagonistic Mechanism
3.2. Heterogeneous Development Model of the SAN and the Essential Physiological Function of the Shox2+/Nkx2-5+ SAN Tail/Peripheral Domain
4. Genome-Wide Studies on Transcription Regulatory Networks and Chromatin Landscape in the Pro-Pacemaker Cells
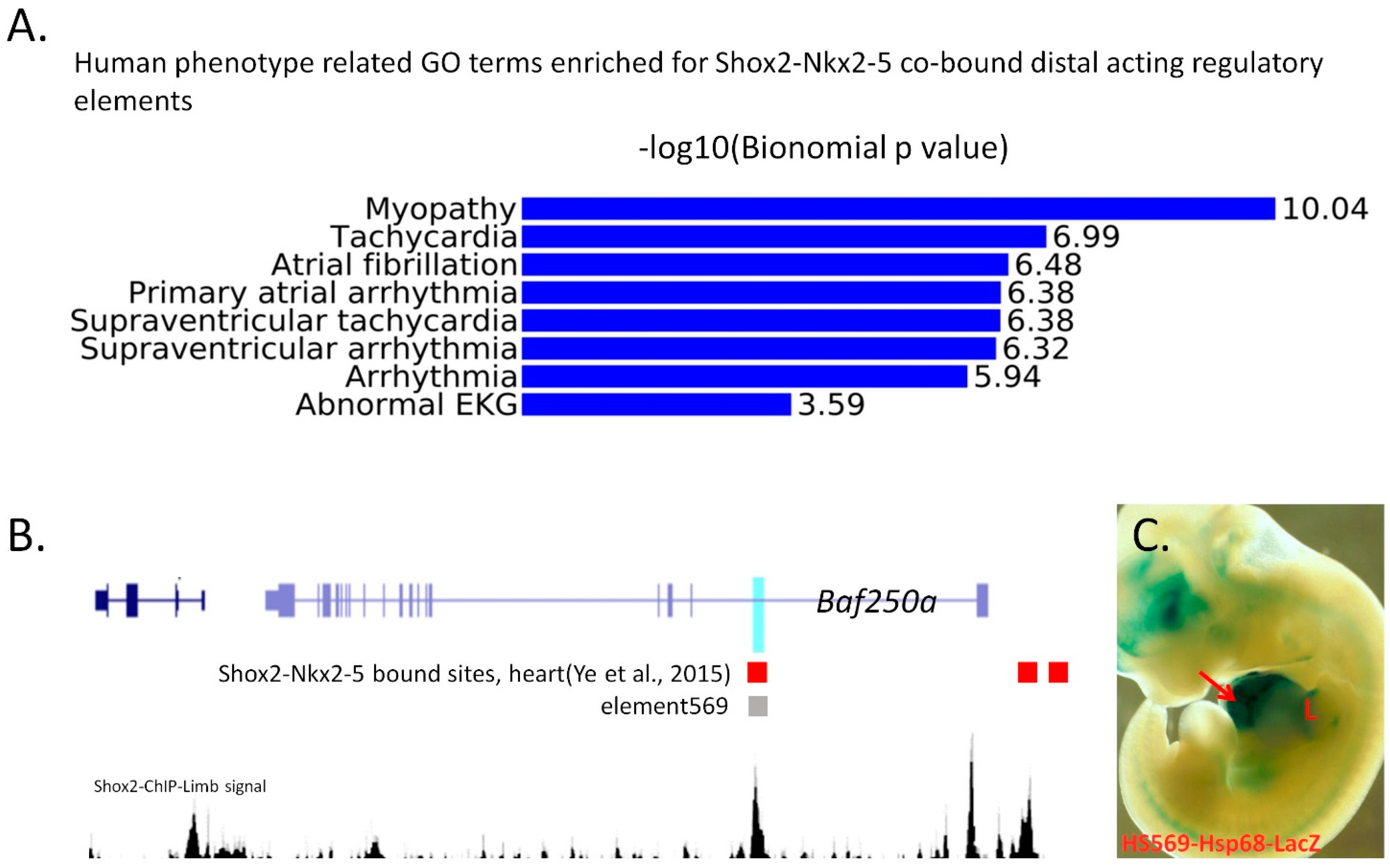
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christoffels, V.M.; Smits, G.J.; Kispert, A.; Moorman, A.F. Development of the pacemaker tissues of the heart. Circ. Res. 2010, 106, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.B.; Benson, D.W. Genetics of sick sinus syndrome. Card. Electrophysiol. Clin. 2010, 2, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189. [Google Scholar] [PubMed]
- Dobrzynski, H.; Boyett, M.R.; Anderson, R.H. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation 2007, 115, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, S.; Fabritz, L.; Layh, B.; Kirchhof, P.; Ludwig, A. Insights into sick sinus syndrome from an inducible mouse model. Cardiovasc. Res. 2011, 90, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Monfredi, O.; Boyett, M.R. Not so fast! Sick sinus syndrome is a complex and incompletely understood disease that might prove hard to model in animals. Cardiovasc. Res. 2011, 92, 178. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.E.; Hollman, A. Discovery of the sinus node by Keith and Flack: On the centennial of their 1907 publication. Heart 2007, 93, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res 2009, 104, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, V.; Galang, G.; Evangelista, M.; Deo, R.C.; Srivastava, D. RNA sequencing of mouse sinoatrial node reveals an upstream regulatory role for Islet-1 in cardiac pacemaker cells. Circ. Res. 2015, 116, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, Q.; Cattaneo, P.; Zhuang, S.; Gong, X.; Spann, N.J.; Jiang, C.; Cao, X.; Zhao, X.; Zhang, X.; et al. Transcription factor ISL1 is essential for pacemaker development and function. J. Clin. Investig. 2015, 125, 3256–3268. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, S.; Vaidya, D.M.; Tamaddon, H.; Degenhardt, K.; Sassoon, D.; Morley, G.E.; Jalife, J.; Fishman, G.I. Visualization and functional characterization of the developing murine cardiac conduction system. Development 2001, 128, 1785–1792. [Google Scholar] [PubMed]
- Blom, N.A.; Gittenberger-de Groot, A.C.; DeRuiter, M.C.; Poelmann, R.E.; Mentink, M.M.; Ottenkamp, J. Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression: Possible relevance for understanding of abnormal atrial automaticity. Circulation 1999, 99, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C.; Mahtab, E.A.; Hahurij, N.D.; Wisse, L.J.; Deruiter, M.C.; Wijffels, M.C.; Poelmann, R.E. Nkx2.5-negative myocardium of the posterior heart field and its correlation with podoplanin expression in cells from the developing cardiac pacemaking and conduction system. Anat. Rec. 2007, 290, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.; et al. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Hoffmann, A.D.; Burnicka-Turek, O.; Friedland-Little, J.M.; Zhang, K.; Moskowitz, I.P. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev. Cell 2012, 23, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Snarr, B.S.; Wirrig, E.E.; Phelps, A.L.; Trusk, T.C.; Wessels, A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev. Dyn. 2007, 236, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Ammirabile, G.; Tessari, A.; Pignataro, V.; Szumska, D.; Sutera Sardo, F.; Benes, J., Jr.; Balistreri, M.; Bhattacharya, S.; Sedmera, D.; Campione, M. Pitx2 confers left morphological, molecular, and functional identity to the sinus venosus myocardium. Cardiovasc. Res. 2012, 93, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, D.; Ye, W.; Liu, C.; Gu, S.; Sinsheimer, N.R.; Song, Z.; Li, X.; Chen, C.; Song, Y.; et al. The short stature homeobox 2 (Shox2)-bone morphogenetic protein (BMP) pathway regulates dorsal mesenchymal protrusion development and its temporary function as a pacemaker during cardiogenesis. J. Biol. Chem. 2015, 290, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.C.; Boukens, B.J.; Wang, T.; Moorman, A.F.; Christoffels, V.M. Evolution of the Sinus Venosus from Fish to Human. J. Cardiovasc. Dev. Dis. 2014, 1, 14–28. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Christoffels, V.M.; Anderson, R.H.; Moorman, A.F. Atrial fibrillation: A developmental point of view. Heart Rhythm 2009, 6, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.; Dominguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.; Kispert, A.; Brown, N.A.; Moorman, A.F.; Christoffels, V.M. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Aanhaanen, W.T.; Mommersteeg, M.T.; Norden, J.; Wakker, V.; de Gier-de Vries, C.; Anderson, R.H.; Kispert, A.; Moorman, A.F.; Christoffels, V.M. Developmental origin, growth, and three-dimensional architecture of the atrioventricular conduction axis of the mouse heart. Circ. Res. 2010, 107, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, G.; Lin, L.; Lowe, J.; Zhang, Q.; Bu, L.; Chen, Y.; Chen, J.; Sun, Y.; Evans, S.M. HCN4 dynamically marks the first heart field and conduction system precursors. Circ. Res. 2013, 113, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Gittenberger-de Groot, A.C. The development of the pulmonary vein revisited. Int. J. Cardiol. 2011, 147, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Douglas, Y.L.; Jongbloed, M.R.; Deruiter, M.C.; Gittenberger-de Groot, A.C. Normal and abnormal development of pulmonary veins: State of the art and correlation with clinical entities. Int. J. Cardiol. 2011, 147, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.M.; Anderson, R.H. Development of the pulmonary vein. Int. J. Cardiol. 2011, 147, 182. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Tessari, A.; Moorman, A.F.; de Boer, P.A.; Hagoort, J.; Soufan, A.T.; Campione, M.; Christoffels, V.M. The transcriptional repressor Tbx3 delineates the developing central conduction system of the heart. Cardiovasc. Res. 2004, 62, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Boukens, B.J.; Mommersteeg, M.T.; Brons, J.F.; Wakker, V.; Moorman, A.F.; Christoffels, V.M. Transcription factor Tbx3 is required for the specification of the atrioventricular conduction system. Circ. Res. 2008, 102, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Hoogaars, W.M.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.U.; Carter, K.L.; Thomas, K.R.; Burr, R.M.; Bakker, M.L.; Coetzee, W.A.; Tristani-Firouzi, M.; Bamshad, M.J.; Christoffels, V.M.; Moon, A.M. Lethal arrhythmias in Tbx3-deficient mice reveal extreme dosage sensitivity of cardiac conduction system function and homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, E154–E163. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, S.; Yang, J.; Tu, Z.; Cai, X.; Cai, C.L.; Wang, Z.; Zhao, Y. Baf250a orchestrates an epigenetic pathway to repress the Nkx2.5-directed contractile cardiomyocyte program in the sinoatrial node. Cell Res. 2014, 24, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Arnolds, D.E.; Liu, F.; Fahrenbach, J.P.; Kim, G.H.; Schillinger, K.J.; Smemo, S.; McNally, E.M.; Nobrega, M.A.; Patel, V.V.; Moskowitz, I.P. TBX5 drives Scn5a expression to regulate cardiac conduction system function. J. Clin. Investig. 2012, 122, 2509–2518. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Mehrkens, D.; Friedrich, F.W.; Stubbendorff, M.; Hua, X.; Muller, J.C.; Schrepfer, S.; Evans, S.M.; Carrier, L.; Eschenhagen, T. Localization of Islet-1-positive cells in the healthy and infarcted adult murine heart. Circ. Res. 2012, 110, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Tessadori, F.; van Weerd, J.H.; Burkhard, S.B.; Verkerk, A.O.; de Pater, E.; Boukens, B.J.; Vink, A.; Christoffels, V.M.; Bakkers, J. Identification and functional characterization of cardiac pacemaker cells in zebrafish. PLoS ONE 2012, 7, e47644. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Liang, W.; Marban, E.; Cho, H.C. Direct conversion of quiescent cardiomyocytes to pacemaker cells by expression of Tbx18. Nat. Biotechnol. 2013, 31, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.V.; Olson, E.N. Translational medicine. Improving cardiac rhythm with a biological pacemaker. Science 2014, 345, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.M.; Grieskamp, T.; Norden, J.; Mommersteeg, M.T.; Rudat, C.; Kispert, A. Tbx18 and the fate of epicardial progenitors. Nature 2009, 458, E8–E9. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, R.J.; Hahurij, N.D.; Kuijper, S.; Just, S.; Wisse, L.J.; Deissler, K.; Maxelon, T.; Anastassiadis, K.; Spitzer, J.; Hardt, S.E.; et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation 2007, 115, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Lewis, R.A.; Yu, L.; He, F.; Liu, H.; Tang, R.; Shi, J.; Sun, X.; Martin, J.F.; Wang, D.; Yang, J.; Chen, Y. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009, 327, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, Y. Revealing the default pacemakers in the venous pole. 2016; to be submitted for publication. [Google Scholar]
- Puskaric, S.; Schmitteckert, S.; Mori, A.D.; Glaser, A.; Schneider, K.U.; Bruneau, B.G.; Blaschke, R.J.; Steinbeisser, H.; Rappold, G. Shox2 mediates Tbx5 activity by regulating Bmp4 in the pacemaker region of the developing heart. Hum. Mol. Genet. 2010, 19, 4625–4633. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, Y.; Department of Cell and Molecular Biology, Tulane University, New Orleans, LA, USA. Unpublished Observation. 2015.
- Mommersteeg, M.T.; Brown, N.A.; Prall, O.W.; de Gier-de Vries, C.; Harvey, R.P.; Moorman, A.F.; Christoffels, V.M. Pitx2c and Nkx2-5 are required for the formation and identity of the pulmonary myocardium. Circ. Res. 2007, 101, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Prall, O.W.; Menon, M.K.; Solloway, M.J.; Watanabe, Y.; Zaffran, S.; Bajolle, F.; Biben, C.; McBride, J.J.; Robertson, B.R.; Chaulet, H.; et al. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 2007, 128, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Yanez, D.A.; Touma, M.; Nakano, H.; Jaroszewicz, A.; Jordan, M.C.; Pellegrini, M.; Roos, K.P.; Nakano, A. Nkx2-5 suppresses the proliferation of atrial myocytes and conduction system. Circ. Res. 2014, 114, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Ai, D.; Liu, W.; Ma, L.; Dong, F.; Lu, M.F.; Wang, D.; Verzi, M.P.; Cai, C.; Gage, P.J.; Evans, S.; et al. Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev. Biol. 2006, 296, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Campione, M. The role of Pitx2 during cardiac development. Linking left-right signaling and congenital heart diseases. Trends Cardiovasc. Med. 2003, 13, 157–163. [Google Scholar] [CrossRef]
- St Amand, T.R.; Ra, J.; Zhang, Y.; Hu, Y.; Baber, S.I.; Qiu, M.; Chen, Y. Cloning and expression pattern of chicken Pitx2: A new component in the SHH signaling pathway controlling embryonic heart looping. Biochem. Biophys. Res. Commun. 1998, 247, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Ros, M.A.; Icardo, J.M.; Piedra, E.; Christoffels, V.M.; Schweickert, A.; Blum, M.; Franco, D.; Moorman, A.F. Pitx2 expression defines a left cardiac lineage of cells: Evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev. Biol. 2001, 231, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Campione, M.; Kelly, R.; Zammit, P.S.; Buckingham, M.; Lamers, W.H.; Moorman, A.F. Multiple transcriptional domains, with distinct left and right components, in the atrial chambers of the developing heart. Circ. Res. 2000, 87, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bai, Y.; Li, N.; Ye, W.; Zhang, M.; Stephanie, B.G.; Tao, Y.; Chen, Y.; Wehrens, X.; Martin, J. Pitx2-microRNA pathway that delimits sinoatrial node development and inhibits predisposition to atrial fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 9181–9186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.; Martin, J.F. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.L.; Christoffels, V.M.; Moorman, A.F. The cardiac pacemaker and conduction system develops from embryonic myocardium that retains its primitive phenotype. J. Cardiovasc. Pharmacol. 2010, 56, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Butters, T.D.; Aslanidi, O.V.; Inada, S.; Boyett, M.R.; Hancox, J.C.; Lei, M.; Zhang, H. Mechanistic links between Na+ channel (SCN5A) mutations and impaired cardiac pacemaking in sick sinus syndrome. Circ. Res. 2010, 107, 126–137. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Kong, S.W.; Ma, Q.; Pu, W.T. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc. Natl. Acad. Sci. USA 2011, 108, 5632–5637. [Google Scholar] [CrossRef] [PubMed]
- Van den Boogaard, M.; Wong, L.Y.; Tessadori, F.; Bakker, M.L.; Dreizehnter, L.K.; Wakker, V.; Bezzina, C.R.; Hoen, P.A.; Bakkers, J.; Barnett, P.; et al. Genetic variation in T-box binding element functionally affects SCN5A/SCN10A enhancer. J. Clin. Investig. 2012, 122, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, H.; Grace, A.A.; Huang, C.L. SCN5A and sinoatrial node pacemaker function. Cardiovasc. Res. 2007, 74, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Attanasio, C.; Nord, A.S.; Zhu, Y.; Blow, M.J.; Li, Z.; Liberton, D.K.; Morrison, H.; Plajzer-Frick, I.; Holt, A.; Hosseini, R.; et al. Fine tuning of craniofacial morphology by distant-acting enhancers. Science 2013, 342. [Google Scholar] [CrossRef] [PubMed]
- Visel, A.; Minovitsky, S.; Dubchak, I.; Pennacchio, L.A. VISTA Enhancer Browser—A database of tissue-specific human enhancers. Nucleic Acids Res. 2007, 35, D88–D92. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, Y. Shox2 functions as a patterning factor in limb development. 2016; to be submitted for publication. [Google Scholar]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Ma, Z.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yue, F.; Li, Y.; Xie, R.; Harper, T.; Patel, N.A.; Muth, K.; Palmer, J.; Qiu, Y.; Wang, J.; et al. Epigenetic priming of enhancers predicts developmental competence of hESC-derived endodermal lineage intermediates. Cell Stem Cell 2015, 16, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Pott, S.; Lieb, J.D. Single-cell ATAC-seq: Strength in numbers. Genome Biol. 2015, 16, 172. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Song, Y.; Huang, Z.; Zhang, Y.; Chen, Y. Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole. J. Cardiovasc. Dev. Dis. 2015, 2, 282-298. https://doi.org/10.3390/jcdd2040282
Ye W, Song Y, Huang Z, Zhang Y, Chen Y. Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole. Journal of Cardiovascular Development and Disease. 2015; 2(4):282-298. https://doi.org/10.3390/jcdd2040282
Chicago/Turabian StyleYe, Wenduo, Yingnan Song, Zhen Huang, Yanding Zhang, and Yiping Chen. 2015. "Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole" Journal of Cardiovascular Development and Disease 2, no. 4: 282-298. https://doi.org/10.3390/jcdd2040282
APA StyleYe, W., Song, Y., Huang, Z., Zhang, Y., & Chen, Y. (2015). Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole. Journal of Cardiovascular Development and Disease, 2(4), 282-298. https://doi.org/10.3390/jcdd2040282