Mid-Term Recovery of Right Ventricular Function and Improvement of Left Ventricular Function After Da Silva Cone Procedure for Ebstein Anomaly
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Transthoracic Echocardiograms
2.3. Clinical Data
3. Results
3.1. Demographics
3.2. Operative Results
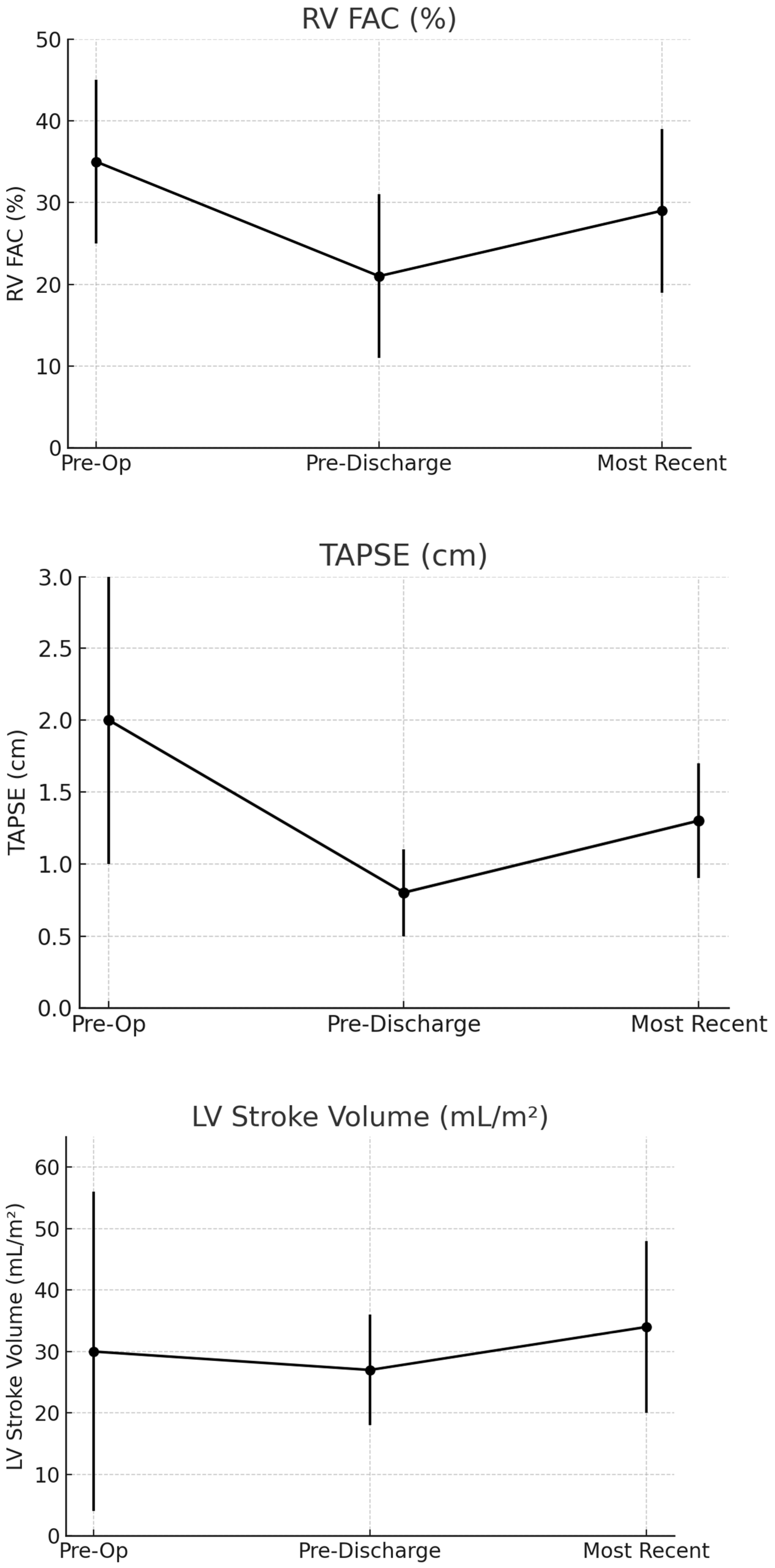
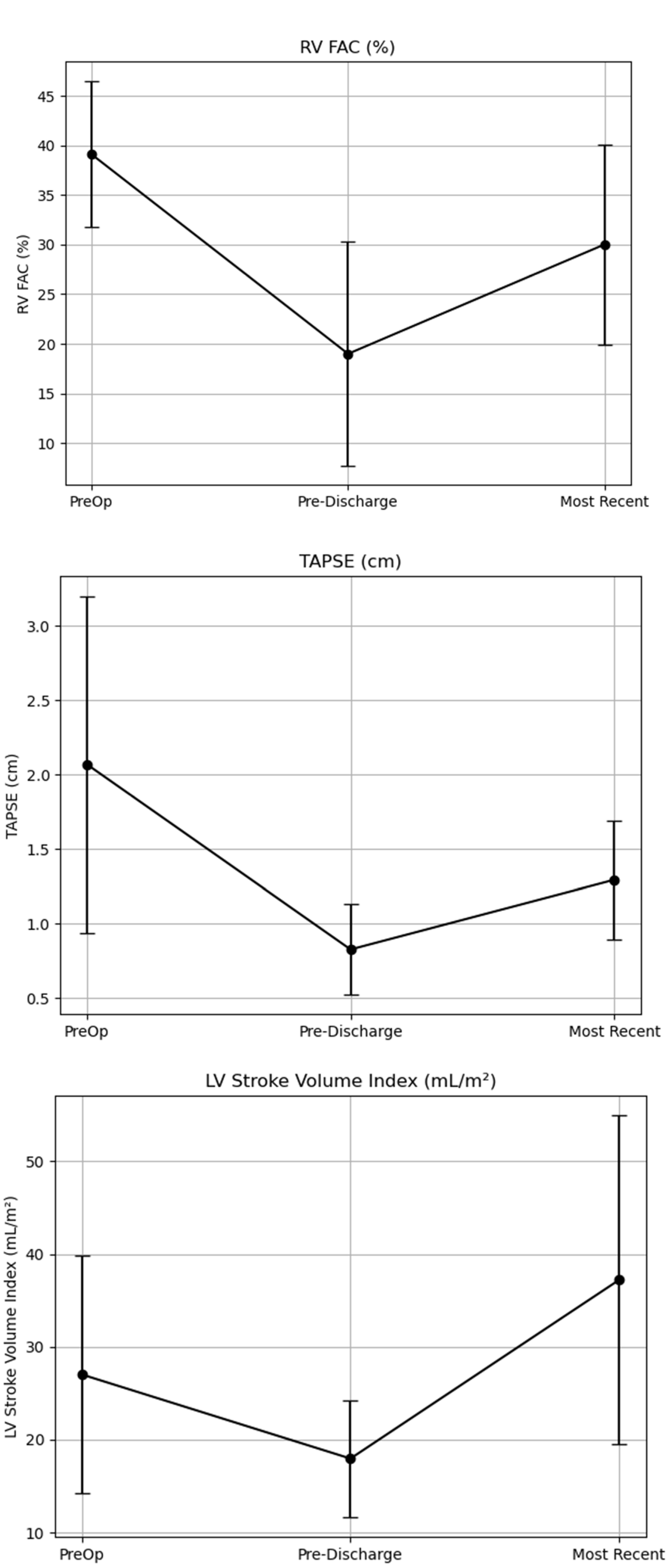
3.3. Change in RV Function
3.4. Change in Left Ventricular Function
3.5. Effect of Previous Glenn Operation on Ventricular Function
3.6. Effect of Previous Starnes Operation on Ventricular Function
3.7. Effect of Ventricular Dilation on Ventricular Function
3.8. Analyzing Patients That Had Functional Parameters at All Three Time Points
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LVEF | Left Ventricular Ejection Fraction |
| RV | Right Ventricle |
| LV | Left Ventricle |
| TR | Tricuspid Regurgitation |
| UPMC | University of Pittsburgh Medical Center |
| MPA | Main Pulmonary Artery |
| ASD | Atrial Septal Defect |
| TV | Tricuspid Valve |
| PV | Pulmonary Valve |
| VSD | Ventricular Septal Defect |
| PDA | Patent Ductus Arteriosus |
| EF | Ejection Fraction |
| BSA | Body Surface Area |
| LVEDVi | BSA Indexed End-Diastolic Volume |
| Mitral valve | E/E′ |
| LVSVi | BSA Indexed LV Stroke Volume |
| FAC | Fractional Area Change |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
References
- Attenhofer Jost, C.H.; Connolly, H.M.; Dearani, J.A.; Edwards, W.D.; Danielson, G.K. Ebstein’s Anomaly. Circulation 2007, 115, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Holst, K.A.; Connolly, H.M.; Dearani, J.A. Ebstein’s Anomaly. Methodist DeBakey Cardiovasc. J. 2019, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Neijenhuis, R.M.L.; Tsang, V.T.; Marek, J.; Issitt, R.; Bonello, B.; Von Klemperer, K.; Hughes, M.L. Cone reconstruction for Ebstein anomaly: Late biventricular function and possible remodeling. J. Thorac. Cardiovasc. Surg. 2021, 161, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Kron, I.L.; Roeser, M.E. Management of Ebstein’s anomaly. Ann. Cardiothorac. Surg. 2017, 6, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.H.; Dearani, J.A. Commentary: Neonatal Ebstein: Starnes procedure first … Fontan not necessarily the last. J. Thorac. Cardiovasc. Surg. 2023, 166, 1744–1745. [Google Scholar] [CrossRef] [PubMed]
- Dobson, C.P.; Christopher, A.B.; Castro-Medina, M.; Viegas, M.L.; Da Silva, J.P.; Da Silva, L.D.F. Using DaSilva Cone Operation to Establish 1.5 or 2 Ventricle Circulation After Initial Single Ventricle Palliation with Starnes Procedure. Pediatr. Cardiol. 2025, 46, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Furtmüller, C.; Baessato, F.; Ferrari, I.; Shehu, N.; Martinoff, S.; Reich, B.; Ewert, P.; Nagdyman, N.; Cleuziou, J.; Stern, H.; et al. Change in Right Ventricular Strain After Cone Reconstruction of Ebstein’s Anomaly: A Cardiovascular Magnetic Resonance-Feature Tracking Study. Appl. Sci. 2025, 15, 2659. [Google Scholar] [CrossRef]
- Perdreau, E.; Tsang, V.; Hughes, M.L.; Ibrahim, M.; Kataria, S.; Janagarajan, K.; Iriart, X.; Khambadkone, S.; Marek, J. Change in biventricular function after cone reconstruction of Ebstein’s anomaly: An echocardiographic study. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Carney, M.; Gupta, A.; Christopher, A.; Olivieri, L.; Da Silva, J.; Diaz Castrillon, C.; Feingold, B.; Dobson, C.P.; De Fonseca Da Silva, L.; Alsaied, T. Large Right Atrial Size on Cardiac MRI is Associated with Post-operative Right Ventricular Dysfunction After the Cone Operation for Ebstein Anomaly. Pediatr. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Castrillon, C.D.; Christopher, A.; Da Silva, J.; Morell, V.O.; Lanford, L.; Goldstein, B.H.; Feingold, B.; Seery, T.; Arora, G.; et al. Cardiac MRI predictors of right ventricular dysfunction after the Da Silva cone operation for Ebstein’s anomaly. Int. J. Cardiol. Congenit. Heart Dis. 2022, 7, 100342. [Google Scholar] [CrossRef] [PubMed]
- Holst, K.A.; Dearani, J.A.; Said, S.; Pike, R.B.; Connolly, H.M.; Cannon, B.C.; Sessions, K.L.; O’Byrne, M.M.; O’Leary, P.W. Improving Results of Surgery for Ebstein Anomaly: Where Are We After 235 Cone Repairs? Ann. Thorac. Surg. 2018, 105, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Beroukhim, R.S.; Jing, L.; Harrild, D.M.; Fornwalt, B.K.; Mejia-Spiegeler, A.; Rhodes, J.; Emani, S.; Powell, A.J. Impact of the cone operation on left ventricular size, function, and dyssynchrony in Ebstein anomaly: A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Suri, R.M.; Thalji, N.M.; Daly, R.C.; Dearani, J.A.; Burkhart, H.M.; Li, Z.; Enriquez-Sarano, M.; Schaff, H.V. Left ventricular dysfunction after mitral valve repair—The fallacy of “normal” preoperative myocardial function. J. Thorac. Cardiovasc. Surg. 2014, 148, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.F.; Silva, J.P.D.; Castro-Medina, M.; Viegas, M.; Alsaied, T.; Seese, L.; Morell, V.O.; Silva, L.D.F.D. The cone repair allows right ventricle rehabilitation with excellent tricuspid valve function following the Starnes procedure. J. Thorac. Cardiovasc. Surg. 2025, 169, 354–361.e3. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Median (Interquartile Range) or % (n) |
|---|---|
| Median Age of Surgery (years) | 7.8 years (IQR): 2.3–17.7) |
| Previous Cardiac Surgery (%) | 29% (n = 39) |
| Males (%) | 47.8% (n = 64) |
| Females (%) | 52.2% (n = 70) |
| Previous surgery or Catheterization Procedure * | Percentage (n) |
|---|---|
| Starnes | 10% (n = 14) |
| Blalock–Taussig–Thomas Shunt | 17% (n = 23) |
| Central Shunt (Aorta to MPA) | 5% (n = 7) |
| Patent Ductus Arteriosus Stent | 4% (n = 6) |
| Glenn | 19% (n = 25) |
| Previous Cone | 4% (n = 6) |
| ASD Device Closure | 6% (n = 8) |
| ASD Surgical Closure | 6% (n = 8) |
| Ablation | 23% (n = 31) |
| TV Repair | 4% (n = 6) |
| TV Replacement | 1% (n = 2) |
| PV Repair | 2% (n = 3) |
| PV Replacement | 1% (n = 2) |
| Other * | 22% (n = 29) |
| Echo Parameter | Time 1 (Pre-Op) | Time 2 (Pre-Discharge) | Time 3 (Most Recent) | Time 1 vs. Time 2 (p-Value) | Time 1 vs. Time 3 (p-Value) | Time 2 vs. Time 3 (p-Value) |
|---|---|---|---|---|---|---|
| RV Fractional Area of Change (%) | 35 ± 10 (n = 53) | 21 ± 10 (n = 79) | 29 ± 10 (n = 35) | <0.001 | <0.001 | 0.039 |
| TAPSE (cm) | 2.0 ±1.0 (n = 118) | 0.8 ± 0.3 (n = 118) | 1.3 ± 0.4 (n = 54) | <0.001 | <0.001 | <0.001 |
| Tricuspid S′ (cm/s) | 13 ± 6 (n = 95) | 5 ± 4 (n = 95) | 7 ± 2 (n = 39) | <0.001 | <0.001 | 0.115 |
| LVEF (%) | 59 ± 6 (n = 129) | 61 ± 6 (n = 122) | 61 ± 3 (n = 64) | 0.011 | 0.227 | 0.988 |
| LV EDVi (mL/m2) | 47 ± 20 (n = 99) | 46 ± 16 (n = 87) | 54 ± 25 (n = 45) | 0.408 | 0.033 | 0.033 |
| LVSVi (mL/m2) | 30 ± 26 (n = 98) | 27 ± 9 (n = 98) | 34 ± 14 (n = 39) | 0.279 | 0.005 | 0.027 |
| MV E/E′ | 7 ± 3 (n = 110) | 11 ± 6 (n = 105) | 7 ± 2 (n = 15) | <0.001 | 0.885 | 0.008 |
| Time 1 (n = 129) | No Glenn (Mean ± SD) N = 106 | Glenn (Mean ± SD) N = 23 | p-Value | No Starnes (Mean ± SD) N = 115 | Starnes (Mean ± SD) N = 14 | p-Value |
|---|---|---|---|---|---|---|
| LVEDVi (mL/m2) | 43.2 ± 15.6 | 65.1 ± 25.9 | <0.001 | 43.4 ± 16 | 72.0 ± 22.0 | <0.001 |
| LVSVI (mL/m2) | 28.5 ± 28.4 | 39.3 ± 16.9 | 0.123 | 28.5 ± 28.1 | 43.4 ± 11.8 | 0.063 |
| LVEF (%) | 59.3 ± 6.3 | 60.0 ± 6.1 | 0.642 | 59.3 ± 6.3 | 60.4 ± 6.0 | 0.535 |
| RV FAC (%) | 35.9 ± 9.8 | 26.9 ± 8.6 | 0.027 | 36.6 ± 8.3 | 22.5 ± 13.0 | <0.001 |
| TAPSE (cm) | 2.4 ± 1.0 | 1.0 ± 0.4 | 0.001 | 2.335 ± 1.0 | 0.7 ± 0.2 | <0.001 |
| Tricuspid S′ (cm/s) | 13.6 ± 5.5 | 7.3 ± 3.9 | 0.001 | 13.4 ± 5.5 | 4.8 ± 1.8 | <0.001 |
| Time 2 (n = 122) | N = 106 | N = 23 | N = 115 | N = 14 | ||
| LVEDVi (mL/m2) | 42.5 ± 13.0 | 56.8 ± 18.9 | <0.001 | 43.7 ± 14.8 | 59.9 ± 14.7 | 0.001 |
| LVSVI (mL/m2) | 26.0 ± 8.0 | 32.9 ± 10.5 | 0.002 | 26.6 ± 9.0 | 34.2 ± 7.8 | 0.009 |
| LVEF (%) | 61.9 ± 5.5 | 58.3 ± 5.3 | 0.004 | 57.0 ± 5.1 | 61.7 ± 5.5 | 0.003 |
| RV FAC (%) | 21.2 ± 9.1 | 19.6 ± 11.7 | 0.555 | 20.0 ± 9.3 | 28.0 ± 9.7 | 0.018 |
| TAPSE (cm) | 0.79 ± 0.3 | 0.7 ± 0.2 | 0.105 | 0.8 ± 0.3 | 0.5 ± 0.2 | 0.001 |
| Tricuspid S′ (cm/s) | 5.22 ± 4.4 | 4.1 ± 1.4 | 0.237 | 5.1 ± 4.0 | 4.2 ± 1.5 | 0.439 |
| Time 3 (n = 64) | N = 55 | N = 24 | N = 59 | N = 5 | ||
| LVEDVi (mL/m2) | 55.4 ± 24.5 | 43.4 ± 30.5 | 0.285 | 54.6 ± 24.6 | 47.2 ± 36.6 | 0.544 |
| LVSVI (mL/m2) | 34.6 ± 13.7 | 33.5 ± 14.7 | 0.865 | 33.9 ± 13.8 | 39.5 ± 12.7 | <0.001 |
| LVEF (%) | 60.8 ± 6.1 | 61.2 ± 10.1 | 0.877 | 60.3 ± 6.5 | 67.6 ± 6.2 | 0.020 |
| RV FAC (%) | 29.9 ± 10.3 | 19.8 ± 6.3 | 0.183 | N/A | N/A | N/A * |
| TAPSE (cm) | 1.36 ± 0.4 | 1.0 ± 0.4 | 0.044 | 1.4 ± 0.4 | 0.8 ± 0.5 | 0.008 |
| Tricuspid S′ (cm/s) | 6.85 ± 1.8 | 7.1 ± 2.8 | 0.826 | 6.9 ± 1.9 | 5.7 ± 1.4 | 0.257 |
| Time 1 | |||
|---|---|---|---|
| Parameter | Mild RV Dilation (N = 36) | Moderate–Severe RV Dilation (N = 67) | p-Value |
| LVEDVi (mL/m2) | 45.6 ± 19.1 | 44.7 ± 15.3 | 0.823 |
| LVSVi (mL/m2) | 27.5 ± 11.1 | 31.2 ± 36.2 | 0.598 |
| LVEF (%) | 60.2 ± 7.5 | 58.9 ± 4.9 | 0.294 |
| RV FAC (%) | 35.0 ± 15.3 | 35.1 ± 6.8 | 0.976 |
| TAPSE (cm) | 1.7 ± 1.0 | 2.4 ± 1.0 | 0.009 |
| S′ (cm/s) | 10.5 ± 5.7 | 14.1 ± 5.3 | 0.009 |
| Time 2 | |||
| Parameter | Mild RV Dilation (N = 43) | Moderate–Severe RV Dilation (N = 64) | p-Value |
| LVEDVi (mL/m2) | 52.9 ± 13.5 | 41.0 ± 15.9 | <0.001 |
| LVSVi (mL/m2) | 32.2 ± 7.6 | 24.8 ± 9.4 | <0.001 |
| LVEF (%) | 61.7 ± 5.0 | 61.5 ± 5.3 | 0.807 |
| RV FAC (%) | 26.9 ± 8.5 | 18.2 ± 8.2 | <0.001 |
| TAPSE (cm) | 0.72 ± 0.3 | 0.81 ± 0.3 | 0.135 |
| S′ (cm/s) | 4.40= ± 1.53 | 5.46 ± 4.8 | 0.215 |
| Time 3 | |||
| Parameter | Mild RV Dilation (N = 32) | Moderate–Severe RV Dilation (N = 28) | p-Value |
| LVEDVi (mL/m2) | 46.6 ± 22.6 | 59.3 ± 27.2 | 0.113 |
| LVESVi (mL/m2) | 20.3 ± 8.1 | 25.1 ± 10.4 | 0.129 |
| LVSVi (mL/m2) | 31.5 ± 10.1 | 36.9 ± 16.5 | 0.240 |
| LVEF (%) | 61.2 ± 5.5 | 60.1 ± 8.0 | 0.539 |
| RV FAC (%) | 31.2 ± 8.4 | 27.5 ± 12.8 | 0.325 |
| TAPSE (cm) | 1.37 ± 0.4 | 1.30 ± 0.34 | 0.536 |
| S′ (cm/s) | 6.55 ± 1.9 | 7.29 ± 1.8 | 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundaram, K.; Stoll, V.; Da Fonseca Da Silva, L.; Christopher, A.; Hoskoppal, A.; Kreutzer, J.; Liddle, D.; Olivieri, L.; Weinberg, J.; Dobson, C.P.; et al. Mid-Term Recovery of Right Ventricular Function and Improvement of Left Ventricular Function After Da Silva Cone Procedure for Ebstein Anomaly. J. Cardiovasc. Dev. Dis. 2025, 12, 276. https://doi.org/10.3390/jcdd12070276
Sundaram K, Stoll V, Da Fonseca Da Silva L, Christopher A, Hoskoppal A, Kreutzer J, Liddle D, Olivieri L, Weinberg J, Dobson CP, et al. Mid-Term Recovery of Right Ventricular Function and Improvement of Left Ventricular Function After Da Silva Cone Procedure for Ebstein Anomaly. Journal of Cardiovascular Development and Disease. 2025; 12(7):276. https://doi.org/10.3390/jcdd12070276
Chicago/Turabian StyleSundaram, Krithika, Veenah Stoll, Luciana Da Fonseca Da Silva, Adam Christopher, Arvind Hoskoppal, Jacqueline Kreutzer, David Liddle, Laura Olivieri, Jacqueline Weinberg, Craig P. Dobson, and et al. 2025. "Mid-Term Recovery of Right Ventricular Function and Improvement of Left Ventricular Function After Da Silva Cone Procedure for Ebstein Anomaly" Journal of Cardiovascular Development and Disease 12, no. 7: 276. https://doi.org/10.3390/jcdd12070276
APA StyleSundaram, K., Stoll, V., Da Fonseca Da Silva, L., Christopher, A., Hoskoppal, A., Kreutzer, J., Liddle, D., Olivieri, L., Weinberg, J., Dobson, C. P., Da Silva, J. P., & Alsaied, T. (2025). Mid-Term Recovery of Right Ventricular Function and Improvement of Left Ventricular Function After Da Silva Cone Procedure for Ebstein Anomaly. Journal of Cardiovascular Development and Disease, 12(7), 276. https://doi.org/10.3390/jcdd12070276







