Association of Pan-Immune-Inflammation Value with All-Cause and Cardiovascular Mortality in Survivors of Myocardial Infarction: NHANES 2001–2018 Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Identification of MI Survivors
2.3. Calculation and Logarithmic Transformation of the PIV
2.4. Mortality and Follow-Up Assessment
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association Between LnPIV and All-Cause and Cardiovascular Mortality in MI Survivors
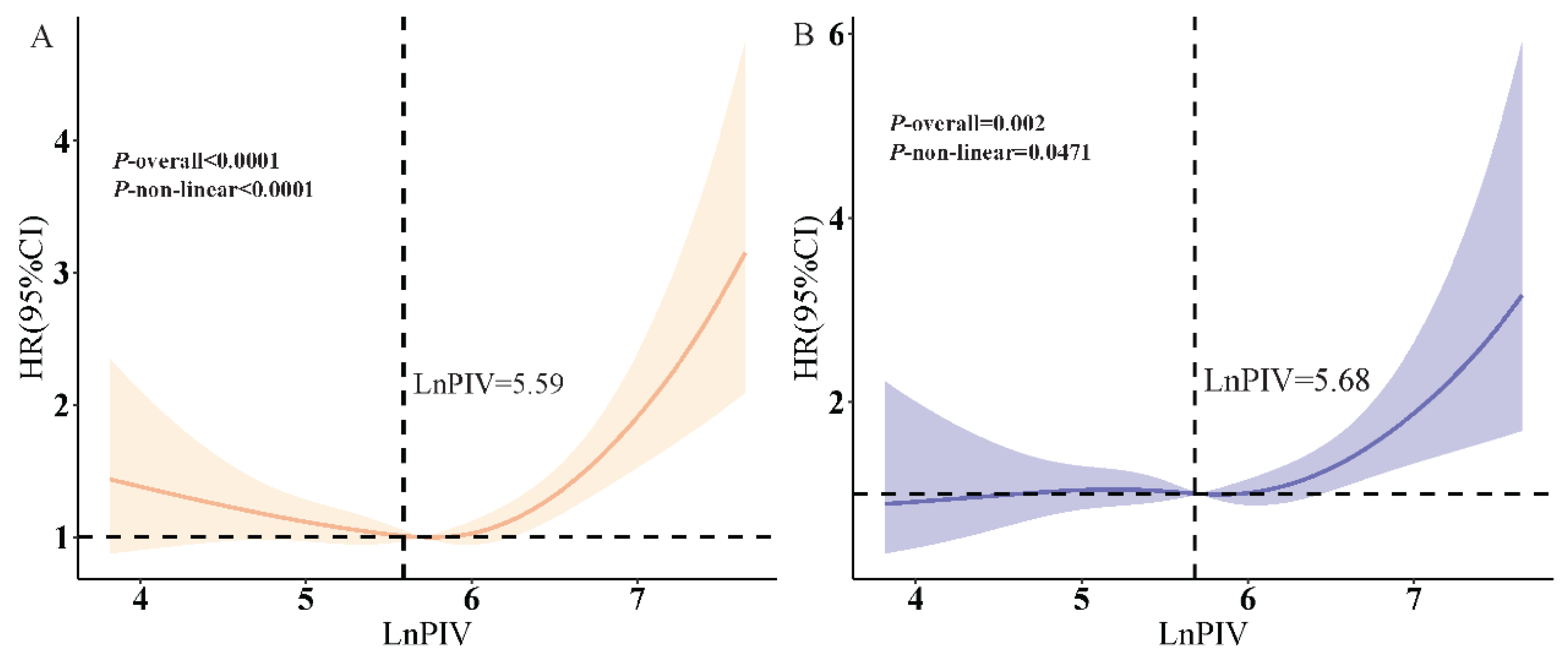
3.3. RCS Analysis
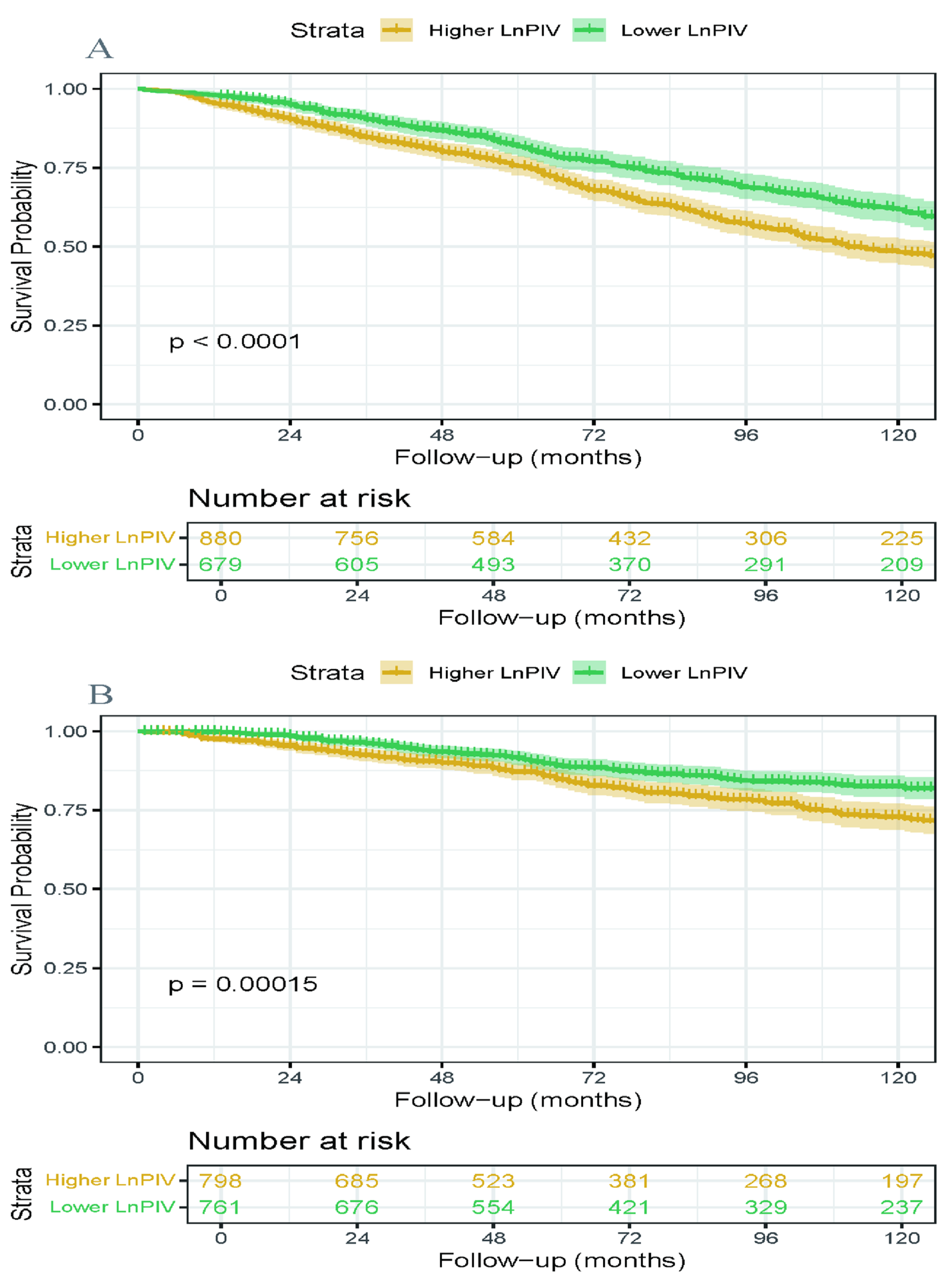
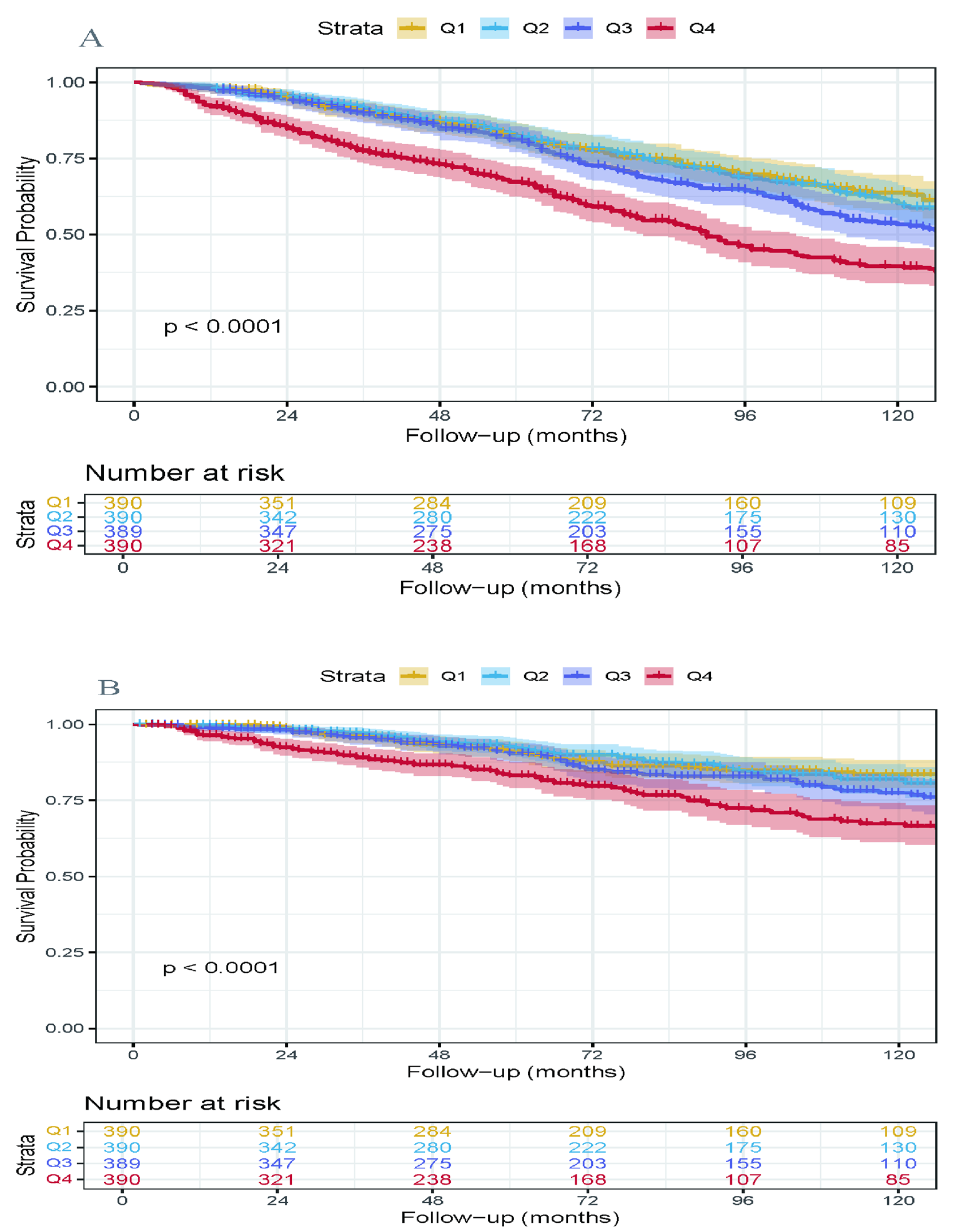
3.4. Kaplan–Meier Survival Analysis
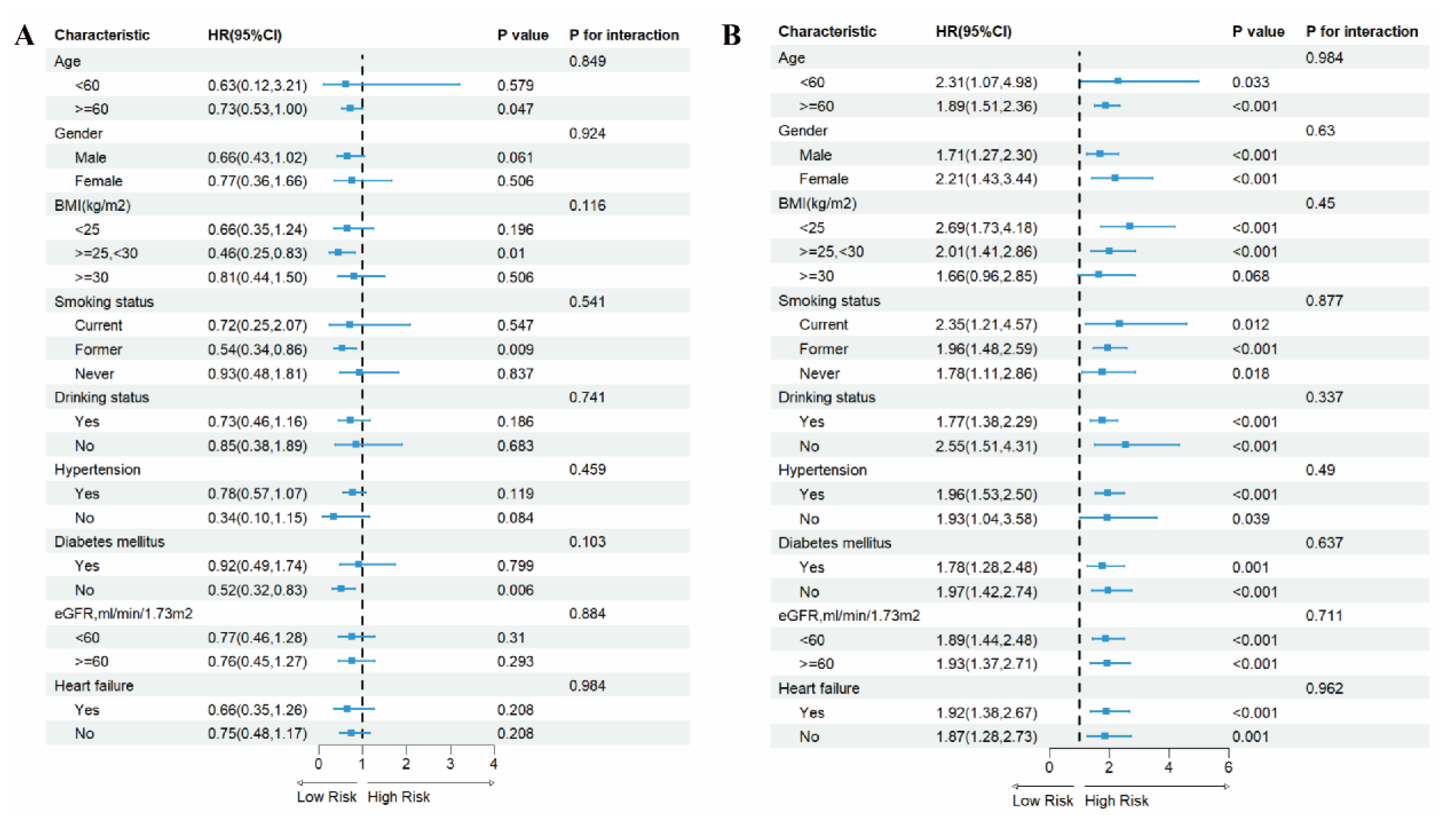
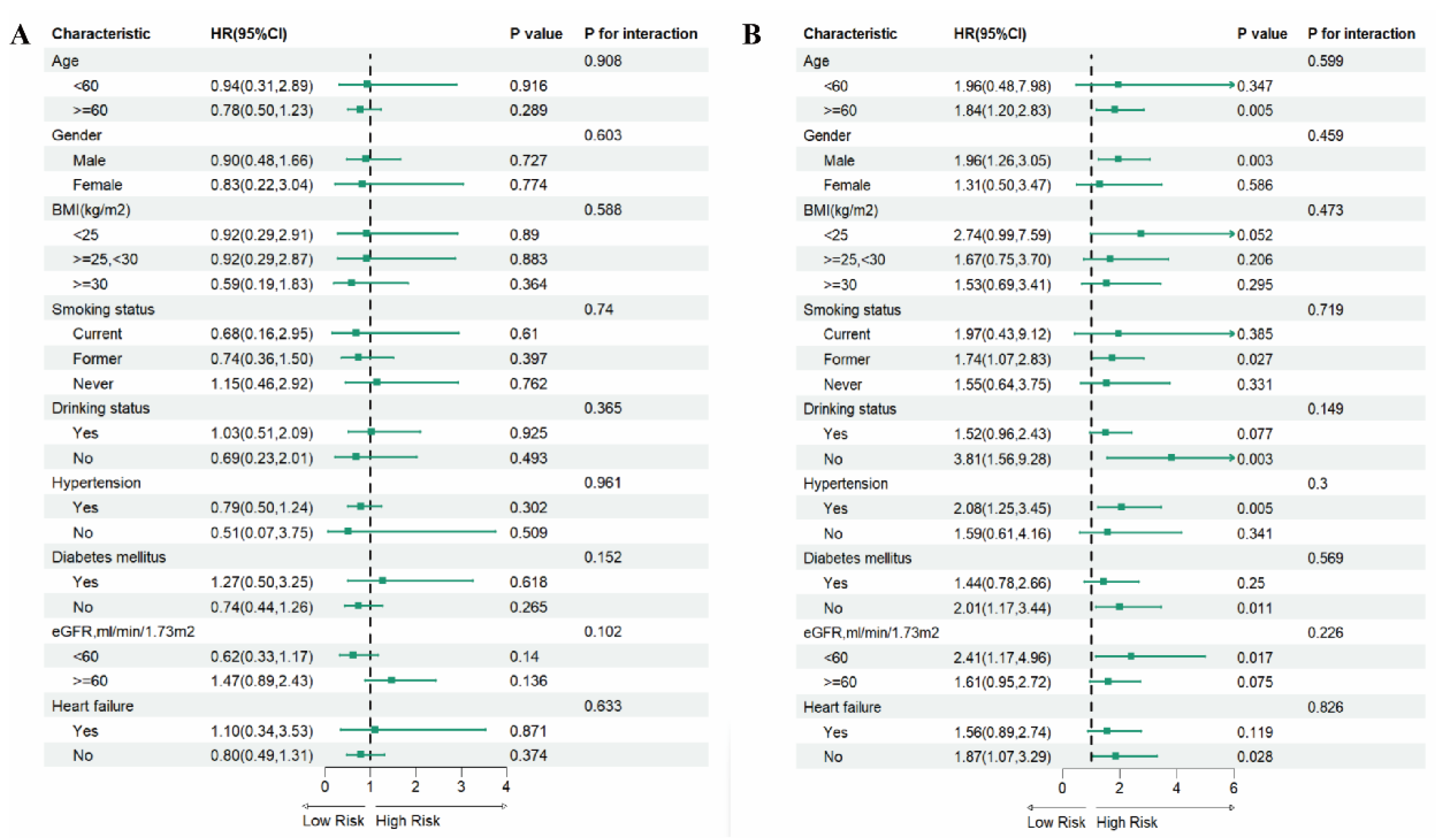
3.5. Subgroup Analysis
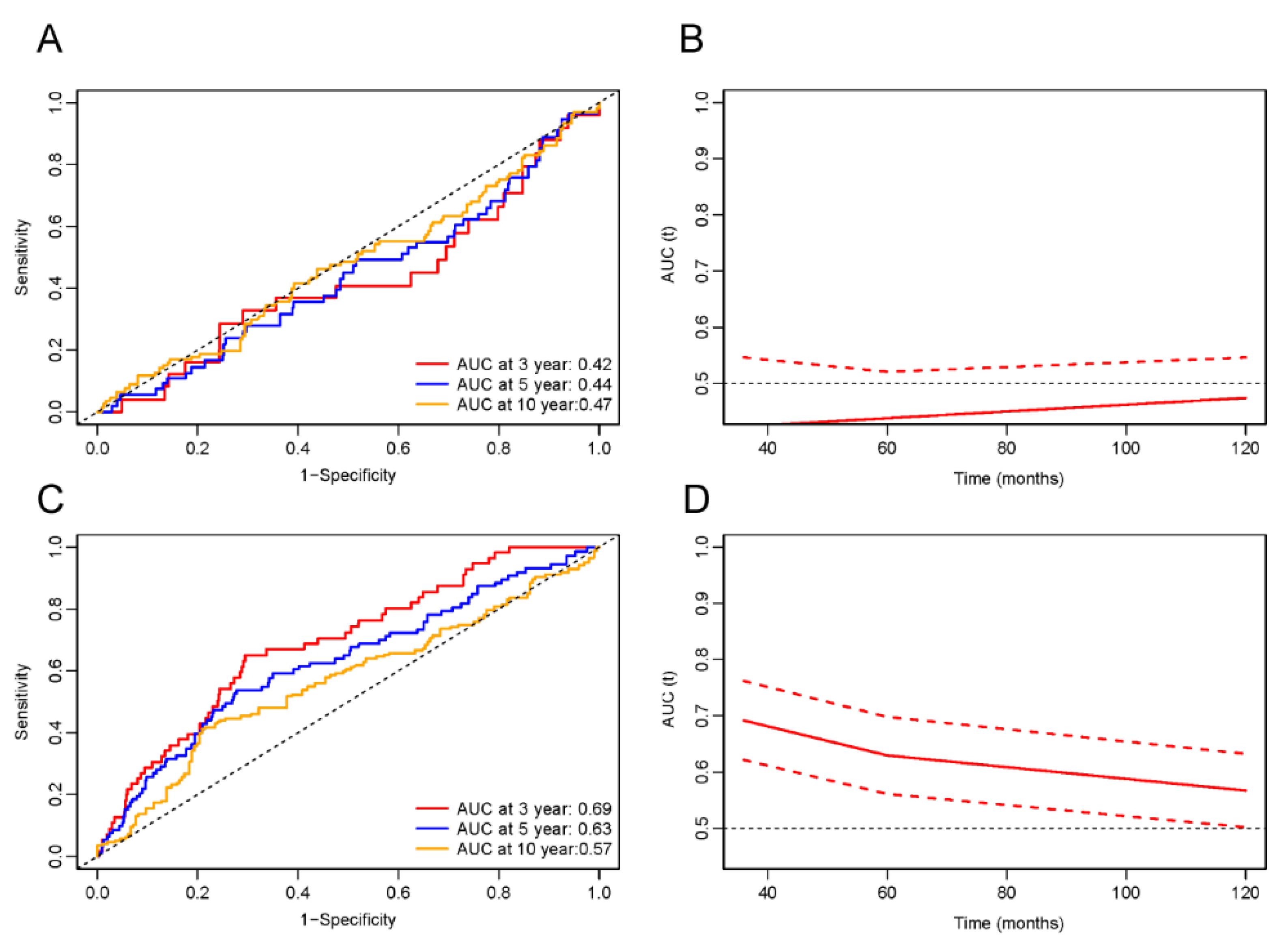
3.6. ROC Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Myocardial Infarction: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute Myocardial Infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.A.; Clayton, T.C.; Damman, P.; Pocock, S.J.; De Winter, R.J.; Tijssen, J.G.P.; Lagerqvist, B.; Wallentin, L. Long-Term Outcome of a Routine Versus Selective Invasive Strategy in Patients with Non–ST-Segment Elevation Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2010, 55, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair, and Remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Innate Immune Cells in Ischaemic Heart Disease: Does Myocardial Infarction Beget Myocardial Infarction? Eur. Heart J. 2016, 37, 868–872. [Google Scholar] [CrossRef]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value Is a New Prognostic Biomarker in Metastatic Colorectal Cancer: Results from a Pooled-Analysis of the Valentino and TRIBE First-Line Trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef]
- Fu, G.; Deng, M.; Neal, M.D.; Billiar, T.R.; Scott, M.J. Platelet–Monocyte Aggregates: Understanding Mechanisms and Functions in Sepsis. Shock 2021, 55, 156–166. [Google Scholar] [CrossRef]
- Gauer, J.S.; Ajjan, R.A.; Ariëns, R.A.S. Platelet–Neutrophil Interaction and Thromboinflammation in Diabetes: Considerations for Novel Therapeutic Approaches. JAHA 2022, 11, e027071. [Google Scholar] [CrossRef]
- Toori, K.U.; Qureshi, M.A.; Chaudhry, A. Lymphopenia: A Useful Predictor of COVID-19 Disease Severity and Mortality. Pak. J. Med. Sci. 2021, 37, 1984. [Google Scholar] [CrossRef]
- Mardan, M.; Zheng, H.; Xu, Q.; Song, S.; Lu, Z.; Deng, H.; Cai, H.; Chen, Q.; Yang, B.; Abuduwufuer, K.; et al. Comprehensive Analysis of Pan-Immune Inflammation and All-Cause Mortality in Rheumatoid Arthritis: A Database-Driven Approach, 1999–2018. Front. Immunol. 2025, 16, 1549955. [Google Scholar] [CrossRef]
- Yang, L.; Guo, J.; Chen, M.; Wang, Y.; Li, J.; Zhang, J. Pan-Immune-Inflammatory Value Is Superior to Other Inflammatory Indicators in Predicting Inpatient Major Adverse Cardiovascular Events and Severe Coronary Artery Stenosis after Percutaneous Coronary Intervention in STEMI Patients. Rev. Cardiovasc. Med. 2024, 25, 294. [Google Scholar] [CrossRef]
- Dervis, E.; Yakut, I.; Inan, D. The Prognostic Significance of the Pan-Immune-Inflammation Value in Patients with Heart Failure with Reduced Ejection Fraction. Diagnostics 2025, 15, 1617. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, C.; Lin, S.; Zhang, Y.; Ding, S.; Song, W. The Relationship between the Pan-Immune-Inflammation Value and Long-Term Prognoses in Patients with Hypertension: National Health and Nutrition Examination Study, 1999–2018. Front. Cardiovasc. Med. 2023, 10, 1099427. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Bhatt, D.L.; Pradhan, A.D.; Glynn, R.J.; MacFadyen, J.G.; Nissen, S.E. Inflammation and Cholesterol as Predictors of Cardiovascular Events among Patients Receiving Statin Therapy: A Collaborative Analysis of Three Randomised Trials. Lancet 2023, 401, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Şen, F.; Kurtul, A.; Bekler, Ö. Pan-Immune-Inflammation Value Is Independently Correlated to Impaired Coronary Flow After Primary Percutaneous Coronary Intervention in Patients With ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2024, 211, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Murat, B.; Murat, S.; Ozgeyik, M.; Bilgin, M. Comparison of Pan-immune-inflammation Value with Other Inflammation Markers of Long-term Survival after ST -segment Elevation Myocardial Infarction. Eur. J. Clin. Investig. 2023, 53, e13872. [Google Scholar] [CrossRef]
- Paulose-Ram, R.; Graber, J.E.; Woodwell, D.; Ahluwalia, N. The National Health and Nutrition Examination Survey (NHANES), 2021–2022: Adapting Data Collection in a COVID-19 Environment. Am. J. Public Health 2021, 111, 2149–2156. [Google Scholar] [CrossRef]
- Li, D.; Ruan, Z.; Xie, S.; Xuan, S.; Zhao, H.; Wu, B. The Relationship between Preserved Ratio Impaired Spirometry and Mortality in the Myocardial Infarction Survivors: A Population-Based Cohort Study. BMC Cardiovasc. Disord. 2023, 23, 331. [Google Scholar] [CrossRef]
- Okura, Y.; Urban, L.H.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J. Agreement between Self-Report Questionnaires and Medical Record Data Was Substantial for Diabetes, Hypertension, Myocardial Infarction and Stroke but Not for Heart Failure. J. Clin. Epidemiol. 2004, 57, 1096–1103. [Google Scholar] [CrossRef]
- Parikh, N.S.; Salehi Omran, S.; Kamel, H.; Elkind, M.S.V.; Willey, J. Symptoms of Depression and Active Smoking among Survivors of Stroke and Myocardial Infarction: An NHANES Analysis. Prev. Med. 2020, 137, 106131. [Google Scholar] [CrossRef]
- Dong, W.; Yang, Z. Trends in Lipid Profile and Lipid Control among Survivors of Stroke or Myocardial Infarction among US Adults, 2001–2018. Front. Endocrinol. 2023, 14, 1128878. [Google Scholar] [CrossRef]
- Shah, N.S.; Huffman, M.D.; Ning, H.; Lloyd-Jones, D.M. Trends in Myocardial Infarction Secondary Prevention: The National Health and Nutrition Examination Surveys (NHANES), 1999–2012. JAHA 2015, 4, e001709. [Google Scholar] [CrossRef]
- Menke, A.; Casagrande, S.; Geiss, L.; Cowie, C.C. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015, 314, 1021. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, Y.; Zhang, B. Associations of Triglyceride-Glucose (TyG) Index with Chest Pain Incidence and Mortality among the U.S. Population. Cardiovasc. Diabetol. 2024, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Han, L.-L.; Luo, W.; Hu, M.; Zhang, H.-Z.; Liao, Y.-L. The Triglyceride-Glucose Index: A Predictor of Mortality Risk among Myocardial Infarction Survivors. Sci. Rep. 2024, 14, 27512. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Iii, A.F.C.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Coresh, J. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, A.; Kyriakou, M.; Sokratous, S.; Karmioti, G.; Drakomathioulakis, M.; Myrianthefs, M.; Ziakas, A.; Tzikas, S.; Kassimis, G. Exploring the Landscape of Anti-Inflammatory Trials: A Comprehensive Review of Strategies for Targeting Inflammation in Acute Myocardial Infraction. Biomedicines 2024, 12, 701. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and the Pathogenesis of Atherosclerosis. Vasc. Pharmacol. 2024, 154, 107255. [Google Scholar] [CrossRef]
- Feng, Q.; Li, Q.; Zhou, H.; Sun, L.; Lin, C.; Jin, Y.; Wang, D.; Guo, G. The Role of Major Immune Cells in Myocardial Infarction. Front. Immunol. 2023, 13, 1084460. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, D.; Cheng, S.; Ni, R.; Yang, M.; Cai, Y.; Chen, J.; Liu, F.; Liu, Y. Inflammatory Cell-Targeted Delivery Systems for Myocardial Infarction Treatment. Bioengineering 2025, 12, 205. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, L.; Li, J.; Zhang, Y.; Shen, Z. Annexin A1-Loaded Alginate Hydrogel Promotes Cardiac Repair via Modulation of Macrophage Phenotypes after Myocardial Infarction. ACS Biomater. Sci. Eng. 2024, 10, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, F.; Cao, F.; Wang, L.; She, J.; He, B.; Xu, X.; Kong, L.; Cai, B. Neutrophils in Tissue Injury and Repair: Molecular Mechanisms and Therapeutic Targets. MedComm 2025, 6, e70184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Zhang, L.; Li, P. Nanozyme-based Visual Diagnosis and Therapeutics for Myocardial Infarction: The Application and Strategy. J. Adv. Res. 2025, 70, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shou, X.; Wu, Y.; Li, D. Immuno-Inflammatory Pathogenesis in Ischemic Heart Disease: Perception and Knowledge for Neutrophil Recruitment. Front. Immunol. 2024, 15, 1411301. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Tucureanu, M.M.; Simionescu, M.; Butoi, E. Anti-Inflammatory Neutrophils Reprogram Macrophages toward a Pro-Healing Phenotype with Increased Efferocytosis Capacity. Cells 2024, 13, 208. [Google Scholar] [CrossRef]
- Antipenko, S.; Mayfield, N.; Jinno, M.; Gunzer, M.; Ismahil, M.A.; Hamid, T.; Prabhu, S.D.; Rokosh, G. Neutrophils Are Indispensable for Adverse Cardiac Remodeling in Heart Failure. J. Mol. Cell. Cardiol. 2024, 189, 1–11. [Google Scholar] [CrossRef]
- Luo, J.; Thomassen, J.Q.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Neutrophil Counts and Cardiovascular Disease. Eur. Heart J. 2023, 44, 4953–4964. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.; Fan, Y.; Fan, X.; Li, A.; Qi, Z.; Zhang, J. Cardiac Repair after Myocardial Infarction: A Two-Sided Role of Inflammation-Mediated. Front. Cardiovasc. Med. 2023, 9, 1077290. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Bao, Z.; Zhu, D.; Chen, G.; Li, W.; Xiao, Y.; Wang, Z.; Zhang, Y.; Liu, H.; et al. Pericardial Delivery of SDF-1 α Puerarin Hydrogel Promotes Heart Repair and Electrical Coupling. Adv. Mater. 2024, 36, 2302686. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Liu, L.; Dang, X.; Zhu, D.; Tian, G. Monocyte/Lymphocyte Ratio Is Related to the Severity of Coronary Artery Disease and Clinical Outcome in Patients with Non-ST-Elevation Myocardial Infarction. Medicine 2019, 98, e16267. [Google Scholar] [CrossRef]
- Martinez Bravo, G.; Annarapu, G.; Carmona, E.; Nawarskas, J.; Clark, R.; Novelli, E.; Mota Alvidrez, R.I. Platelets in Thrombosis and Atherosclerosis. Am. J. Pathol. 2024, 194, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Song, P.S.; Ahn, K.T.; Jeong, J.-O.; Jeon, K.-H.; Song, Y.B.; Gwon, H.-C.; Rha, S.-W.; Jeong, M.H.; Seong, I.-W. KAMIR-NIH Investigators Association of Baseline Platelet Count with All-Cause Mortality after Acute Myocardial Infarction. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Martín, P.; Sánchez-Madrid, F. T Cells in Cardiac Health and Disease. J. Clin. Investig. 2025, 135, e185218. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.; Liang, T.; Zhou, Y.; Su, X.; Li, X.; Zeng, J.; Qu, P.; Wang, Y.; Chen, F.; et al. The Roles of Th Cells in Myocardial Infarction. Cell Death Discov. 2024, 10, 287. [Google Scholar] [CrossRef]
- Learmonth, M.; Corker, A.; Dasgupta, S.; DeLeon-Pennell, K.Y. Regulation of Cardiac Fibroblasts by Lymphocytes after a Myocardial Infarction: Playing in the Major League. Am. J. Physiol.-Heart Circ. Physiol. 2023, 325, H553–H561. [Google Scholar] [CrossRef]
- Qin, Y.; Li, M.; Liu, H. Regulatory T Cells: A Promising New Therapeutic Target in Ventricular Remodeling after Myocardial Infarction. Front. Immunol. 2025, 16, 1514335. [Google Scholar] [CrossRef]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and Pathogenic Th2 Cells in Inflammation, Tissue Repair, and Fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, C.; Bo, X.; Lu, Z.; Qian, H.; Chen, L. The Prognostic Value of Admission Lymphocyte-to-Monocyte Ratio in Critically Ill Patients with Acute Myocardial Infarction. BMC Cardiovasc. Disord. 2022, 22, 308. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, R.; Wang, X.; Zhang, W.; Li, M.; Ni, T.; Weng, W.; Li, Q. The Neutrophil-to-Lymphocyte Ratio Is Associated with All-Cause and Cardiovascular Mortality among Individuals with Hypertension. Cardiovasc. Diabetol. 2024, 23, 117. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Li, J. Inflammation and Inflammatory Cells in Myocardial Infarction and Reperfusion Injury: A Double-Edged Sword. Clin. Med. Insights Cardiol. 2016, 10, CMC.S33164. [Google Scholar] [CrossRef]
- Sun, K.; Li, Y.; Jin, J. A Double-Edged Sword of Immuno-Microenvironment in Cardiac Homeostasis and Injury Repair. Sig. Transduct. Target. Ther. 2021, 6, 79. [Google Scholar] [CrossRef]
- Sun, M.; Dubé, M.-P.; Hennessy, T.; Schultz, C.J.; Barhdadi, A.; Rhainds, D.; Hillis, G.S.; Tardif, J.-C. Low-Dose Colchicine and High-Sensitivity C-Reactive Protein after Myocardial Infarction: A Combined Analysis Using Individual Patient Data from the COLCOT and LoDoCo-MI Studies. Int. J. Cardiol. 2022, 363, 20–22. [Google Scholar] [CrossRef]
- Zheng, W.C.; Chan, W.; Dart, A.; Shaw, J.A. Novel Therapeutic Targets and Emerging Treatments for Atherosclerotic Cardiovascular Disease. Eur. Heart J.-Cardiovasc. Pharmacother. 2024, 10, 53–67. [Google Scholar] [CrossRef]
- Canton, L.; Fedele, D.; Bergamaschi, L.; Foà, A.; Di Iuorio, O.; Tattilo, F.P.; Rinaldi, A.; Angeli, F.; Armillotta, M.; Sansonetti, A.; et al. Sex- and Age-Related Differences in Outcomes of Patients with Acute Myocardial Infarction: MINOCA vs. MIOCA. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 604–614. [Google Scholar] [CrossRef]
- Dong, G.; Gan, M.; Xu, S.; Xie, Y.; Zhou, M.; Wu, L. The Neutrophil–Lymphocyte Ratio as a Risk Factor for All-Cause and Cardiovascular Mortality among Individuals with Diabetes: Evidence from the NHANES 2003–2016. Cardiovasc. Diabetol. 2023, 22, 267. [Google Scholar] [CrossRef]


| Characteristic | Total Participants (n = 1559) | Surviving Participants (n = 884) | Dead Participants (n = 675) | p-Value |
|---|---|---|---|---|
| Age, years | 65.01 ± 12.28 | 61.59 ± 12.35 | 70.82 ± 9.73 | <0.0001 |
| Gender, n (%) | 0.4602 | |||
| male | 1051 (63.69) | 575 (63.00) | 476 (64.86) | |
| female | 508 (36.31) | 309 (37.00) | 199 (35.14) | |
| Race, n (%) | 0.0003 | |||
| Mexican American | 140 (3.27) | 86 (3.71) | 54 (2.52) | |
| Other Hispanic | 101 (3.36) | 76 (4.33) | 25 (1.73) | |
| Non-Hispanic White | 960 (79.05) | 484 (75.78) | 476 (84.59) | |
| Non-Hispanic Black | 278 (9.08) | 178 (9.71) | 100 (8.01) | |
| Other Race | 80 (5.23) | 60 (6.46) | 20 (3.15) | |
| Education Level, n (%) | <0.0001 | |||
| Under high school | 535 (25.51) | 258 (20.68) | 277 (33.72) | |
| High school or equivalent | 388 (28.57) | 218 (28.27) | 170 (29.09) | |
| Above high school | 636 (45.91) | 408 (51.05) | 228 (37.19) | |
| PIR | 2.66 ± 1.61 | 2.84 ± 1.68 | 2.34 ± 1.42 | <0.0001 |
| Marital status, n (%) | ||||
| Married/Living with a partner | 893 (61.48) | 535 (65.55) | 358 (54.57) | |
| Never married | 94 (5.44) | 60 (5.95) | 34 (4.58) | |
| Widowed/Divorced/Separated | 572 (33.08) | 289 (28.50) | 283 (40.85) | |
| BMI, kg/m2 | 30.32 ± 6.81 | 30.87 ± 6.83 | 29.39 ± 6.67 | <0.0001 |
| Hypercholesterolemia, n (%) | 0.1065 | |||
| Yes | 1190 (77.46) | 695 (78.78) | 495 (75.24) | |
| No | 369 (22.54) | 189 (21.22) | 180 (24.76) | |
| Smoking, n (%) | 0.0001 | |||
| never | 496 (31.08) | 299 (32.94) | 197 (27.93) | |
| former | 698 (44.45) | 352 (40.39) | 346 (51.35) | |
| current | 365 (24.46) | 233 (26.66) | 132 (20.73) | |
| Drinking, n (%) | 0.0017 | |||
| Yes | 1155 (76.33) | 679 (78.92) | 476 (71.94) | |
| No | 404 (23.67) | 205 (21.08) | 199 (28.06) | |
| Hypertension, n (%) | 0.0012 | |||
| Yes | 1176 (72.18) | 646 (69.36) | 530 (76.97) | |
| No | 383 (27.82) | 238 (30.64) | 145 (23.03) | |
| Diabetes mellitus, n (%) | 0.0003 | |||
| Yes | 637 (38.44) | 344 (35.06) | 293 (44.18) | |
| No | 922 (61.56) | 540 (64.94) | 382 (55.82) | |
| Heart failure, n (%) | <0.0001 | |||
| Yes | 520 (30.93) | 251(25.38) | 269 (40.36) | |
| No | 1039 (69.07) | 633 (74.62) | 406 (59.64) | |
| Stroke, n (%) | 0.0073 | |||
| Yes | 275 (16.19) | 138 (14.27) | 137 (19.45) | |
| No | 1284 (83.81) | 746 (85.73) | 538 (80.55) | |
| Cancer, n (%) | <0.0001 | |||
| Yes | 332 (21.49) | 154 (17.67) | 178 (27.98) | |
| No | 1227 (78.51) | 730 (82.33) | 497 (72.02) | |
| eGFR (mL/min/1.73 m2) | <0.0001 | |||
| <60 | 510 (28.25) | 196 (19.71) | 314 (42.75) | |
| ≥60 | 1049 (71.75) | 688 (80.29) | 361 (57.25) | |
| PIV | 380.33 ± 320.41 | 343.49 ± 256.88 | 442.89 ± 398.40 | <0.0001 |
| LnPIV | 5.69 ± 0.71 | 5.62 ± 0.67 | 5.81 ± 0.75 | <0.0001 |
| LnPIV | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| All-cause mortality | ||||||||
| Continuous | 1.40 (1.19, 1.64) | <0.001 | 1.21 (1.02, 1.44) | 0.026 | 1.39 (1.17, 1.64) | <0.001 | 1.17 (0.99, 1.39) | 0.070 |
| Categorical | ||||||||
| Q1 (2.09–5.23) | Reference | Reference | Reference | Reference | ||||
| Q2 (5.23–5.71) | 0.98 (0.68, 1.41) | 0.913 | 0.90 (0.65, 1.24) | 0.518 | 0.94 (0.63, 1.40) | 0.757 | 0.87 (0.61, 1.25) | 0.445 |
| Q3 (5.71–6.13) | 1.17 (0.86, 1.60) | 0.327 | 0.86 (0.62, 1.19) | 0.373 | 1.15 (0.84, 1.58) | 0.391 | 0.83 (0.61, 1.15) | 0.267 |
| Q4 (6.13–8.20) | 1.83 (1.35, 2.47) | <0.001 | 1.39 (1.04, 1.88) | 0.029 | 1.69 (1.22, 2.34) | 0.002 | 1.29 (0.94, 1.77) | 0.112 |
| p for trend | <0.001 | 0.023 | <0.001 | 0.077 | ||||
| Cardiovascular mortality | ||||||||
| Continuous | 1.49 (1.17, 1.89) | 0.001 | 1.30 (1.01, 1.67) | 0.043 | 1.50 (1.17, 1.91) | 0.001 | 1.26 (0.97, 1.64) | 0.083 |
| Categorical | ||||||||
| Q1 (2.09–5.23) | Reference | Reference | Reference | Reference | ||||
| Q2 (5.23–5.71) | 1.16 (0.75, 1.79) | 0.499 | 1.07 (0.70, 1.65) | 0.749 | 1.14 (0.72, 1.79) | 0.580 | 1.04 (0.67, 1.62) | 0.858 |
| Q3 (5.71–6.13) | 1.36 (0.86, 2.16) | 0.188 | 1.02 (0.64, 1.62) | 0.946 | 1.40 (0.88, 2.22) | 0.157 | 1.00 (0.63, 1.60) | 0.991 |
| Q4 (6.13–8.20) | 2.01 (1.31, 3.08) | 0.001 | 1.51 (0.96, 2.39) | 0.074 | 1.90 (1.22, 2.96) | 0.005 | 1.41 (0.88, 2.27) | 0.151 |
| p for trend | 0.001 | 0.087 | 0.003 | 0.162 |
| Adjusted HR (95%CI), p Value | |
|---|---|
| All-cause mortality | |
| Total | 1.17 (0.99, 1.39), 0.070 |
| Cutoff value | 5.59 |
| LnPIV < 5.59 | 0.76 (0.51, 1.13), 0.176 |
| LnPIV ≥ 5.59 | 1.85 (1.49, 2.28), <0.001 |
| p for Log-likelihood ratio | <0.001 |
| Cardiovascular mortality | |
| Total | 1.26 (0.97, 1.64), 0.083 |
| Cutoff value | 5.68 |
| LnPIV < 5.68 | 0.98 (0.63, 1.52), 0.914 |
| LnPIV ≥ 5.68 | 1.77 (1.20, 2.63), 0.004 |
| p for Log-likelihood ratio | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yang, W.; Zhang, R.; Guo, X.; Wei, Y. Association of Pan-Immune-Inflammation Value with All-Cause and Cardiovascular Mortality in Survivors of Myocardial Infarction: NHANES 2001–2018 Analysis. J. Cardiovasc. Dev. Dis. 2025, 12, 363. https://doi.org/10.3390/jcdd12090363
Liu Q, Yang W, Zhang R, Guo X, Wei Y. Association of Pan-Immune-Inflammation Value with All-Cause and Cardiovascular Mortality in Survivors of Myocardial Infarction: NHANES 2001–2018 Analysis. Journal of Cardiovascular Development and Disease. 2025; 12(9):363. https://doi.org/10.3390/jcdd12090363
Chicago/Turabian StyleLiu, Qingyi, Wenling Yang, Ruiyu Zhang, Xiaopeng Guo, and Yumiao Wei. 2025. "Association of Pan-Immune-Inflammation Value with All-Cause and Cardiovascular Mortality in Survivors of Myocardial Infarction: NHANES 2001–2018 Analysis" Journal of Cardiovascular Development and Disease 12, no. 9: 363. https://doi.org/10.3390/jcdd12090363
APA StyleLiu, Q., Yang, W., Zhang, R., Guo, X., & Wei, Y. (2025). Association of Pan-Immune-Inflammation Value with All-Cause and Cardiovascular Mortality in Survivors of Myocardial Infarction: NHANES 2001–2018 Analysis. Journal of Cardiovascular Development and Disease, 12(9), 363. https://doi.org/10.3390/jcdd12090363