Challenging the Paradigm: Long-Term Outcomes in Dialysis-Dependent Patients Undergoing CABG
Abstract
1. Introduction
2. Methods
2.1. Patients and Data Collection
2.2. Long-Term Survival
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgical Details
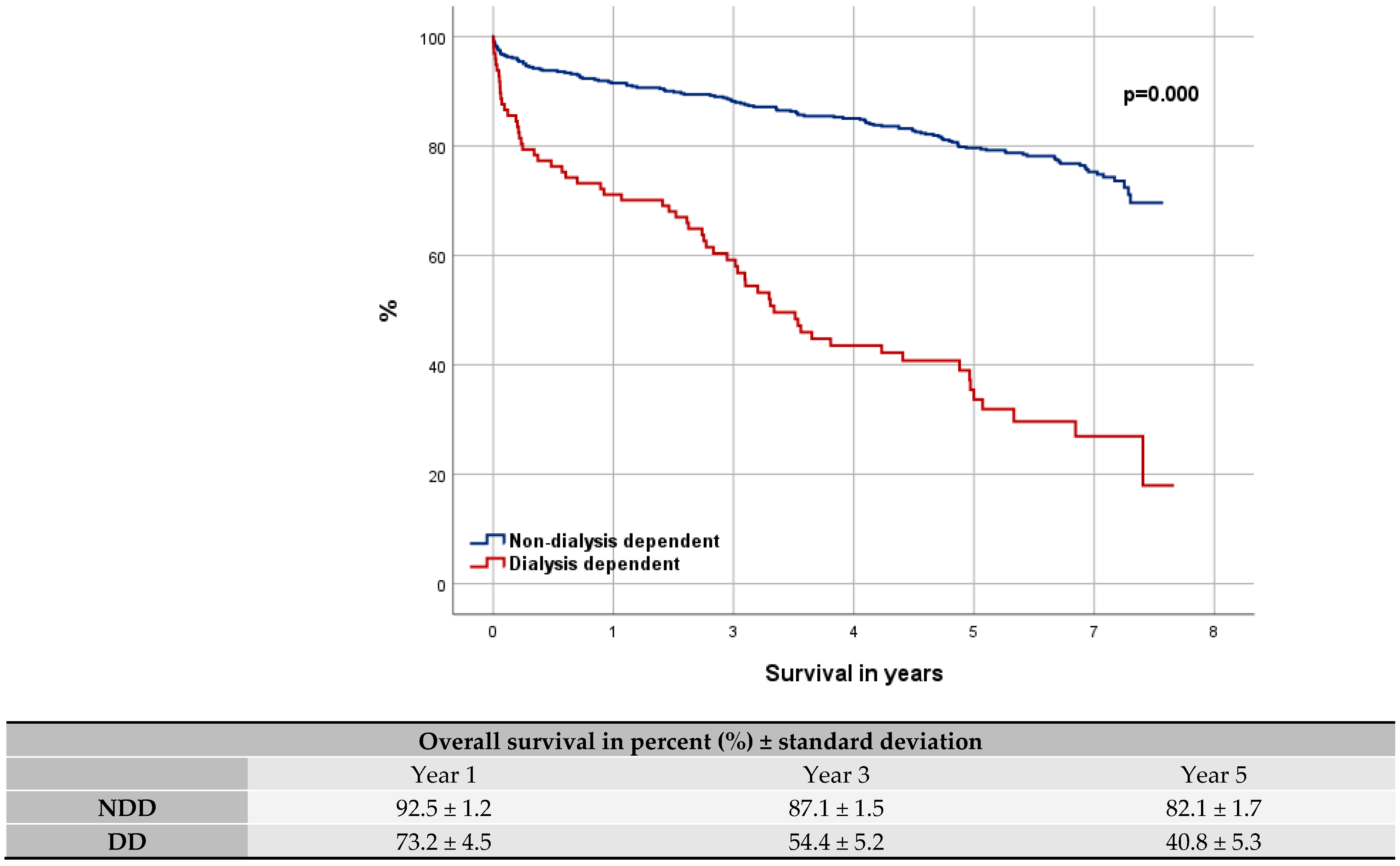
3.3. Overall Survival
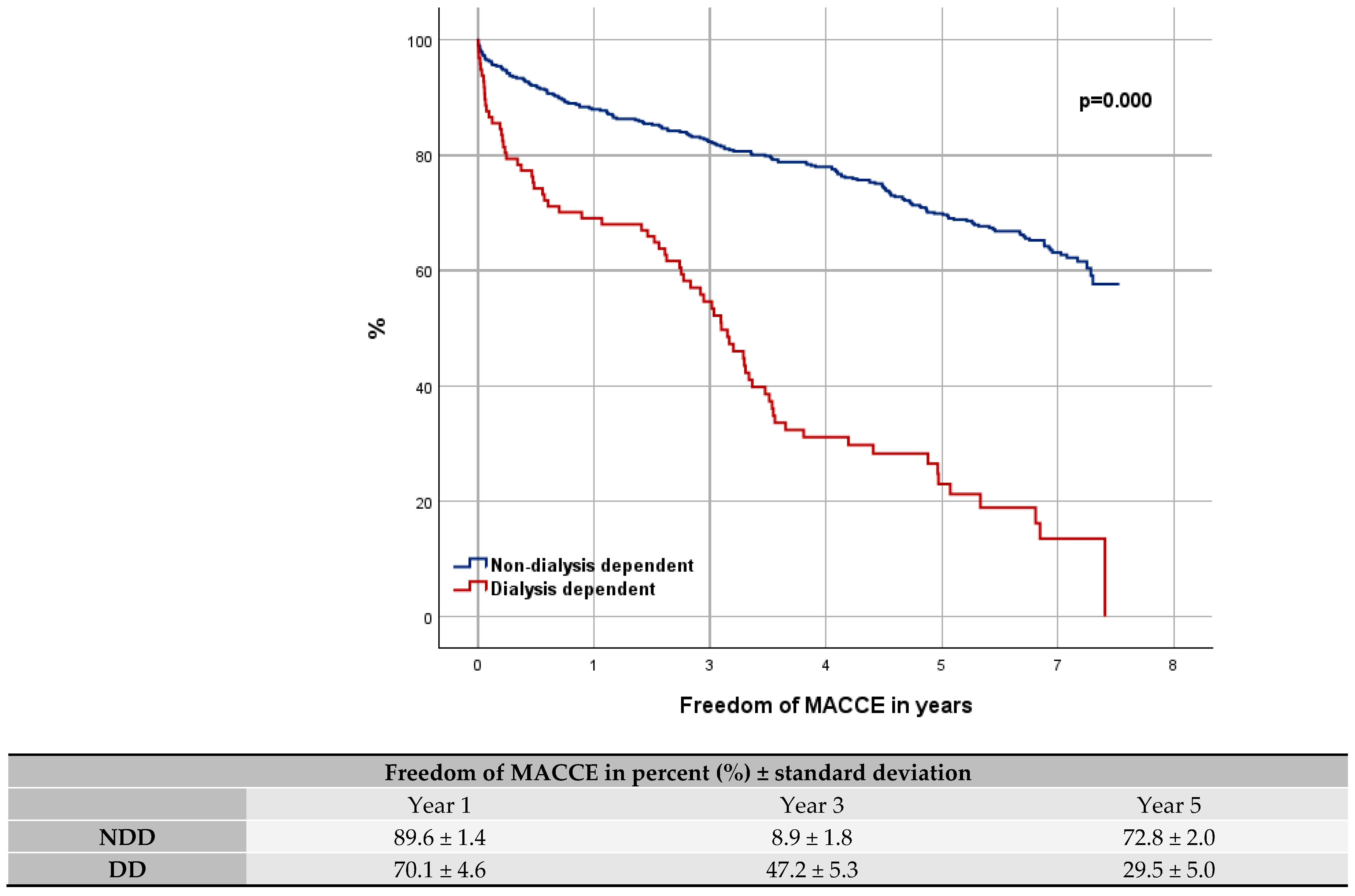
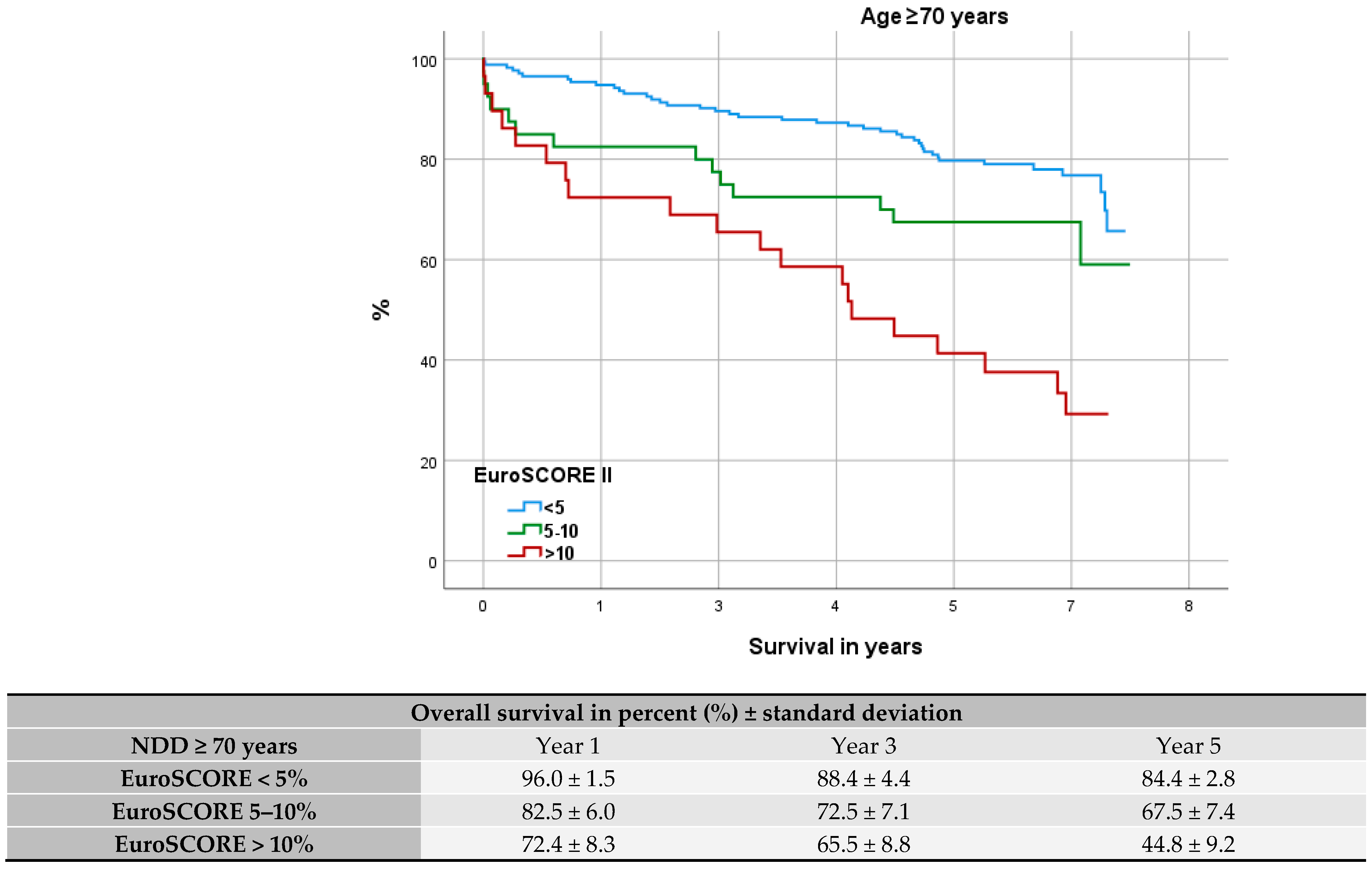
3.4. Comparison with Elderly and High-Risk Patients
3.5. Uni- and Multivariate Analysis
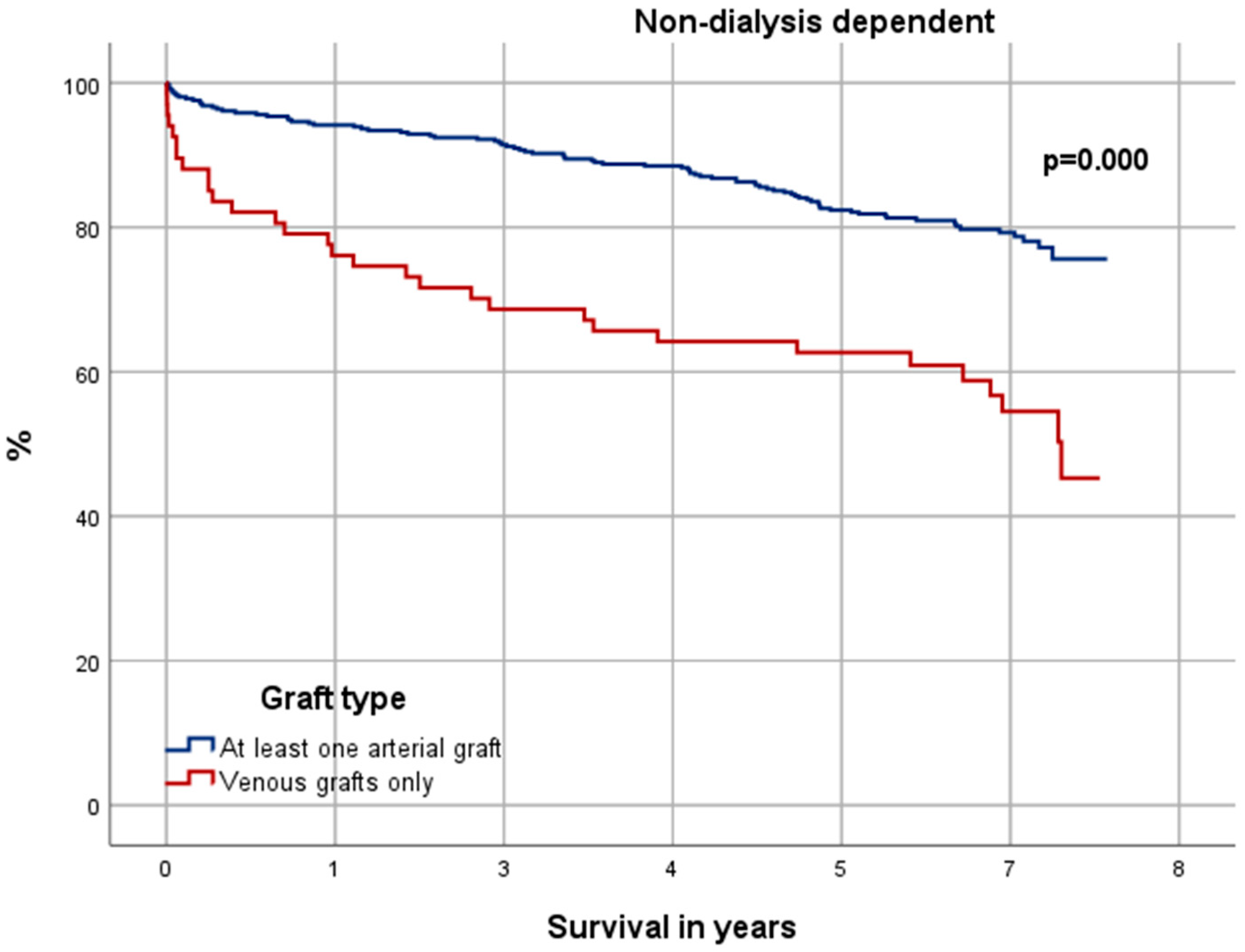
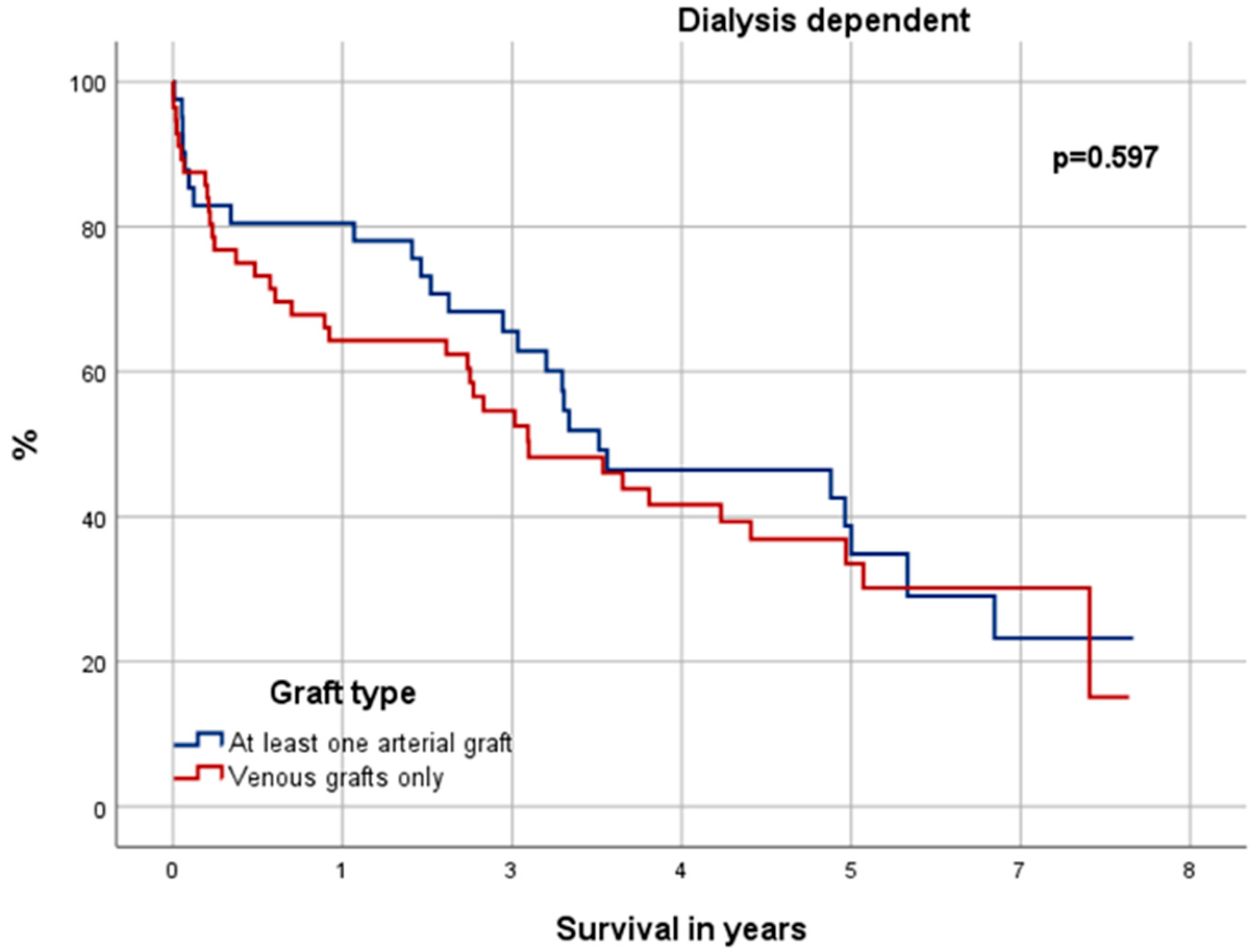
3.6. Significance in Choice of Graft Material
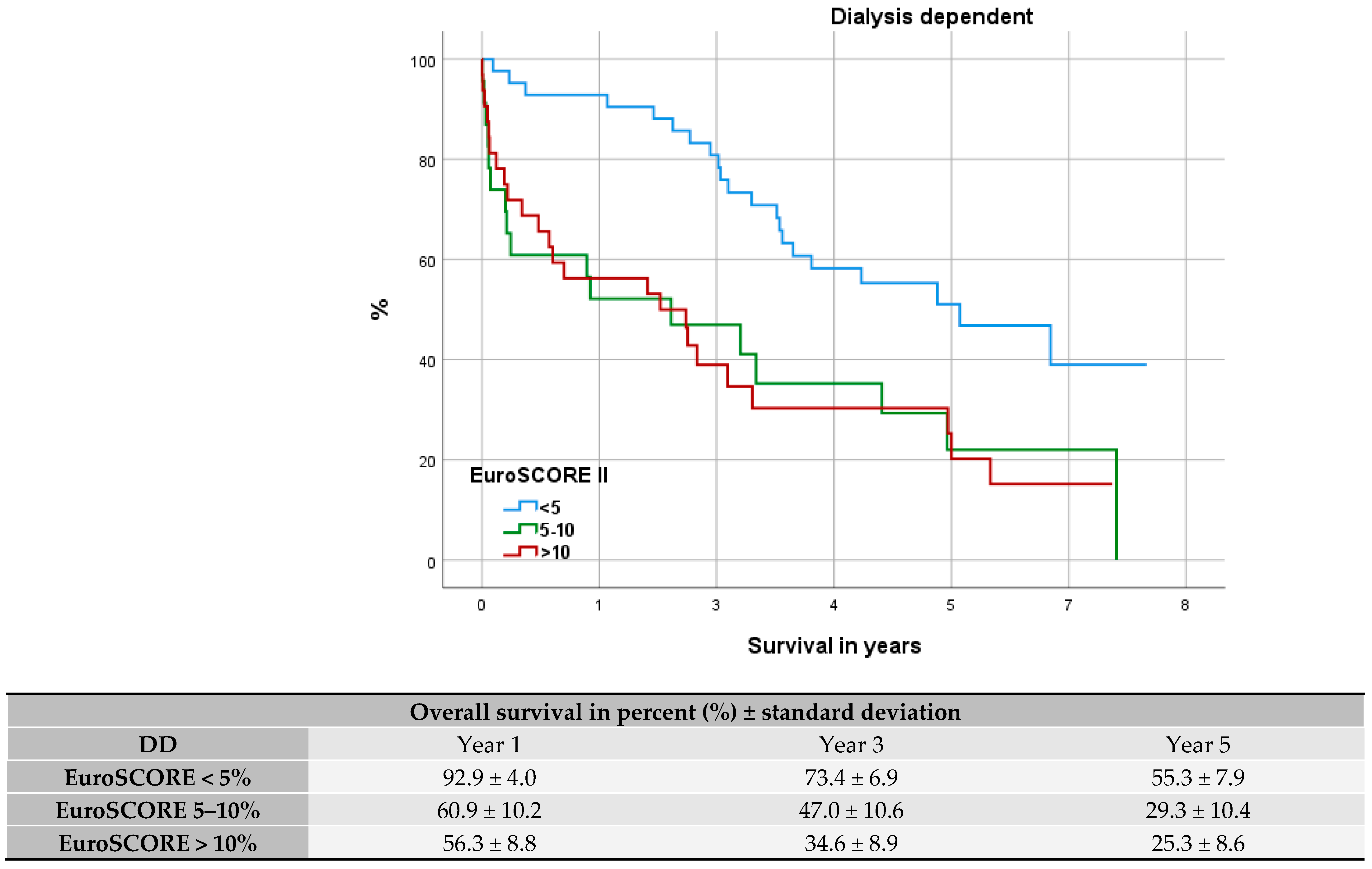
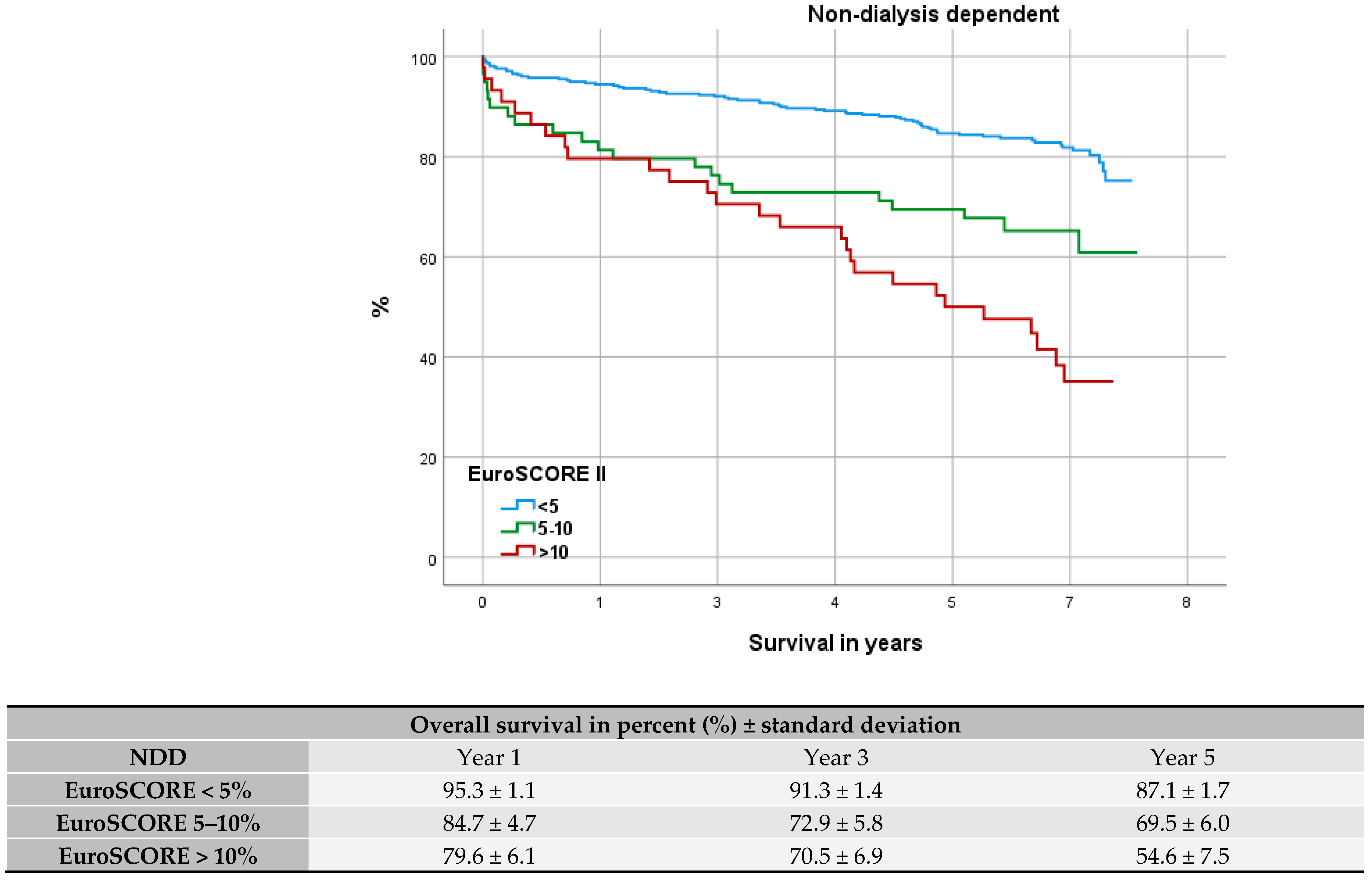
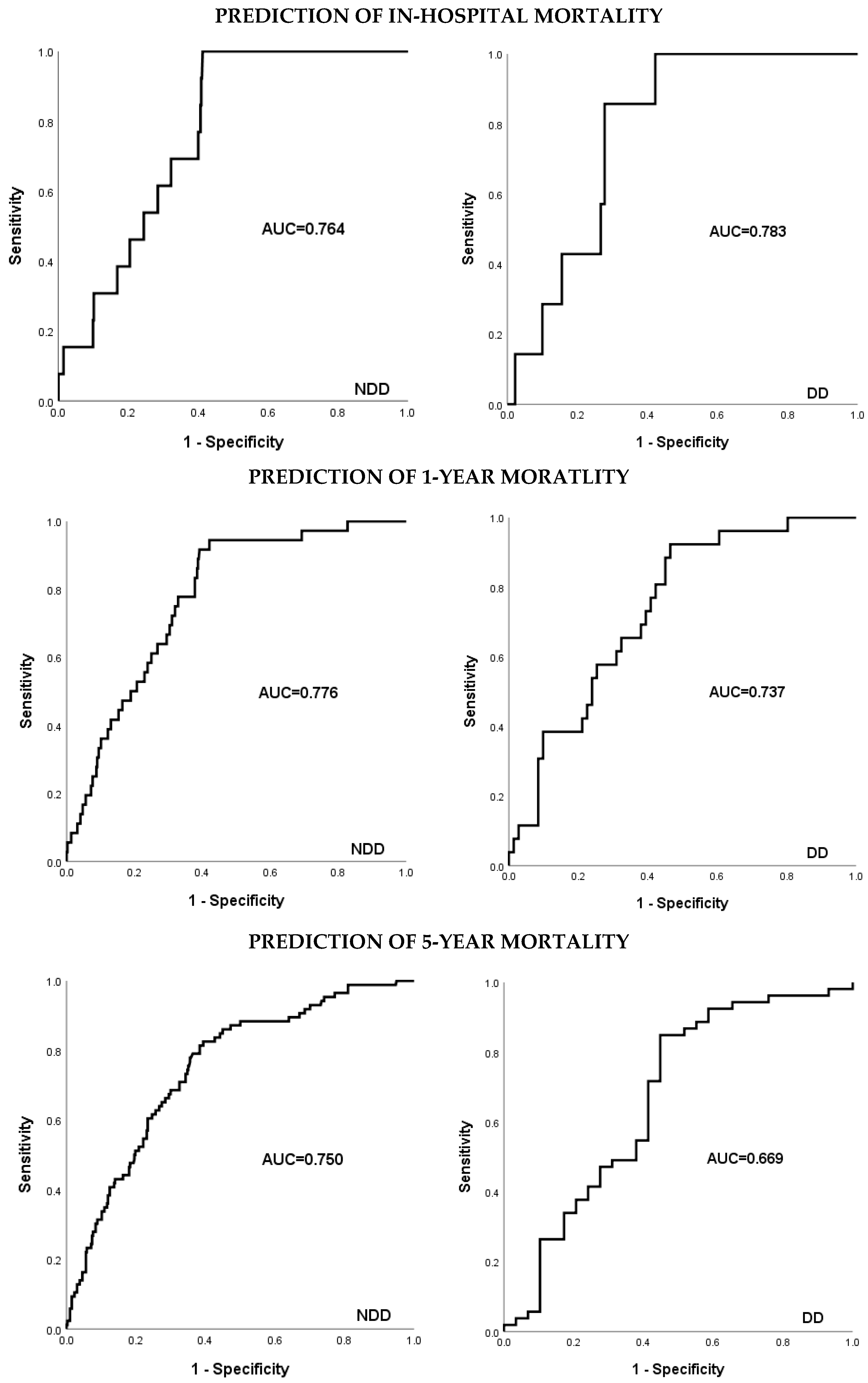
3.7. Evaluation of Fit: EuroSCORE II
4. Discussion
4.1. Overall Survival
4.2. Uni- and Multivariate Analysis
4.3. Comparison to High-Risk and Elderly Patients
4.4. Significance in Choice of Graft Material
4.5. ONCAB vs. OPCAB
4.6. Evaluation of Fit: EuroSCORE II
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Head, S.J.; Kieser, T.M.; Falk, V.; Huysmans, H.A.; Kappetein, A.P. Coronary artery bypass grafting: Part 1—The evolution over the first 50 years. Eur. Heart J. 2013, 34, 2862–2872. [Google Scholar] [CrossRef]
- McNeely, C.; Markwell, S.; Vassileva, C. Trends in Patient Characteristics and Outcomes of Coronary Artery Bypass Grafting in the 2000 to 2012 Medicare Population. Ann. Thorac. Surg. 2016, 102, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bianco, V.; Kilic, A.; Gleason, T.G.; Aranda-Michel, E.; Navid, F.; Sultan, I. Longitudinal outcomes of dialysis-dependent patients undergoing isolated coronary artery bypass grafting. J. Card. Surg. 2019, 34, 110–117. [Google Scholar] [CrossRef]
- Leontyev, S.; Davierwala, P.M.; Gaube, L.M.; Röhrig, K.A.; Lehmann, S.; Holzhey, D.M.; Seeburger, J.; Noack, T.; Misfeld, M.; Mohr, F.W. Outcomes of Dialysis-Dependent Patients After Cardiac Operations in a Single-Center Experience of 483 Patients. Ann. Thorac. Surg. 2017, 103, 1270–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mourad, F.; Cleve, N.; Nowak, J.; Wendt, D.; Sander, A.; Demircioglu, E.; El Gabry, M.; Jakob, H.; Shehada, S.-E. Long-Term Single-Center Outcomes of Patients With Chronic Renal Dialysis Undergoing Cardiac Surgery. Ann. Thorac. Surg. 2020, 109, 1442–1448. [Google Scholar] [CrossRef]
- Rahmanian, P.B.; Adams, D.H.; Castillo, J.G.; Vassalotti, J.; Filsoufi, F. Early and late outcome of cardiac surgery in dialysis-dependent patients: Single-center experience with 245 consecutive patients. J. Thorac. Cardiovasc. Surg. 2008, 135, 915–922. [Google Scholar] [CrossRef]
- Häckl, D.; Kossack, N.; Schoenfelder, T. [Prevalence, Costs of Medical Treatment and Modalities of Dialysis-dependent Chronic Renal Failure in Germany: Comparison of Dialysis Care of Nursing Home Residents and in Outpatient Units]. Gesundheitswesen 2021, 83, 818–828. [Google Scholar]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.-h.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; House, A.; Gorini, A.; Santoboni, A.; Russo, D.; Ronco, C. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 2015, 20, 259–272. [Google Scholar] [CrossRef]
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Bhurtu, A.; Chen, M.H. Impact of coronary artery bypass surgery and percutaneous coronary intervention on mortality in patients with chronic kidney disease and on dialysis: A systematic review and meta-analysis. Medicine 2016, 95, e4129. [Google Scholar] [CrossRef]
- Barili, F.; Pacini, D.; D’Ovidio, M.; Dang, N.C.; Alamanni, F.; Di Bartolomeo, R.; Grossi, C.; Davoli, M.; Fusco, D.; Parolari, A. The Impact of EuroSCORE II Risk Factors on Prediction of Long-Term Mortality. Ann. Thorac. Surg. 2016, 102, 1296–1303. [Google Scholar] [CrossRef]
- Alramadan, M.J.; Karim, M.N.; Hossain, M.N.; Smith, J.A.; Cochrane, A.; Reid, C.M.; Billah, B. Renal Disease Is Associated With Poor Outcomes Following Isolated Coronary Artery Bypass Grafting. Glob. Heart 2019, 14, 347–353. [Google Scholar]
- Hillis, G.S.; Croal, B.L.; Buchan, K.G.; El-Shafei, H.; Gibson, G.; Jeffrey, R.R.; Millar, C.G.M.; Prescott, G.J.; Cuthbertson, B.H. Renal function and outcome from coronary artery bypass grafting: Impact on mortality after a 2.3-year follow-up. Circulation 2006, 113, 1056–1062. [Google Scholar]
- Hori, D.; Yamaguchi, A.; Adachi, H. Coronary Artery Bypass Surgery in End-Stage Renal Disease Patients. Ann. Vasc. Dis. 2017, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardiothorac. Surg. 2012, 41, 734–744; discussion 744–745. [Google Scholar]
- Habib, A.M.; Dhanji, A.R.; Mansour, S.A.; Wood, A.; Awad, W.I. The EuroSCORE: A neglected measure of medium-term survival following cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 427–434. [Google Scholar] [PubMed]
- De Maria, R.; Mazzoni, M.; Parolini, M.; Gregori, D.; Bortone, F.; Arena, V.; Parodi, O. Predictive value of EuroSCORE on long term outcome in cardiac surgery patients: A single institution study. Heart 2005, 91, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, K.; Bagiatis, V.; Deutsch, O.; Kemkes, B.M.; Antonopoulos, N.; Karangelis, D.; Haschemi, A.; Gansera, B. Performance of EuroSCORE II compared to EuroSCORE I in predicting operative and mid-term mortality of patients from a single center after combined coronary artery bypass grafting and aortic valve replacement. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 103–111. [Google Scholar] [CrossRef]
- Grimes, D.A.; Schulz, K.F. Compared to what? Finding controls for case-control studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.S.; Alemzadeh-Ansari, M.J.; Alemzadeh-Ansari, M.H.; Beladi-Mousavi, M. Long-term survival of patients with end-stage renal disease on maintenance hemodialysis: A multicenter study in Iran. Iran. J. Kidney Dis. 2012, 6, 452–456. [Google Scholar]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Afentakis, N.; Ambuhl, P.M.; Andrusev, A.M.; Fuster, E.A.; Monzón, F.E.A.; Åsberg, A.; et al. The European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: A summary. Clin. Kidney J. 2018, 11, 108–122. [Google Scholar] [CrossRef]
- Dacey, L.J.; Liu, J.Y.; Braxton, J.H.; Weintraub, R.M.; DeSimone, J.; Charlesworth, D.C.; Lahey, S.J.; Ross, C.S.; Hernandez, F.; Leavitt, B.J.; et al. Long-term survival of dialysis patients after coronary bypass grafting. Ann. Thorac. Surg. 2002, 74, 458–462; discussion 462–463. [Google Scholar] [CrossRef]
- Gao, D.; Grunwald, G.K.; Rumsfeld, J.S.; Schooley, L.; MacKenzie, T.; Shroyer, A.L. Time-varying risk factors for long-term mortality after coronary artery bypass graft surgery. Ann. Thorac. Surg. 2006, 81, 793–799. [Google Scholar]
- Takami, Y.; Tajima, K.; Kato, W.; Fujii, K.; Hibino, M.; Munakata, H.; Sakai, Y. Predictors for early and late outcomes after coronary artery bypass grafting in hemodialysis patients. Ann. Thorac. Surg. 2012, 94, 1940–1945. [Google Scholar] [CrossRef]
- Pang, P.Y.K.; Teow, C.K.J.; Huang, M.J.; Naik, M.J.; Lim, S.L.; Chao, V.T.T.; Tan, T.E.; Chua, Y.L.; Sin, Y.K. Long-term prognosis in patients with end-stage renal disease after coronary artery bypass grafting. J. Thorac. Dis. 2020, 12, 6722–6730. [Google Scholar] [CrossRef]
- Axelsson, T.A.; Mennander, A.; Malmberg, M.; Gunn, J.; Jeppsson, A.; Gudbjartsson, T. Is emergency and salvage coronary artery bypass grafting justified? The Nordic Emergency/Salvage coronary artery bypass grafting study. Eur. J. Cardiothorac. Surg. 2016, 49, 1451–1456. [Google Scholar]
- Bechtel, J.F.M.; Detter, C.; Fischlein, T.; Krabatsch, T.; Osswald, B.R.; Rieß, F.-C.; Scholz, F.; Schönburg, M.; Stamm, C.; Sievers, H.-H.; et al. Cardiac Surgery in Patients on Dialysis: Decreased 30-Day Mortality, Unchanged Overall Survival. Ann. Thorac. Surg. 2008, 85, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Klester, E.B.; Shoykhet, Y.N.; Elykomov, V.A.; Rudakova, D.M. Co morbidity as a risk factor for CABG patients of elderly and senile age. MOJ Gerontol. Geriatr. 2019, 4, 87–88. [Google Scholar] [CrossRef]
- Rogers, C.A.; Pike, K.; Campbell, H.; Reeves, B.C.; Angelini, G.D.; Gray, A.; Altman, D.G.; Miller, H.; Wells, S.; Taggart, D.P. Coronary artery bypass grafting in high-RISk patients randomised to off- or on-Pump surgery: A randomised controlled trial (the CRISP trial). Health Technol. Assess. 2014, 18, v–xx. [Google Scholar] [PubMed]
- Nappi, F.; Bellomo, F.; Nappi, P.; Chello, C.; Iervolino, A.; Chello, M.; Acar, C. The Use of Radial Artery for CABG: An Update. Biomed. Res. Int. 2021, 2021, 5528006. [Google Scholar]
- Tinica, G.; Chistol, R.O.; Enache, M.; Leon Constantin, M.M.; Ciocoiu, M.; Furnica, C. Long-term graft patency after coronary artery bypass grafting: Effects of morphological and pathophysiological factors. Anatol. J. Cardiol. 2018, 20, 275–282. [Google Scholar] [CrossRef]
- Fleissner, F.; Engelke, H.; Rojas-Hernandez, S.; Ismail, I.; Stiefel, P.; Cebotari, S.; Haverich, A.; Shrestha, M.; Martens, A. Long-term follow-up of total arterial revascularization with left internal thoracic artery and radial artery T-grafts: Survival, cardiac morbidity and quality of life. Eur. J. Cardiothorac. Surg. 2016, 49, 1195–1200. [Google Scholar] [CrossRef]
- Collins, P.; Webb, C.M.; Chong, C.F.; Moat, N.E. Radial artery versus saphenous vein patency randomized trial: Five-year angiographic follow-up. Circulation 2008, 117, 2859–2864. [Google Scholar] [CrossRef]
- Santoro, D.; Benedetto, F.; Mondello, P.; Spinelli, F.; Ricciardi, C.A.; Cernaro, V.; Buemi, M.; Pipitò, N.; Barillà, D. Vascular access for hemodialysis: Current perspectives. Int. J. Nephrol. Renovasc. Dis. 2014, 7, 281–294. [Google Scholar] [CrossRef]
- Li, D.; Gu, S.; Liu, Y.; Zhang, X.; An, X.; Yan, J.; Wang, H.; Guo, Y.; Su, P. Outcomes of left internal mammary artery with saphenous vein composite graft to bypass the left anterior descending artery: A propensity-matched study. J. Thorac. Dis. 2020, 12, 6629–6639. [Google Scholar] [CrossRef] [PubMed]
- Sajja, L.R. Strategies to reduce deep sternal wound infection after bilateral internal mammary artery grafting. Int. J. Surg. 2015, 16, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shilane, D.; Hlatky, M.A.; Winkelmayer, W.C.; Chang, T.I. Coronary artery bypass graft type and outcomes in maintenance dialysis. J. Cardiovasc. Surg. 2015, 56, 463–471. [Google Scholar]
- Hake, U.; Dahm, M.; Böning, A.; Massoudy, P.; Schmitz, C.; Tzanova, I. Koronaroperationen ohne Herz-Lungen-Maschine. Dtsch. Arztebl. Int. 2007, 104, 2127–2132. [Google Scholar]
- Zangrillo, A.; Crescenzi, G.; Landoni, G.; Leoni, A.; Marino, G.; Calabrò, M.G.; Corno, C.; Pappalardo, F.; Alfieri, O. Off-pump coronary artery bypass grafting reduces postoperative neurologic complications. J. Cardiothorac. Vasc. Anesth. 2005, 19, 193–196. [Google Scholar]
- Haverich, A. OPCAB-Eingriffe an der Kardiochirurgie der Medizinischen Hochschule Hannover. Z. Für Herz-Thorax-Und Gefäßchirurgie 2011, 25, 21. [Google Scholar] [CrossRef][Green Version]
- Matkovic, M.; Tutus, V.; Bilbija, I.; Milin Lazovic, J.; Savic, M.; Cubrilo, M.; Aleksic, N.; Atanasijevic, I.; Andrijasevic, V.; Putnik, S. Long Term Outcomes of The Off-Pump and On-Pump Coronary Artery Bypass Grafting In A High-Volume Center. Sci. Rep. 2019, 9, 8567. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Reitz, M.; Cebotari, S.; Kaufeld, T.; Haverich, A.; Shrestha, M.; Ismail, I.; Martens, A. Total Arterial Revascularization with Radial Artery T-grafts in Patients with Significant Left Main Stem Stenosis Is Not Associated with Higher Perioperative Risk. Thorac. Cardiovasc. Surg. 2016, 64, 197–203. [Google Scholar]
- Obed, D.; Fleissner, F.; Martens, A.; Cebotari, S.; Haverich, A.; Warnecke, G.; Ismail, I. Total Arterial Revascularization with Radial Artery and Internal Thoracic Artery T-Grafts Is Associated with Superior Long-Term Survival in Patients Undergoing Coronary Artery Bypass Grafting. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 30–39. [Google Scholar] [CrossRef]
- Ad, N.; Holmes, S.D.; Patel, J.; Pritchard, G.; Shuman, D.J.; Halpin, L. Comparison of EuroSCORE II, Original EuroSCORE, and The Society of Thoracic Surgeons Risk Score in Cardiac Surgery Patients. Ann. Thorac. Surg. 2016, 102, 573–579. [Google Scholar] [CrossRef] [PubMed]
| Variable | NDD n = 488 | DD n = 97 | p-Value |
|---|---|---|---|
| Gender (male) | 75.8% | 79.4% | 0.045 |
| Age | 67.9 ± 9.8 | 66.9 ± 9.9 | 0.362 |
| Age ≥ 70 | 49.8% | 48.5% | 0.809 |
| Systemic hypertension | 84.4% | 86.6% | 0.586 |
| Diabetes | 28.3% | 43.3% | 0.003 |
| Hyperlipidemia | 64.5% | 44.3% | 0.000 |
| PAD | 19.3% | 45.4% | 0.000 |
| BMI > 30 kg/m2 | 31.1% | 26.8% | 0.396 |
| Previous stroke | 4.1% | 6.2% | 0.362 |
| CLD | 10.2% | 17.5% | 0.040 |
| MI ≤ 90 days | 21.9% | 36.1% | 0.003 |
| ND | 1.6% | 14.4% | 0.000 |
| Active endocarditis | 0.4% | 2.1% | 0.071 |
| AP | 19.9% | 19.6% | 0.949 |
| Critical preoperative state | 4.3% | 3.1% | 0.583 |
| Previous cardiac surgery | 3.3% | 6.2% | 0.169 |
| Previous PCI | 23.3% | 22.9% | 0.903 |
| LVEF | |||
| good (≥50%) | 66.0% | 50.5% | 0.004 |
| moderate (50–31%) | 19.3% | 19.6% | 0.941 |
| poor (30–21%) | 8.4% | 19.6% | 0.001 |
| very poor (≤20%) | 6.4% | 10.3% | 0.163 |
| NYHA | |||
| I | 41.0% | 13.4% | 0.000 |
| II | 36.9% | 50.5% | 0.012 |
| III | 18.9% | 30.9% | 0.008 |
| IV | 3.3% | 5.2% | 0.364 |
| PHT | |||
| good | 93.2% | 82.5% | 0.001 |
| moderate | 6.1% | 12.4% | 0.030 |
| severe | 0.6% | 5.2% | 0.000 |
| Priority | |||
| elective | 58.6% | 56.7% | 0.728 |
| urgent | 32.4% | 34.0% | 0.753 |
| emergency | 8.8% | 9.3% | 0.883 |
| salvage | 0.2% | 0% | 0.655 |
| EuroSCORE II | 4.4 ± 7.4 | 9.8 ± 9.8 | 0.000 |
| Variable | NDD n = 488 | DD n = 97 | p-Value |
|---|---|---|---|
| Surgery weight | |||
| Isolated CABG | 69.9% | 59.8% | 0.051 |
| 2 Procedures | 21.7% | 23.7% | 0.666 |
| 3 Procedures | 8.4% | 16.5% | 0.014 |
| Aorta surgery | 5.3% | 6.2% | 0.734 |
| Surgery duration (min) | 207.3 ± 67.1 | 216.8 ± 69.1 | 0.220 |
| Bypass duration (min) | 98.7 ± 44.7 | 114.3 ± 52.7 | 0.001 |
| Clamp time (min) | 54.7 ± 28.5 | 56.9 ± 36.3 | 0.843 |
| Number of anastomosis | 3.8 ± 1.3 | 3.8 ± 1.3 | 0.475 |
| OPCAB | 3.1% | 0.0% | 0.206 |
| Grafts | |||
| LITA | 84.4% | 42.3% | 0.000 |
| RITA | 3.7% | 1.0% | 0.173 |
| BITA | 2.0% | 1.0% | 0.500 |
| A. radialis | 21.6% | 2.1% | 0.000 |
| TAR | 23.0% | 0.0% | 0.000 |
| Venous | 65.8% | 100.0% | 0.000 |
| Venous only | 13.9% | 57.7% | 0.000 |
| LAD | 91.9% | 85.6% | 0.048 |
| RCX | 73.9% | 66.0% | 0.113 |
| RCA | 67.2% | 69.1% | 0.722 |
| Vascularization of | |||
| 1 Coronary artery | 15.6% | 19.6% | 0.328 |
| 2 Coronary arteries | 35.0% | 40.2% | 0.333 |
| 3 Coronary arteries | 48.2% | 40.2% | 0.152 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Dialysis dependency | 4.464 (3.260–6.113) | 0.000 | 3.110 (2.148–4.513) | 0.000 |
| Gender (male) | 0.718 (0.527–1.008) | 0.056 | ||
| Age | 1.046 (1.029–1.064) | 0.000 | 1.057 (1.037–1.077) | 0.000 |
| LVEF | 0.975 (0.965–0.984) | 0.000 | 0.980 (0.968–0.992) | 0.001 |
| Hypertension | 1.270 (0.820–1.968) | 0.284 | ||
| Diabetes | 1.765 (1.313–2.373) | 0.000 | 1.560 (1.143–2.129) | 0.005 |
| PAD | 2.407 (1.779–3.257) | 0.000 | 1.646 (1.182–2.291) | 0.003 |
| CLD | 2.598 (1.821–3.707) | 0.000 | 1.691 (1.161–2.464) | 0.006 |
| EuroSCORE II | 1.050 (1.039–1.061) | 0.000 | 0.997 (0.977–1.017) | 0.733 |
| Previous stroke | 1.210 (0.614–2.366) | 0.577 | ||
| MI ≥ 90 days | 1.758 (1.288–2.401) | 0.000 | 1.408 (0.992–1.998) | 0.055 |
| Previous cardiac surgery | 1.430 (0.732–2.797) | 0.295 | ||
| Critical preoperative state | 1.098 (0.540–2.232) | 0.795 | ||
| Emergency procedure | 1.158 (0.711–1.885) | 0.566 | ||
| Concomitant surgery | 2.045 (1.525–2.742) | 0.000 | 1.244 (0.863–1.793) | 0.241 |
| Aorta surgery | 1.754 (1.034–2.976) | 0.037 | 2.473 (1.353–54.624) | 0.005 |
| Venous grafts only | 2.989 (2.204–4.054) | 0.000 | 1.467 (1.009–2.132) | 0.034 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Gender (male) | 0.621 (0.340–1.134) | 0.121 | ||
| Age | 1.044 (1.015–1.074) | 0.003 | 1.043 (1.013–1.075) | 0.005 |
| LVEF | 0.988 (0.971–1.006) | 0.201 | ||
| Hypertension | 1.028 (0.488–2.163) | 0.943 | ||
| Diabetes | 1.731 (1.039–2.884) | 0.035 | 1.792 (1.045–3.074) | 0.034 |
| PAD | 1.272 (0.767–2.109) | 0.350 | ||
| CLD | 1.117 (0.604–2.064) | 0.724 | ||
| EuroSCORE II | 1.032 (1.010–1.055) | 0.005 | 1.011 (0.984–1.040) | 0.420 |
| Previous stroke | 1.260 (0.456–3.482) | 0.657 | ||
| MI ≥ 90 days | 1.871 (1.117–3.132) | 0.017 | 1.549 (0.901–2.662) | 0.113 |
| Previous cardiac surgery | 0.741 (0.265–2.070) | 0.568 | ||
| Critical preoperative state | 7.622 (2.184–26.96) | 0.001 | 9.723 (2.027–46.643) | 0.004 |
| Emergency procedure | 0.953 (0.381–2.381) | 0.918 | ||
| Concomitant surgery | 1.618 (0.979–2.676) | 0.061 | ||
| Aorta surgery | 1.652 (0.661–4.130) | 0.283 | ||
| Venous grafts only | 1.146 (0.691–1.899) | 0.597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deniz, E.; Brunkhorst, T.; Helms, F.; Hanke, J.; Merzah, A.; Ali-Hasan Al-Saegh, S.; Zubarevich, A.; Fleissner, F.; Ismail, I.; Warnecke, G.; et al. Challenging the Paradigm: Long-Term Outcomes in Dialysis-Dependent Patients Undergoing CABG. J. Cardiovasc. Dev. Dis. 2025, 12, 356. https://doi.org/10.3390/jcdd12090356
Deniz E, Brunkhorst T, Helms F, Hanke J, Merzah A, Ali-Hasan Al-Saegh S, Zubarevich A, Fleissner F, Ismail I, Warnecke G, et al. Challenging the Paradigm: Long-Term Outcomes in Dialysis-Dependent Patients Undergoing CABG. Journal of Cardiovascular Development and Disease. 2025; 12(9):356. https://doi.org/10.3390/jcdd12090356
Chicago/Turabian StyleDeniz, Ezin, Tonita Brunkhorst, Florian Helms, Jasmin Hanke, Ali Merzah, Sadeq Ali-Hasan Al-Saegh, Alina Zubarevich, Felix Fleissner, Issam Ismail, Gregor Warnecke, and et al. 2025. "Challenging the Paradigm: Long-Term Outcomes in Dialysis-Dependent Patients Undergoing CABG" Journal of Cardiovascular Development and Disease 12, no. 9: 356. https://doi.org/10.3390/jcdd12090356
APA StyleDeniz, E., Brunkhorst, T., Helms, F., Hanke, J., Merzah, A., Ali-Hasan Al-Saegh, S., Zubarevich, A., Fleissner, F., Ismail, I., Warnecke, G., Dogan, G., Schmitto, J. D., Schmack, B., Weymann, A., Ruhparwar, A., & Popov, A.-F. (2025). Challenging the Paradigm: Long-Term Outcomes in Dialysis-Dependent Patients Undergoing CABG. Journal of Cardiovascular Development and Disease, 12(9), 356. https://doi.org/10.3390/jcdd12090356








