Circulating Biomarkers in Failing Fontan Circulation: Current Evidence and Future Directions
Abstract
1. Introduction
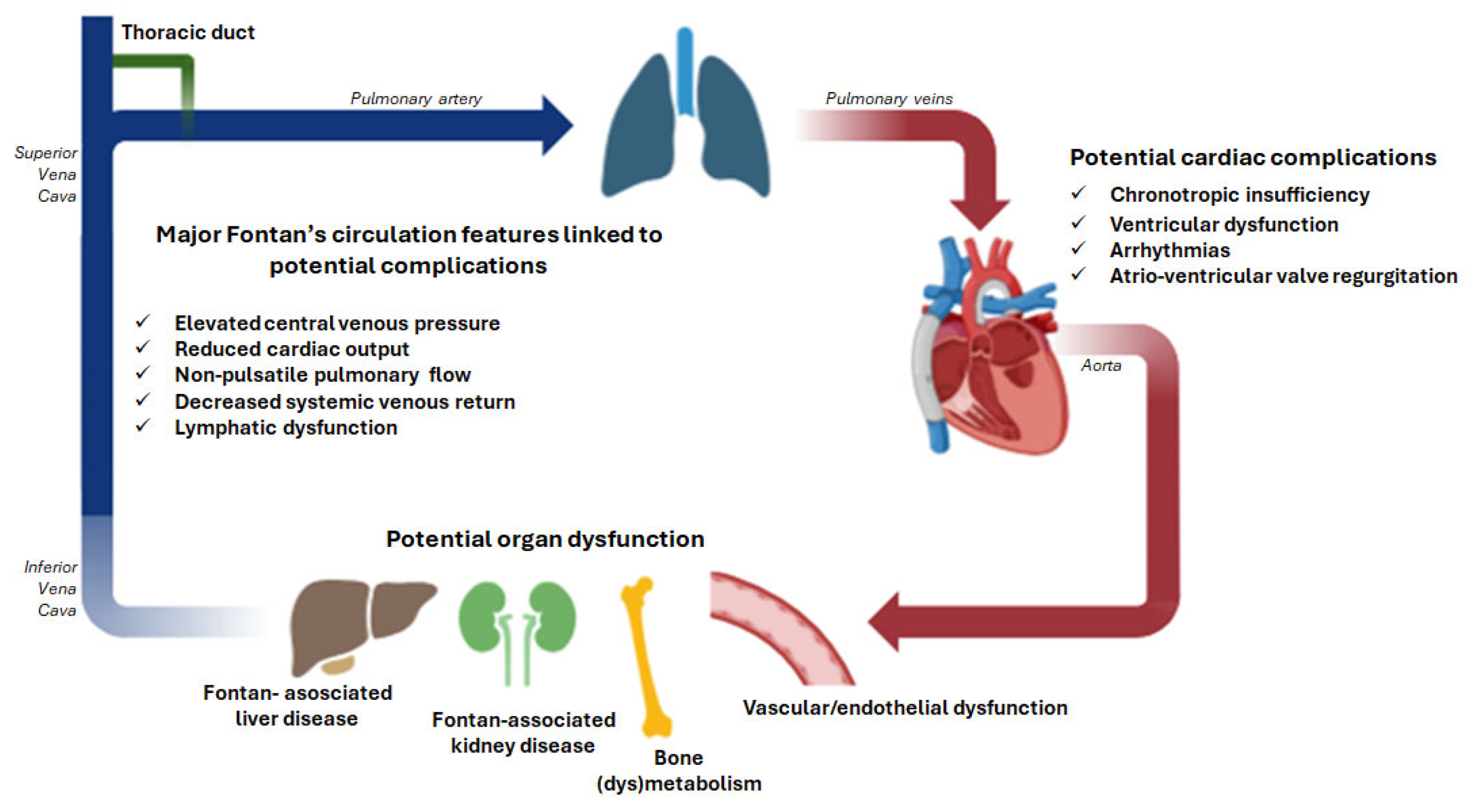
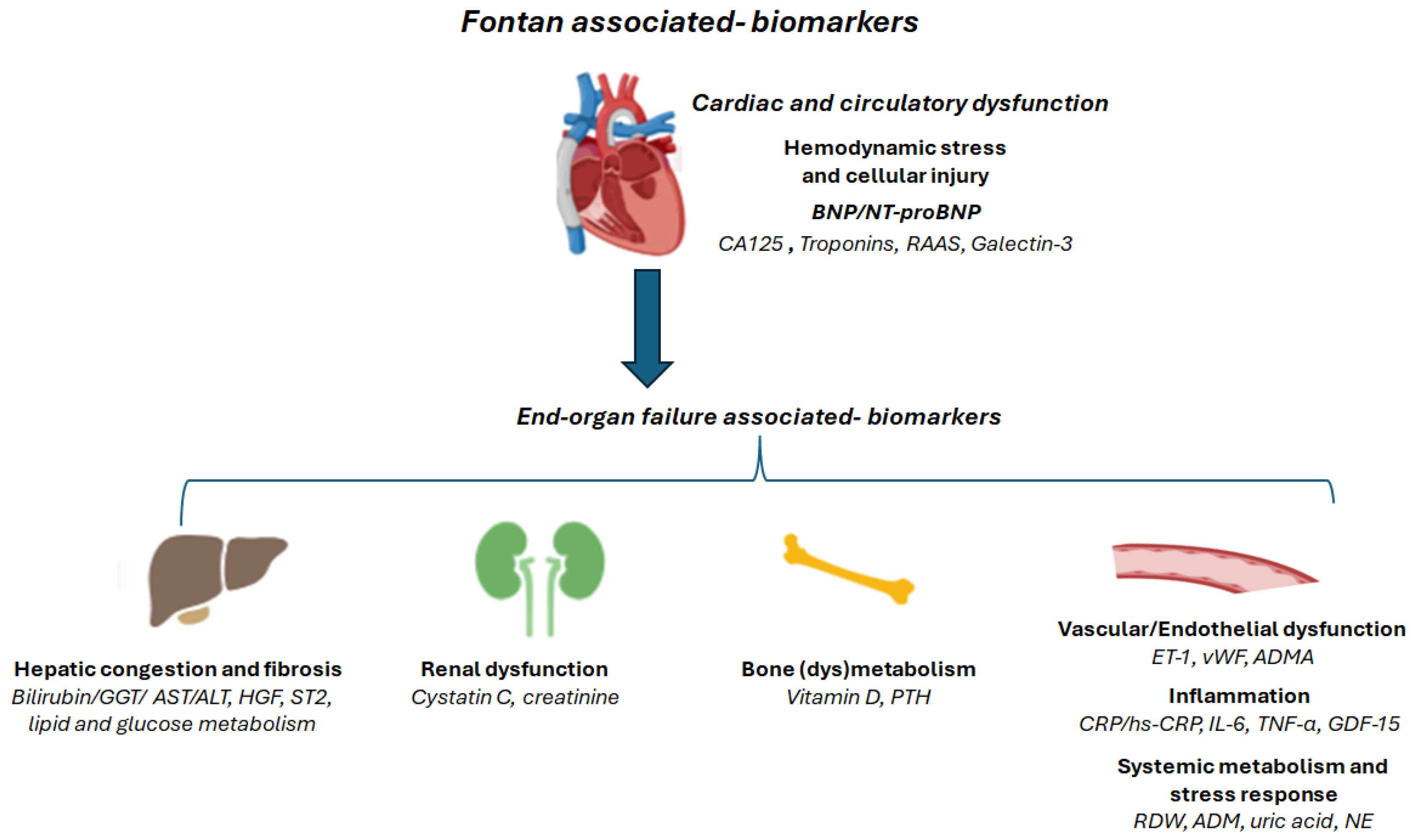
2. Pathophysiological Pathways Reflected by Circulating Biomarkers
2.1. Hemodynamic Stress and Cellular Injury
2.1.1. BNP and NT-proBNP
2.1.2. Troponins
2.1.3. The Renin-Angiotensin-Aldosterone System (RAAS)
2.1.4. Galectin-3
2.2. Inflammation
2.2.1. C-Reactive Protein
2.2.2. Pro-Inflammatory Cytokines (IL-6, TNF-α)
2.3. Endothelial Dysfunction
2.3.1. Endothelin-1
2.3.2. Von Willebrand Factor (vWF)
2.3.3. Asymmetric Dimethylarginine (ADMA)
2.4. End-Organ Involvement: Hepatic Congestion and Fibrosis
2.4.1. Bilirubin, GGT, AST and ALT
2.4.2. Hepatocyte Growth Factor
2.4.3. Suppression of Tumorigenicity 2
2.4.4. Cholesterol Metabolism
2.4.5. Glucose Metabolism
2.5. End-Organ Involvement: Renal Dysfunction
Cystatin C and Creatinine
2.6. End-Organ Involvement: Bone (dys)Metabolism
2.6.1. Vitamin D
2.6.2. Parathyroid Hormone
2.7. Systemic Metabolism and Stress Response
2.7.1. Red Blood Cell Distribution Width
2.7.2. Adrenomedullin
2.7.3. Uric Acid
2.7.4. Norepinephrine
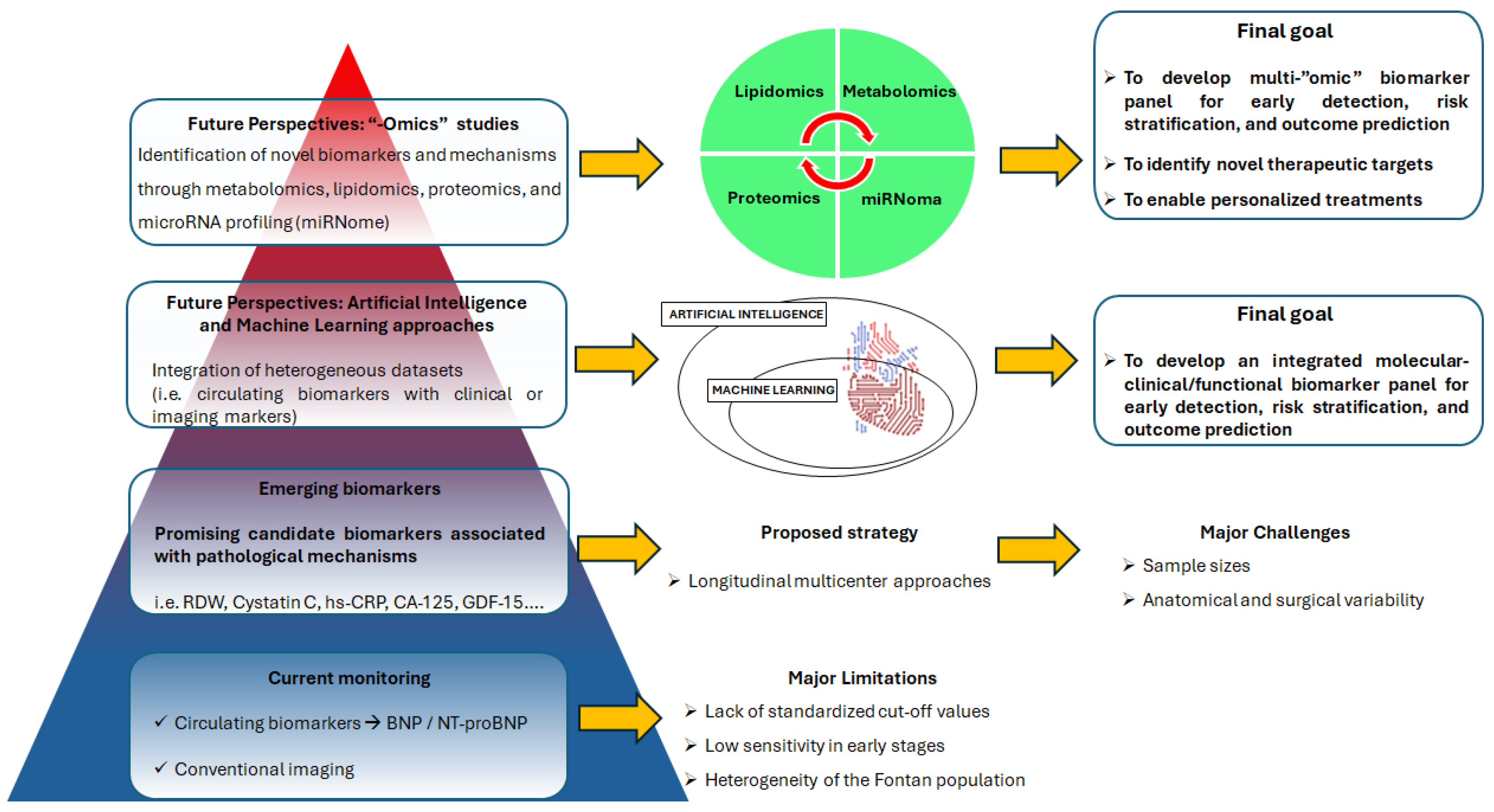
3. Methodological Challenges of Biomarker Research in the Fontan Population
4. Future Perspectives
4.1. From Single Biomarkers to an Integrated Biomarker Panel
4.2. The Emerging “-omics” Technologies
5. Conclusions
Funding
Conflicts of Interest
References
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef] [PubMed]
- Wittczak, A.; Dryżek, P.; Maciejewski, M.; Kula-Mazurek, A.; Moszura, T.; Bikiewicz, A.; Bielecka-Dabrowa, A. Successful complex percutaneous intervention in patient with Fontan circulation and severe heart failure: A case report. Clin. Case Rep. 2023, 11, e7222. [Google Scholar] [CrossRef]
- Rychik, J.; Atz, A.M.; Celermajer, D.S.; Deal, B.J.; Gatzoulis, M.A.; Gewillig, M.H.; Hsia, T.Y.; Hsu, D.T.; Kovacs, A.H.; McCrindle, B.W.; et al. Evaluation and Management of the Child and Adult with Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e234–e284. [Google Scholar] [CrossRef] [PubMed]
- Fontan, F.; Baudet, E. Surgical repair of tricuspid atresia. Thorax 1971, 26, 240–248. [Google Scholar] [CrossRef]
- Wittczak, A.; Mazurek-Kula, A.; Banach, M.; Piotrowski, G.; Bielecka-Dabrowa, A. Blood Biomarkers as a Non-Invasive Method for the Assessment of the State of the Fontan Circulation. J. Clin. Med. 2025, 14, 496. [Google Scholar] [CrossRef]
- Kverneland, L.S.; Kramer, P.; Ovroutski, S. Five decades of the Fontan operation: A systematic review of international reports on outcomes after univentricular palliation. Congenit. Heart Dis. 2018, 13, 181–193. [Google Scholar] [CrossRef]
- Plappert, L.; Edwards, S.; Senatore, A.; De Martini, A. The Epidemiology of Persons Living with Fontan in 2020 and Projections for 2030: Development of an Epidemiology Model Providing Multinational Estimates. Adv. Ther. 2022, 39, 1004–1015. [Google Scholar] [CrossRef]
- Kumar, T.K.S. The failing Fontan. Indian. J. Thorac. Cardiovasc. Surg. 2021, 37, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Goldberg, D.J. Failure of the Fontan Circulation. Heart Fail. Clin. 2014, 10, 105–116. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Carlomagno, N.; Incollingo, P.; Tammaro, V.; Peluso, G.; Rupealta, N.; Chiacchio, G.; Sandoval Sotelo, M.L.; Minieri, G.; Pisani, A.; Riccio, E.; et al. Diagnostic, Predictive, Prognostic, and Therapeutic Molecular Biomarkers in Third Millennium: A Breakthrough in Gastric Cancer. BioMed Res. Int. 2017, 2017, 7869802. [Google Scholar] [CrossRef]
- Morrow, D.A.; de Lemos, J.A. Benchmarks for the Assessment of Novel Cardiovascular Biomarkers. Circulation 2007, 115, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Alshawabkeh, L.; Opotowsky, A.R. Current Role of Blood and Urine Biomarkers in the Clinical Care of Adults with Congenital Heart Disease. Curr. Cardiol. Rep. 2017, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Zhu, B. BNP and NT-proBNP as Diagnostic Biomarkers for Cardiac Dysfunction in Both Clinical and Forensic Medicine. Int. J. Mol. Sci. 2019, 20, 1820. [Google Scholar] [CrossRef]
- Ohuchi, H.; Takasugi, H.; Ohashi, H.; Yamada, O.; Watanabe, K.; Yagihara, T.; Echigo, S. Abnormalities of Neurohormonal and Cardiac Autonomic Nervous Activities Relate Poorly to Functional Status in Fontan Patients. Circulation 2004, 110, 2601–2608. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Hayama, Y.; Sasaki, O.; Taniguchi, Y.; Noritake, K.; Miyazaki, A.; Yamada, O. Hyperuricemia reflects global Fontan pathophysiology and associates with morbidity and mortality in patients after the Fontan operation. Int. J. Cardiol. 2015, 184, 623–630. [Google Scholar] [CrossRef]
- Miyamoto, K.; Takeuchi, D.; Inai, K.; Shinohara, T.; Nakanishi, T. Prognostic value of multiple biomarkers for cardiovascular mortality in adult congenital heart disease: Comparisons of single-/two-ventricle physiology, and systemic morphologically right/left ventricles. Heart Vessel. 2016, 31, 1834–1847. [Google Scholar] [CrossRef]
- Burchill, L.J.; Redington, A.N.; Silversides, C.K.; Ross, H.J.; Jimenez-Juan, L.; Mital, S.; Oechslin, E.N.; Dragulescu, A.; Slorach, C.; Mertens, L.; et al. Renin–angiotensin–aldosterone system genotype and serum BNP in a contemporary cohort of adults late after Fontan palliation. Int. J. Cardiol. 2015, 197, 209–215. [Google Scholar] [CrossRef]
- Law, Y.M.; Ettedgui, J.; Beerman, L.; Maisel, A.; Tofovic, S. Comparison of Plasma B-Type Natriuretic Peptide Levels in Single Ventricle Patients with Systemic Ventricle Heart Failure Versus Isolated Cavopulmonary Failure. Am. J. Cardiol. 2006, 98, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Man, B.; Cheung, Y. Plasma brain natriuretic peptide and systemic ventricular function in asymptomatic patients late after the Fontan procedure. Heart Vessel. 2007, 22, 398–403. [Google Scholar] [CrossRef]
- Koch, A.M.E.; Zink, S.; Singer, H.; Dittrich, S. B-type natriuretic peptide levels in patients with functionally univentricular hearts after total cavopulmonary connection. Eur. J. Heart Fail. 2008, 10, 60–62. [Google Scholar] [CrossRef]
- Atz, A.M.; Zak, V.; Breitbart, R.E.; Colan, S.D.; Pasquali, S.K.; Hsu, D.T.; Lu, M.; Mahony, L.; Paridon, S.M.; Puchalski, M.D.; et al. Factors Associated with Serum Brain Natriuretic Peptide Levels after the Fontan Procedure. Congenit. Heart Dis. 2011, 6, 313–321. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Li, S.; Dolgner, S.J.; Steinberg, Z.L.; Buber, J. Utility of biomarkers in adult Fontan patients with decompensated heart failure. Cardiol. Young 2020, 30, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lechner, E.; Gitter, R.; Mair, R.; Pinter, M.; Schreier-Lechner, E.; Vondrys, D.; Tulzer, G. Aminoterminal Brain Natriuretic Peptide Levels in Children and Adolescents After Fontan Operation Correlate with Congestive Heart Failure. Pediatr. Cardiol. 2008, 29, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, S.J.; Opotowsky, A.R.; Harrild, D.M.; Powell, A.J.; Azcue, N.; Ahmad, S.; Clair, N.S.; Bradwin, G.; Rathod, R.H. Characterization of Circulating and Urinary Biomarkers in the Fontan Circulation and Their Correlation With Cardiac Imaging. Am. J. Cardiol. 2022, 162, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cindik, N.; Gökdemir, M.; Varan, B.; Ulubay, G.; Ozkan, M.; Tokel, N.K. Comparison of serum N-terminal pro-brain natriuretic peptide levels, conventional echocardiography, exercise parameters, and dyssynchrony measurements in Fontan patients. Cardiol. Young 2023, 33, 1706–1712. [Google Scholar] [CrossRef]
- Palm, J.; Ono, M.; Niedermaier, C.; Hörer, J.; Hoffmann, G.; Holdenrieder, S.; Klawonn, F.; Ewert, P. Quantification of Ventricular Stress in Univentricular Hearts during Early Childhood Using Age-Independent Zlog-NT-ProBNP. Int. J. Cardiol. 2024, 406, 131983. [Google Scholar] [CrossRef]
- Kolcz, J.; Tomkiewicz-Pajak, L.; Wojcik, E.; Podolec, P.; Skalski, J. Prognostic Significance and Correlations of Neurohumoral Factors in Early and Late Postoperative Period after Fontan Procedure. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 40–45. [Google Scholar] [CrossRef]
- Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Schut, A.-R.W.; Cuypers, J.A.A.E.; Witsenburg, M.; de Waart, M.; van Schaik, R.H.N.; Zijlstra, F.; Boersma, E.; et al. Prognostic Value of N-Terminal Pro-B-Type Natriuretic Peptide, Troponin-T, and Growth-Differentiation Factor 15 in Adult Congenital Heart Disease. Circulation 2017, 135, 264–279. [Google Scholar] [CrossRef]
- van den Bosch, E.; Bossers, S.S.M.; Kamphuis, V.P.; Boersma, E.; Roos-Hesselink, J.W.; Breur, J.M.P.J.; Ten Harkel, A.D.J.; Kapusta, L.; Bartelds, B.; Roest, A.A.W.; et al. Associations Between Blood Biomarkers, Cardiac Function, and Adverse Outcome in a Young Fontan Cohort. J. Am. Heart Assoc. 2021, 10, e015022. [Google Scholar] [CrossRef]
- Perrone, M.A.; Pomiato, E.; Palmieri, R.; Di Già, G.; Piemonte, F.; Porzio, O.; Gagliardi, M.G. The Effects of Exercise Training on Cardiopulmonary Exercise Testing and Cardiac Biomarkers in Adult Patients with Hypoplastic Left Heart Syndrome and Fontan Circulation. J. Cardiovasc. Dev. Dis. 2022, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Buendía Fuentes, F.; Jover Pastor, P.; Arnau Vives, M.Á.; Lozano Edo, S.; Rodríguez Serrano, M.; Aguero, J.; Osa Sáez, A.; Conde Amiel, I.; Aguilera Sancho-Tello, V.; Martínez-Dolz, L.; et al. CA125: A new biomarker in patients with Fontan circulation. Rev. Española Cardiol. (Engl. Ed.) 2023, 76, 112–120. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Miñana, G.; Santas, E.; Llácer, P.; Núñez, E.; Palau, P.; Bodí, V.; Chorro, F.J.; Sanchis, J.; et al. Antigen carbohydrate 125 as a biomarker in heart failure: A narrative review. Eur. J. Heart Fail. 2021, 23, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Lazar, D.R.; Lazar, F.L.; Homorodean, C.; Cainap, C.; Focsan, M.; Cainap, S.; Olinic, D.M. High-Sensitivity Troponin: A Review on Characteristics, Assessment, and Clinical Implications. Dis. Markers 2022, 2022, 9713326. [Google Scholar] [CrossRef]
- Raber, I.; McCarthy, C.P.; Januzzi, J.L. A Test in Context: Interpretation of High-Sensitivity Cardiac Troponin Assays in Different Clinical Settings. J. Am. Coll. Cardiol. 2021, 77, 1357–1367. [Google Scholar] [CrossRef]
- Willinger, L.; Brudy, L.; Meyer, M.; Oberhoffer-Fritz, R.; Ewert, P.; Müller, J. Prognostic value of non-acute high sensitive troponin-T for cardiovascular morbidity and mortality in adults with congenital heart disease: A systematic review. J. Cardiol. 2021, 78, 206–212. [Google Scholar] [CrossRef]
- Schmieder, R.E.; Hilgers, K.F.; Schlaich, M.P.; Schmidt, B.M. Renin-angiotensin system and cardiovascular risk. Lancet 2007, 369, 1208–1219. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Ono, S.; Miyake, A.; Toyota, N.; Tamaki, W.; Miyazaki, A.; Yamada, O. Hyponatremia and Its Association with the Neurohormonal Activity and Adverse Clinical Events in Children and Young Adult Patients after the Fontan Operation. Congenit. Heart Dis. 2011, 6, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Inai, K.; Nakanishi, T.; Nakazawa, M. Clinical correlation and prognostic predictive value of neurohumoral factors in patients late after the Fontan operation. Am. Heart J. 2005, 150, 588–594. [Google Scholar] [CrossRef]
- Sugimoto, M.; Saiki, H.; Tamai, A.; Seki, M.; Inuzuka, R.; Masutani, S.; Senzaki, H. Ventricular fibrogenesis activity assessed by serum levels of procollagen type III N-terminal amino peptide during the staged Fontan procedure. J. Thorac. Cardiovasc. Surg. 2016, 151, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Kanayama, T.; Noguchi, K.; Niwa, A.; Matsuo, M.; Kuroda, T.; Hatano, Y.; Okada, H.; Tomita, H. Galectin-3: A Potential Prognostic and Diagnostic Marker for Heart Disease and Detection of Early Stage Pathology. Biomolecules 2020, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Baraona, F.; Owumi, J.; Loukas, B.; Singh, M.N.; Valente, A.M.; Wu, F.; Cheng, S.; Veldtman, G.; Rimm, E.B.; et al. Galectin-3 Is Elevated and Associated with Adverse Outcomes in Patients With Single-Ventricle Fontan Circulation. J. Am. Heart Assoc. 2016, 5, e002706. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Wienecke, L.M.; Cohen, S.; Bauersachs, J.; Mebazaa, A.; Chousterman, B.G. Immunity and inflammation: The neglected key players in congenital heart disease? Heart Fail. Rev. 2022, 27, 1957–1971. [Google Scholar] [CrossRef]
- Stosio, M.; Ruszkowski, J.; Mikosik-Roczyńska, A.; Haponiuk, I.; Witkowski, J.M. The significance of neonatal thymectomy for shaping the immune system in children with congenital heart defects. Pol. J. Cardio-Thorac. Surg. 2017, 4, 258–262. [Google Scholar] [CrossRef]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of High-Sensitivity C-reactive Protein (Hs-CRP) in Non-communicable Diseases: A Review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Valente, A.M.; Alshawabkeh, L.; Cheng, S.; Bradley, A.; Rimm, E.B.; Landzberg, M.J. Prospective cohort study of C-reactive protein as a predictor of clinical events in adults with congenital heart disease: Results of the Boston adult congenital heart disease biobank. Eur. Heart J. 2018, 39, 3253–3261. [Google Scholar] [CrossRef]
- Ohuchi, H.; Asano, R.; Mori, A.; Ishibashi, T.; Motooka, D.; Nakai, M.; Nakaoka, Y. Gut Dysbiosis in Patients with Fontan Circulation. J. Am. Heart Assoc. 2024, 13, e034538. [Google Scholar] [CrossRef]
- Mainwaring, R.D.; Lamberti, J.J.; Hugli, T.E. Complement Activation and Cytokine Generation After Modified Fontan Procedure. Ann. Thorac. Surg. 1998, 65, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A.; Beshish, A.G.; Bush, L.B.; Lowery, R.E.; Wong, J.H.; Schumacher, K.R.; Halligan, N.L.N.; Cornell, T.T.; Rocchini, A.P. Analysis of Inflammatory Cytokines in Postoperative Fontan Pleural Drainage. Pediatr. Cardiol. 2019, 40, 744–752. [Google Scholar] [CrossRef]
- Saraf, A.; De Staercke, C.; Everitt, I.; Haouzi, A.; Ko, Y.A.; Jennings, S.; Kim, J.H.; Rodriguez, F.H.; Kalogeropoulos, A.P.; Quyyumi, A.; et al. Biomarker profile in stable Fontan patients. Int. J. Cardiol. 2020, 305, 56–62. [Google Scholar] [CrossRef]
- Ostrow, A.M.; Freeze, H.; Rychik, J. Protein-Losing Enteropathy After Fontan Operation: Investigations Into Possible Pathophysiologic Mechanisms. Ann. Thorac. Surg. 2006, 82, 695–700. [Google Scholar] [CrossRef]
- Meyer, S.L.; Wolff, D.; Ridderbos, F.S.; Eshuis, G.; Hillege, H.; Willems, T.P.; Ebels, T.; van Melle, J.P.; Berger, R.M.F. GDF-15 (Growth Differentiation Factor 15) Is Associated with Hospitalization and Mortality in Patients with a Fontan Circulation. J. Am. Heart Assoc. 2020, 9, e015521. [Google Scholar] [CrossRef]
- Lambert, E.; d’Udekem, Y.; Cheung, M.; Sari, C.I.; Inman, J.; Ahimastos, A.; Eikelis, N.; Pathak, A.; King, I.; Grigg, L.; et al. Sympathetic and vascular dysfunction in adult patients with Fontan circulation. Int. J. Cardiol. 2013, 167, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.H.; Sandelin, A.M.; Golbus, J.R.; Warnke, N.; Gooding, L.; King, K.K.; Donohue, J.E.; Yu, S.; Gurney, J.G.; Goldberg, C.S.; et al. Impact of Vitamin C on Endothelial Function and Exercise Capacity in Patients with a Fontan Circulation. Congenit. Heart Dis. 2012, 7, 226–234. [Google Scholar] [CrossRef]
- Hauser, J.A.; Muthurangu, V.; Pandya, B.; Michel-Behnke, I.; Taylor, A.M.; Demyanets, S. Postprandial variability of novel heart failure biomarkers in Fontan patients compared to healthy volunteers. Int. J. Cardiol. Congenit. Heart Dis. 2021, 3, 100127. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz-Pajak, L.; Wojcik, T.; Chłopicki, S.; Olszowska, M.; Pajak, J.; Podolec, J.; Sitek, B.; Musiałek, P.; Rubis, P.; Komar, M.; et al. Aspirin resistance in adult patients after Fontan surgery. Int. J. Cardiol. 2015, 181, 19–26. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Imai, Y.; Takanashi, Y.; Seo, K.; Terada, M.; Aoki, M.; Nakazawa, M. Time course of endothelin-1 and adrenomedullin after the Fontan procedure. Ann. Thorac. Surg. 1999, 68, 169–172. [Google Scholar] [CrossRef] [PubMed]
- d’Udekem, Y.; Cheung, M.M.; Setyapranata, S.; Iyengar, A.J.; Kelly, P.; Buckland, N.; Grigg, L.E.; Weintraub, R.G.; Vance, A.; Brizard, C.P.; et al. How Good Is a Good Fontan? Quality of Life and Exercise Capacity of Fontans Without Arrhythmias. Ann. Thorac. Surg. 2009, 88, 1961–1969. [Google Scholar] [CrossRef]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood 2015, 125, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Tomkiewicz-Pajak, L.; Plazak, W.; Kolcz, J.; Pajak, J.; Kopec, G.; Dluzniewska, N.; Olszowska, M.; Moryl-Bujakowska, A.; Podolec, P. Iron deficiency and hematological changes in adult patients after Fontan operation. J. Cardiol. 2014, 64, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Hayama, Y.; Miike, H.; Suzuki, D.; Nakajima, K.; Iwasa, T.; Konagai, N.; Sakaguchi, H.; Miyazaki, A.; Shiraishi, I.; et al. Prognostic value of von Willebrand factor in adult patients with congenital heart disease. Heart 2020, 106, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Agarwal, S.C.; Home, P.D.; Boger, R.H. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr. Cardiol. Rev. 2010, 6, 82–90. [Google Scholar] [CrossRef]
- Tutarel, O.; Denecke, A.; Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Lovric, S.; Bauersachs, J.; Schieffer, B.; Westhoff-Bleck, M.; Kielstein, J.T. Asymmetrical Dimethylarginine—More Sensitive than NT-proBNP to Diagnose Heart Failure in Adults with Congenital Heart Disease. PLoS ONE 2012, 7, e33795. [Google Scholar] [CrossRef]
- Michel, M.; Dubowy, K.-O.; Entenmann, A.; Karall, D.; Adam, M.G.; Zlamy, M.; Odri Komazec, I.; Geiger, R.; Niederwanger, C.; Salvador, C.; et al. Targeted Metabolomic Analysis of Serum Amino Acids in the Adult Fontan Patient with a Dominant Left Ventricle. Sci. Rep. 2020, 10, 8930. [Google Scholar] [CrossRef]
- Emamaullee, J.; Khan, S.; Weaver, C.; Goldbeck, C.; Yanni, G.; Kohli, R.; Genyk, Y.; Zhou, S.; Shillingford, N.; Sullivan, P.M.; et al. Non-Invasive Biomarkers of Fontan-Associated Liver Disease. JHEP Rep. 2021, 3, 100362. [Google Scholar] [CrossRef]
- Goldberg, D.J.; Surrey, L.F.; Glatz, A.C.; Dodds, K.; O’Byrne, M.L.; Lin, H.C.; Fogel, M.; Rome, J.J.; Rand, E.B.; Russo, P.; et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity Is Associated with Time from Surgery: A Liver Biopsy and Hemodynamic Study. J. Am. Heart Assoc. 2017, 6, e004809. [Google Scholar] [CrossRef]
- Surrey, L.F.; Russo, P.; Rychik, J.; Goldberg, D.J.; Dodds, K.; O’Byrne, M.L.; Glatz, A.C.; Rand, E.B.; Lin, H.C. Prevalence and Characterization of Fibrosis in Surveillance Liver Biopsies of Patients with Fontan Circulation. Hum. Pathol. 2016, 57, 106–115. [Google Scholar] [CrossRef]
- Rychik, J.; Veldtman, G.; Rand, E.; Russo, P.; Rome, J.J.; Krok, K.; Goldberg, D.J.; Cahill, A.M.; Wells, R.G. The Precarious State of the Liver After a Fontan Operation: Summary of a Multidisciplinary Symposium. Pediatr. Cardiol. 2012, 33, 1001–1012. [Google Scholar] [CrossRef]
- Emamaullee, J.; Zaidi, A.N.; Schiano, T.; Kahn, J.; Valentino, P.L.; Hofer, R.E.; Taner, T.; Wald, J.W.; Olthoff, K.M.; Bucuvalas, J.; et al. Fontan-Associated Liver Disease. Circulation 2020, 142, 591–604. [Google Scholar] [CrossRef]
- De Bruyne, R.; Vandekerckhove, K.; Van Overschelde, H.; Hendricx, F.; Vande Walle, C.; De Groote, K.; Panzer, J.; De Wolf, D.; Van Biervliet, S.; Bové, T.; et al. Non-Invasive Assessment of Liver Abnormalities in Pediatric Fontan Patients. Eur. J. Pediatr. 2022, 181, 159–169. [Google Scholar] [CrossRef]
- Sethasathien, S.; Silvilairat, S.; Sittiwangkul, R.; Makonkawkeyoon, K.; Pongprot, Y. Associated Factors of Liver Disease After Fontan Operation in Relation to Ultrasound Liver Elastography. Pediatr. Cardiol. 2020, 41, 1639–1644. [Google Scholar] [CrossRef]
- Kogiso, T.; Ogasawara, Y.; Taniai, M.; Shimada, E.; Inai, K.; Tokushige, K.; Nakai, Y. Importance of Gamma-glutamyl Transferase Elevation in Patients with Fontan-associated Liver Disease. Hepatol. Res. 2024, 54, 1205–1214. [Google Scholar] [CrossRef]
- Ferraro, R.A.; Ogunmoroti, O.; Zhao, D.; Ndumele, C.E.; Rao, V.; Pandey, A.; Larson, N.B.; Bielinski, S.J.; Michos, E.D. Hepatocyte Growth Factor and Incident Heart Failure Subtypes: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Card. Fail. 2021, 27, 981–990. [Google Scholar] [CrossRef]
- Kim, G.B.; Kwon, B.S.; Bae, E.J.; Noh, C., II; Choi, J.Y. Significance of Circulating Hepatocyte Growth Factor in Protein-Losing Enteropathy After Fontan Operation. Pediatr. Cardiol. 2011, 32, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Taki, M.; Toda, K.; Muraji, S.; Yoshiba, S.; Kobayshi, T.; Sumitomo, N. Hepatocyte Growth Factor Predicts Failure of Fontan Circulation. ESC Heart Fail. 2020, 7, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Cortese, F.; Gesualdo, M.; Riccardi, R.; Di Nunzio, D.; Moncelli, M.; Iacoviello, M.; Scicchitano, P. A Novel Cardiac Bio-Marker: ST2: A Review. Molecules 2013, 18, 15314–15328. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of SST2 in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Laqqan, M.; Schwaighofer, C.; Graeber, S.; Raedle-Hurst, T. Predictive Value of Soluble ST2 in Adolescent and Adult Patients with Complex Congenital Heart Disease. PLoS ONE 2018, 13, e0202406. [Google Scholar] [CrossRef] [PubMed]
- Geenen, L.W.; Baggen, V.J.M.; van den Bosch, A.E.; Eindhoven, J.A.; Cuypers, J.A.A.E.; Witsenburg, M.; Boersma, E.; Roos-Hesselink, J.W. Prognostic Value of Soluble ST2 in Adults with Congenital Heart Disease. Heart 2019, 105, 999–1006. [Google Scholar] [CrossRef]
- Whiteside, W.; Tan, M.; Yu, S.; Rocchini, A. Low Total, Low-Density Lipoprotein, High-Density Lipoprotein, and Non–High-Density Lipoprotein Cholesterol Levels in Patients with Complex Congenital Heart Disease after Fontan Palliation. J. Pediatr. 2013, 162, 1199–1204. [Google Scholar] [CrossRef]
- Whiteside, W.; Tan, M.; Ostlund, R.E.; Yu, S.; Ma, L.; Rocchini, A. Altered Cholesterol Metabolism and Hypocholesterolemia in Patients with Single Ventricle Following Fontan Palliation. J. Pediatr. 2016, 171, 73–77. [Google Scholar] [CrossRef]
- Lubert, A.M.; Alsaied, T.; Palermo, J.J.; Anwar, N.; Urbina, E.M.; Brown, N.M.; Alexander, C.; Almeneisi, H.; Wu, F.; Leventhal, A.R.; et al. Fontan-Associated Dyslipidemia. J. Am. Heart Assoc. 2021, 10, e019578. [Google Scholar] [CrossRef]
- Lu, C.-W.; Wu, C.-H.; Huang, M.-T.; Lee, C.-S.; Chen, H.-L.; Lin, M.-T.; Chiu, S.-N.; Tseng, W.-C.; Chen, C.-A.; Wang, J.-K.; et al. Liver Fibrosis Detected by Diffusion-Weighted Magnetic Resonance Imaging and Its Functional Correlates in Fontan Patients. Eur. J. Cardio-Thorac. Surg. 2024, 66, ezae249. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; Dubowy, K.-O.; Zlamy, M.; Karall, D.; Adam, M.G.; Entenmann, A.; Keller, M.A.; Koch, J.; Odri Komazec, I.; Geiger, R.; et al. Targeted Metabolomic Analysis of Serum Phospholipid and Acylcarnitine in the Adult Fontan Patient with a Dominant Left Ventricle. Ther. Adv. Chronic. Dis. 2020, 11, 2040622320916031. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Hayama, Y.; Miike, H.; Suzuki, D.; Nakajima, K.; Konagai, N.; Iwasa, T.; Sakaguchi, H.; Kurosaki, K.; et al. Abnormal Glucose Metabolism in Patients with Fontan Circulation: Unique Characteristics and Associations with Fontan Pathophysiology. Am. Heart J. 2019, 216, 125–135. [Google Scholar] [CrossRef]
- Ohuchi, H.; Miyamoto, Y.; Yamamoto, M.; Ishihara, H.; Takata, H.; Miyazaki, A.; Yamada, O.; Yagihara, T. High Prevalence of Abnormal Glucose Metabolism in Young Adult Patients with Complex Congenital Heart Disease. Am. Heart J. 2009, 158, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dodeja, A.K.; Toro-Salazar, O.; Fairchild, W.; Han, F. Cardiorenal Syndrome in Adults with Congenital Heart Disease. J. Clin. Med. 2025, 14, 4392. [Google Scholar] [CrossRef]
- Filler, G.; Huang, S.-H.S.; Yasin, A. The Usefulness of Cystatin C and Related Formulae in Pediatrics. Clin. Chem. Lab. Med. (CCLM) 2012, 50, 2081–2091. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Baraona, F.R.; Mc Causland, F.R.; Loukas, B.; Landzberg, E.; Landzberg, M.J.; Sabbisetti, V.; Waikar, S.S. Estimated Glomerular Filtration Rate and Urine Biomarkers in Patients with Single-Ventricle Fontan Circulation. Heart 2017, 103, 434–442. [Google Scholar] [CrossRef]
- Opotowsky, A.R.; Carazo, M.; Singh, M.N.; Dimopoulos, K.; Cardona-Estrada, D.A.; Elantably, A.; Waikar, S.S.; Mc Causland, F.R.; Veldtman, G.; Grewal, J.; et al. Creatinine versus Cystatin C to Estimate Glomerular Filtration Rate in Adults with Congenital Heart Disease: Results of the Boston Adult Congenital Heart Disease Biobank. Am. Heart J. 2019, 214, 142–155. [Google Scholar] [CrossRef]
- Katz, D.A.; Gao, Z.; Freytag, J.; Mahendran, A.; Szugye, C.; Woodly, S.; Alvarez, T.C.E.; Lubert, A.M.; Alsaied, T.; Goldstein, S.L.; et al. Associations Between Characteristics of Individuals with Fontan Circulation with Blood and Urine Biomarkers of Kidney Injury and Dysfunction. J. Am. Heart Assoc. 2023, 12, e029130. [Google Scholar] [CrossRef]
- D’Ambrosio, P.; Tran, D.; Verrall, C.E.; Attard, C.; Singh, M.F.; Ayer, J.; d’Udekem, Y.; Twigg, S.; Celermajer, D.S.; Cordina, R. Prevalence and Risk Factors for Low Bone Density in Adults with a Fontan Circulation. Congenit. Heart Dis. 2019, 14, 987–995. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Haider, F.; Ghafoor, H.; Hassan, O.F.; Farooqui, K.; Bel Khair, A.O.M.; Shoaib, F. Vitamin D and Cardiovascular Diseases: An Update. Cureus 2023, 15, e49734. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.M.; Leonard, M.B.; Zemel, B.S.; Brodsky, J.L.; Lee, D.; Dodds, K.; Hayden-Rush, C.; Whitehead, K.K.; Goldmuntz, E.; Paridon, S.M.; et al. Lean Mass Deficits, Vitamin D Status and Exercise Capacity in Children and Young Adults after Fontan Palliation. Heart 2014, 100, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.M.; Goldberg, D.J.; Zemel, B.S.; Brodsky, J.L.; Dodds, K.; Hayden-Rush, C.; Whitehead, K.K.; Goldmuntz, E.; Rychik, J.; Leonard, M.B. Deficits in Bone Density and Structure in Children and Young Adults Following Fontan Palliation. Bone 2015, 77, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Holler, F.; Hannes, T.; Germund, I.; Emmel, M.; Hoyer-Kuhn, H.; Khalil, M.; Sreeram, N.; Udink ten Cate, F.E.A. Low Serum 25-Hydroxyvitamin D Levels and Secondary Hyperparathyroidism in Fontan Patients. Cardiol. Young 2016, 26, 876–884. [Google Scholar] [CrossRef]
- Diab, S.G.; Godang, K.; Müller, L.O.; Almaas, R.; Lange, C.; Brunvand, L.; Hansen, K.M.; Myhre, A.G.; Døhlen, G.; Thaulow, E.; et al. Progressive Loss of Bone Mass in Children with Fontan Circulation. Congenit. Heart Dis. 2019, 14, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, S.J.; Dodds, K.M.; Burstein, D.S.; Huang, J.; Rand, E.B.; Mancilla, E.; Heimall, J.R.; McBride, M.G.; Paridon, S.M.; Goldberg, D.J.; et al. End-Organ Function and Exercise Performance in Patients with Fontan Circulation: What Characterizes the High Performers? J. Am. Heart Assoc. 2020, 9, e016850. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, W.; Ma, J.; Lin, B. Parathyroid Hormone and Risk of Heart Failure in the General Population. Medicine 2016, 95, e4810. [Google Scholar] [CrossRef]
- Sharma, S.; Ruebner, R.L.; Furth, S.L.; Dodds, K.M.; Rychik, J.; Goldberg, D.J. Assessment of Kidney Function in Survivors Following Fontan Palliation. Congenit. Heart Dis. 2016, 11, 630–636. [Google Scholar] [CrossRef]
- Dopsaj, V.; Mikovic-Golubovic, G.; Martinovic, J.; Kalimanovska-Ostric, D. Evaluation of Derived Coulter Red Blood Cell Parameters for the Assessment of Iron Deficiency in Adults with Congenital Heart Disease. Int. J. Lab. Hematol. 2012, 34, 461–472. [Google Scholar] [CrossRef] [PubMed]
- van Kimmenade, R.R.J.; Mohammed, A.A.; Uthamalingam, S.; van der Meer, P.; Felker, G.M.; Januzzi, J.L. Red Blood Cell Distribution Width and 1-year Mortality in Acute Heart Failure. Eur. J. Heart Fail. 2010, 12, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Yasuhara, J.; Kumamoto, T.; Shimizu, H.; Yoshiba, S.; Kobayashi, T.; Sumitomo, N. Usefulness of the Red Blood Cell Distribution Width to Predict Heart Failure in Patients with a Fontan Circulation. Am. J. Cardiol. 2015, 116, 965–968. [Google Scholar] [CrossRef]
- Voors, A.A.; Kremer, D.; Geven, C.; ter Maaten, J.M.; Struck, J.; Bergmann, A.; Pickkers, P.; Metra, M.; Mebazaa, A.; Düngen, H.; et al. Adrenomedullin in Heart Failure: Pathophysiology and Therapeutic Application. Eur. J. Heart Fail. 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Watanabe, K.; Nishikimi, T.; Takamuro, M.; Yasuda, K.; Ishikawa, Y.; Tanabe, S.; Yamada, O.; Yagihara, T.; Suga, S.; Kangawa, K.; et al. Possible Role of Adrenomedullin in the Regulation of Fontan Circulation: Mature Form of Plasma Adrenomedullin Is Extracted in the Lung in Patients with Fontan Procedure. Regul. Pept. 2007, 141, 129–134. [Google Scholar] [CrossRef]
- Kaiser, R.; Abdul-Khaliq, H.; Wilkens, H.; Herrmann, E.; Raedle-Hurst, T.M. Mid-regional Pro-adrenomedullin: An Indicator of the Failing Fontan Circuit in Patients with Univentricular Hearts? Eur. J. Heart Fail. 2014, 16, 1082–1088. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric Acid and Cardiovascular Disease: A Clinical Review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kumrić, M.; Borovac, J.A.; Kurir, T.T.; Božić, J. Clinical Implications of Uric Acid in Heart Failure: A Comprehensive Review. Life 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Liaudet, L.; Calderari, B.; Pacher, P. Pathophysiological Mechanisms of Catecholamine and Cocaine-Mediated Cardiotoxicity. Heart Fail. Rev. 2014, 19, 815–824. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Ohuchi, H.; Negishi, J.; Miyake, A.; Sakaguchi, H.; Miyazaki, A.; Yamada, O. Long-Term Prognostic Value of Cardiac Autonomic Nervous Activity in Postoperative Patients with Congenital Heart Disease. Int. J. Cardiol. 2011, 151, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Tamarappoo, B.K.; Lin, A.; Commandeur, F.; McElhinney, P.A.; Cadet, S.; Goeller, M.; Razipour, A.; Chen, X.; Gransar, H.; Cantu, S.; et al. Machine Learning Integration of Circulating and Imaging Biomarkers for Explainable Patient-Specific Prediction of Cardiac Events: A Prospective Study. Atherosclerosis 2021, 318, 76–82. [Google Scholar] [CrossRef]
- Truong, V.T.; Moore, R.A.; Lubert, A.M.; Taylor, M.D.; Mazur, W.; Alsaied, T.; Goldstein, B.H. Association of Plasma Biomarkers and Interstitial Myocardial Fibrosis in Fontan Population: A Machine Learning Approach. Int. J. Cardiol. Congenit. Heart Dis. 2022, 7, 100321. [Google Scholar] [CrossRef]
- Böger, R.H.; Bode-Böger, S.M.; Szuba, A.; Tsao, P.S.; Chan, J.R.; Tangphao, O.; Blaschke, T.F.; Cooke, J.P. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction. Circulation 1998, 98, 1842–1847. [Google Scholar] [CrossRef]
- Michel, M.; Renaud, D.; Schmidt, R.; Einkemmer, M.; Laser, L.V.; Michel, E.; Dubowy, K.O.; Karall, D.; Laser, K.T.; Scholl-Bürgi, S. Altered Serum Proteins Suggest Inflammation, Fibrogenesis and Angiogenesis in Adult Patients with a Fontan Circulation. Int. J. Mol. Sci. 2024, 25, 5416. [Google Scholar] [CrossRef]
- O’Connell, T.M.; Logsdon, D.L.; Mitscher, G.; Payne, R.M. Metabolic Profiles Identify Circulating Biomarkers Associated with Heart Failure in Young Single Ventricle Patients. Metabolomics 2021, 17, 95. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Meese, E.; Saleh, M.A.; Keller, A.; Abdul-Khaliq, H.; Raedle-Hurst, T. MicroRNA-29b/c-3p Indicate Advanced Liver Fibrosis/Cirrhosis in Univentricular Heart Patients with and Without Fontan Palliation. Front. Cardiovasc. Med. 2021, 7, 619083. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, J.; Toka, O.; Lukassen, S.; Ekici, A.B.; Mackensen, A.; Völkl, S.; Dittrich, S. Lymphocyte Immune Response and T Cell Differentiation in Fontan Patients with Protein-Losing Enteropathy. Thorac. Cardiovasc. Surg. 2021, 69, e10–e20. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, J.; Yamakuchi, M.; Ueno, K.; Hashiguchi, T.; Okamoto, Y. MiR-25-3p Regulates Pulmonary Arteriovenous Malformation after Glenn Procedure in Patients with Univentricular Heart via the PHLPP2-HIF-1α Axis. Sci. Rep. 2025, 15, 4138. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecoli, C.; Ait-Alì, L.; Storti, S.; Foffa, I. Circulating Biomarkers in Failing Fontan Circulation: Current Evidence and Future Directions. J. Cardiovasc. Dev. Dis. 2025, 12, 358. https://doi.org/10.3390/jcdd12090358
Vecoli C, Ait-Alì L, Storti S, Foffa I. Circulating Biomarkers in Failing Fontan Circulation: Current Evidence and Future Directions. Journal of Cardiovascular Development and Disease. 2025; 12(9):358. https://doi.org/10.3390/jcdd12090358
Chicago/Turabian StyleVecoli, Cecilia, Lamia Ait-Alì, Simona Storti, and Ilenia Foffa. 2025. "Circulating Biomarkers in Failing Fontan Circulation: Current Evidence and Future Directions" Journal of Cardiovascular Development and Disease 12, no. 9: 358. https://doi.org/10.3390/jcdd12090358
APA StyleVecoli, C., Ait-Alì, L., Storti, S., & Foffa, I. (2025). Circulating Biomarkers in Failing Fontan Circulation: Current Evidence and Future Directions. Journal of Cardiovascular Development and Disease, 12(9), 358. https://doi.org/10.3390/jcdd12090358





