Right Ventricular Phenotyping Can Lead to Pulmonary Vascular Therapy Response in Those with Pulmonary Hypertension with COPD: A Single-Center Cohort Study
Abstract
1. Introduction
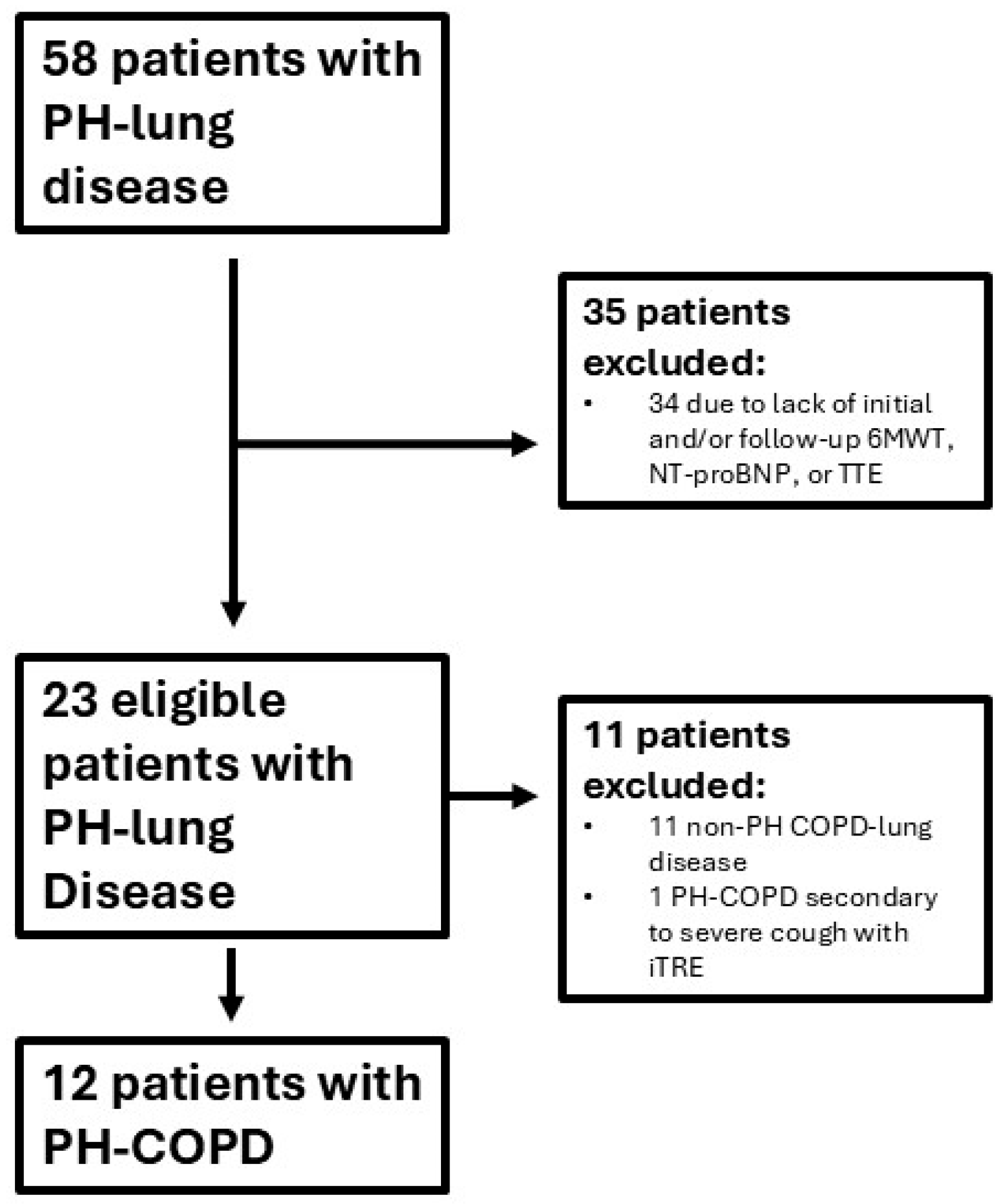
2. Materials and Methods
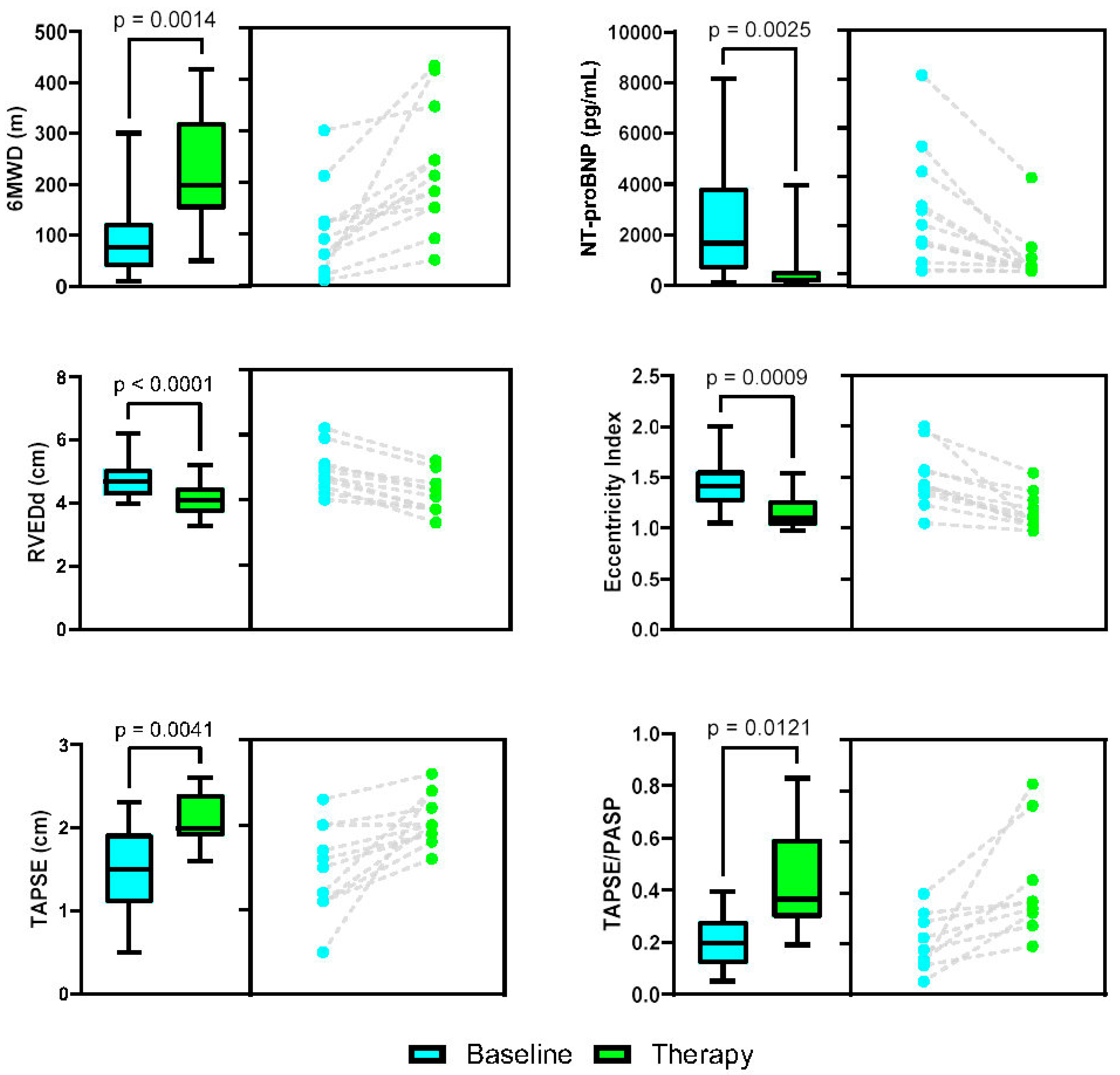
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.H.; Iversen, M.; Kjaergaard, J.; Mortensen, J.; Nielsen-Kudsk, J.E.; Bendstrup, E.; Videbaek, R.; Carlsen, J. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J. Hear. Lung Transplant. 2012, 31, 373–380. [Google Scholar] [CrossRef]
- Kovacs, G.; Avian, A.; Bachmaier, G.; Troester, N.; Tornyos, A.; Douschan, P.; Foris, V.; Sassmann, T.; Zeder, K.; Lindenmann, J.; et al. Severe Pulmonary Hypertension in COPD. Chest 2022, 162, 202–212. [Google Scholar] [CrossRef]
- Nathan, S.D.; Barbera, J.A.; Gaine, S.P.; Harari, S.; Martinez, F.J.; Olschewski, H.; Olsson, K.M.; Peacock, A.J.; Pepke-Zaba, J.; Provencher, S.; et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur. Respir. J. 2019, 53, 1801914. [Google Scholar] [CrossRef]
- Shlobin, O.A.; Adir, Y.; Barbera, J.A.; Cottin, V.; Harari, S.; Jutant, E.-M.; Pepke-Zaba, J.; Ghofrani, H.-A.; Channick, R. Pulmonary hypertension associated with lung diseases. Eur. Respir. J. 2024, 64, 2401200. [Google Scholar] [CrossRef]
- Maron, B.A.; Choudhary, G.; Goldstein, R.L.; Garshick, E.; Jankowich, M.; Tucker, T.J.S.; LaCerda, K.A.; Hattler, B.; Dempsey, E.C.; Sadikot, R.T.; et al. Tadalafil for veterans with chronic obstructive pulmonary disease—Pulmonary hypertension: A multicenter, placebo-controlled randomized trial. Pulm. Circ. 2022, 12, e12043. [Google Scholar] [CrossRef]
- Vitulo, P.; Stanziola, A.; Confalonieri, M.; Libertucci, D.; Oggionni, T.; Rottoli, P.; Paciocco, G.; Tuzzolino, F.; Martino, L.; Beretta, M.; et al. Sildenafil in severe pulmonary hypertension associated with chronic obstructive pulmonary disease: A randomized controlled multicenter clinical trial. J. Hear. Lung Transplant. 2017, 36, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiéry, J.-L.; Gibbs, S.; Lang, I.M.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.V.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Russ. J. Cardiol. 2016, 37, 5–64. [Google Scholar] [CrossRef][Green Version]
- Seeger, W.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, S.; et al. Pulmonary Hypertension in Chronic Lung Diseases. JACC 2013, 62, D109–D116. [Google Scholar] [CrossRef]
- Arif, R.; Pandey, A.; Zhao, Y.; Arsenault-Mehta, K.; Khoujah, D.; Mehta, S. Treatment of pulmonary hypertension associated with COPD: A systematic review. ERJ Open Res. 2021, 8, 00348-2021. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhu, Y.; Mao, Y.; Xu, J.; He, X.; Huang, S.; Wang, M.; Qiu, J. Corrigendum to “Efficacy and safety of Sildenafil treatment in pulmonary hypertension caused by chronic obstructive pulmonary disease: A meta-analysis” [Life Sci. 257 (15) (September 2020) 118001]. Life Sci. 2020, 259, 118471. [Google Scholar] [CrossRef] [PubMed]
- Prins, K.W.; Duval, S.; Markowitz, J.; Pritzker, M.; Thenappan, T. Chronic use of PAH-specific therapy in World Health Organization Group III Pulmonary Hypertension: A systematic review and meta-analysis. Pulm. Circ. 2017, 7, 145–155. [Google Scholar] [CrossRef]
- Vizza, C.D.; Hoeper, M.M.; Huscher, D.; Pittrow, D.; Benjamin, N.; Olsson, K.M.; Ghofrani, H.A.; Held, M.; Klose, H.; Lange, T.; et al. Pulmonary Hypertension in Patients With COPD. Chest 2021, 160, 678–689. [Google Scholar] [CrossRef]
- Hurdman, J.; Condliffe, R.; Elliot, C.A.; Swift, A.; Rajaram, S.; Davies, C.; Hill, C.; Hamilton, N.; Armstrong, I.J.; Billings, C.; et al. Pulmonary hypertension in COPD: Results from the ASPIRE registry. Eur. Respir. J. 2012, 41, 1292–1301. [Google Scholar] [CrossRef]
- Waxman, A.; Restrepo-Jaramillo, R.; Thenappan, T.; Ravichandran, A.; Engel, P.; Bajwa, A.; Allen, R.; Feldman, J.; Argula, R.; Smith, P.; et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N. Engl. J. Med. 2021, 384, 325–334. [Google Scholar] [CrossRef]
- Nathan, S.D.; Argula, R.; Trivieri, M.G.; Aziz, S.; Gay, E.; Medarov, B.; Parambil, J.; Raina, A.; Risbano, M.G.; Thenappan, T.; et al. Inhaled treprostinil in pulmonary hypertension associated with COPD: PERFECT study results. Eur. Respir. J. 2024, 63, 2400172. [Google Scholar] [CrossRef]
- Nathan, S.D.; Lacasse, V.; Bell, H.; Sista, P.; Di Marino, M.; Bull, T.; Tapson, V.; Waxman, A. COPD associated pulmonary hypertension: A post hoc analysis of the PERFECT study. Pulm. Circ. 2024, 14, e12430. [Google Scholar] [CrossRef]
- Lange, T.J.; Baron, M.; Seiler, I.; Arzt, M.; Pfeifer, M. Outcome of Patients with Severe PH due to Lung Disease with and without Targeted Therapy. Cardiovasc. Ther. 2014, 32, 202–208. [Google Scholar] [CrossRef]
- Brewis, M.J.; Church, A.C.; Johnson, M.K.; Peacock, A.J. Severe pulmonary hypertension in lung disease: Phenotypes and response to treatment. Eur. Respir. J. 2015, 46, 1378–1389. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A.R.; Lipworth, B.J.; Hopkinson, P.J.; Wei, L.; Struthers, A.D. Tadalafil in patients with chronic obstructive pulmonary disease: A randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2014, 2, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Golbus, J.R.; Vedage, N.A.; Mazurek, J.; Raza, F.; Forfia, P.R. Virtual echocardiography screening tool to differentiate hemodynamic profiles in pulmonary hypertension. Pulm. Circ. 2020, 10, 2045894020950225. [Google Scholar] [CrossRef] [PubMed]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the Right Ventricular Doppler Envelope Predicts Hemodynamics and Right Heart Function in Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef] [PubMed]
- López-Candales, A.; Edelman, K. Shape of the right ventricular outflow Doppler envelope and severity of pulmonary hypertension. Eur. Hear. J.—Cardiovasc. Imaging 2011, 13, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Voswinckel, R.; Reichenberger, F.; Enke, B.; Kreckel, A.; Krick, S.; Gall, H.; Schermuly, R.T.; Grimminger, F.; Rubin, L.J.; Olschewski, H.; et al. Acute effects of the combination of sildenafil and inhaled treprostinil on haemodynamics and gas exchange in pulmonary hypertension. Pulm. Pharmacol. Ther. 2008, 21, 824–832. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | ||||
|---|---|---|---|---|
| Total number of patients | COPD (n = 12) | |||
| Female | 7 (58.0%) | |||
| Age (Years) | 73.8 (57–85) | |||
| Comorbidities a | ||||
| BMI (kg/m2) | 24 (16.5–33.7) | |||
| CAD | 5 (41.7%) | |||
| HTN | 8 (66.7%) | |||
| DM II | 3 (25.0%) | |||
| Atrial fibrillation/Flutter | 3 (25.0%) | |||
| OSA | 6 (50.0%) | |||
| Pulmonary Data | ||||
| Combined | Subgroup A | Subgroup B | p value | |
| n = 12 | n = 6 | n = 6 | ||
| FEV1 (%) | 65 +/− 12.8 (39–80) | 59.2 +/− 14.5 | 70.8 +/− 8.3 | 0.1175 |
| FVC (%) | 83.8 +/− 19.9 (57–127) | 85 +/− 17.3 | 82 +/− 23.8 | |
| FEV1/FVC (%) | 70.5 +/− 17.8 (48–95) | 58.2 +/− 7.1 | 82.8 +/− 16.8 | 0.0079 |
| DLCO (%) | 30.8 +/− 9.5 (17–46) | 28 +/− 7.2 | 33.7 +/− 11.7 | 0.3251 |
| Supplemental O2 (L/min) | 12 (100%) 3.4 +/− 1.4 (2–5 L/min) | |||
| Emphysema on CT Chest | 12 (100%) | 6 (50%) | 6 (50%) | |
| Mild | 4 (33.3%) | 2 (33%) | 2 (33%) | |
| Moderate to severe | 8 (66.7%) | 4 (67%) | 4 (67%) | |
| Echocardiographic Parameters | ||||
| Pre—Treatment Mean ± STDev | On—Treatment Mean ± STDev | p value | ||
| RVEDd (cm)—basal width | 4.8 +/− 0.67 | 4.1 +/− 0.59 | <0.0001 | |
| LV systolic EI index | 1.46 +/− 0.29 | 1.15 +/− 0.17 | <0.0009 | |
| TAPSE (cm) | 1.47 +/− 0.50 | 2.1 +/− 0.30 | <0.0041 | |
| TAPSE/PASP ratio | 0.20 +/− 0.10 | 0.43 +/− 0.21 | <0.0121 | |
| RVOT VTI (cm) | 11.2 +/− 4.5 | 15.6 +/− 2.5 | <0.02 | |
| LVOT VTI (cm) | 17.3 +/− 4.9 | 22.2 +/− 2.8 | <0.0093 | |
| RVOT PW Doppler notching | ||||
| Mid-systolic notch | 11 (92%) | 4 (33%) | <0.036 | |
| Late-systolic notch | 1 (8%) | 8 (67%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salako, O.; Singh, A. Right Ventricular Phenotyping Can Lead to Pulmonary Vascular Therapy Response in Those with Pulmonary Hypertension with COPD: A Single-Center Cohort Study. J. Cardiovasc. Dev. Dis. 2025, 12, 366. https://doi.org/10.3390/jcdd12090366
Salako O, Singh A. Right Ventricular Phenotyping Can Lead to Pulmonary Vascular Therapy Response in Those with Pulmonary Hypertension with COPD: A Single-Center Cohort Study. Journal of Cardiovascular Development and Disease. 2025; 12(9):366. https://doi.org/10.3390/jcdd12090366
Chicago/Turabian StyleSalako, Oluwafeyijimi, and Abhishek Singh. 2025. "Right Ventricular Phenotyping Can Lead to Pulmonary Vascular Therapy Response in Those with Pulmonary Hypertension with COPD: A Single-Center Cohort Study" Journal of Cardiovascular Development and Disease 12, no. 9: 366. https://doi.org/10.3390/jcdd12090366
APA StyleSalako, O., & Singh, A. (2025). Right Ventricular Phenotyping Can Lead to Pulmonary Vascular Therapy Response in Those with Pulmonary Hypertension with COPD: A Single-Center Cohort Study. Journal of Cardiovascular Development and Disease, 12(9), 366. https://doi.org/10.3390/jcdd12090366






