Higher Physical Activity Is Associated with Improved Ventricular–Arterial Coupling: Assessment Using the cfPWV/GLS Ratio in Primary Care—A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Assessment and Aortic Stiffness
2.3. Echocardiographic Parameters
2.4. Ventricular–Arterial Coupling (VAC) Assessment
2.5. Statistical Analysis
3. Results
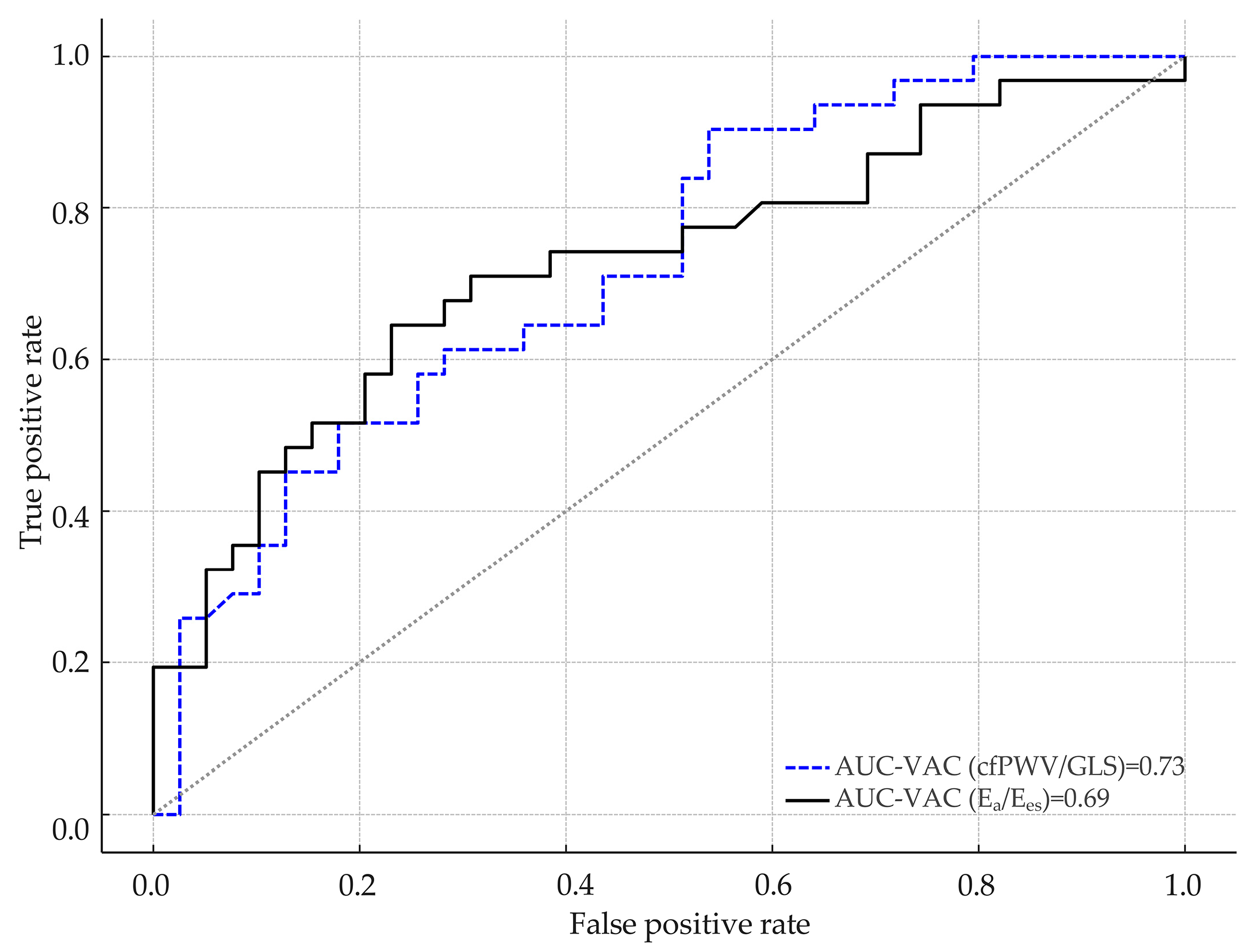
3.1. Comparative Analysis Between VAC Derived from Ea/Ees and the cfPWV/GLS Ratio
3.2. Comparative Assessment Between VAC and Echocardiographic Parameters
4. Discussion
4.1. Study Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2C | Apical two-chamber view |
| A3C | Apical three-chamber view |
| A4C | Apical four-chamber view |
| ABI | Ankle brachial index |
| AUC | Area under the curve |
| baPWV | Brachial–ankle pulse wave velocity |
| bfPWV | Brachial–femoral pulse wave velocity |
| BMI | Body mass index |
| BP | Blood pressure |
| BSA | Body surface area |
| CAD | Coronary artery disease |
| CAVI | Cardio–ankle vascular index |
| cfPWV | Carotid–femoral pulse wave velocity |
| CI | Confidence interval |
| CO | Cardiac output |
| CRF | Cardio-respiratory fitness |
| CVD | Cardiovascular disease |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| Ea | Arterial elastance |
| Ees | Left ventricular end-systolic elastance |
| eNOS | Endothelial nitric oxide synthase |
| GLS | Global longitudinal strain |
| HF | Heart failure |
| HFpEF | Heart failure with preserved ejection fraction |
| HTN | Hypertension |
| ICA | Invasive coronary angiography |
| IQR | Interquartile range |
| LAA | Left atrial area |
| LAESD | Left atrial end systolic diameter |
| LAV | Left atrial volume |
| LV | Left ventricle or left ventricular |
| LVEF | Left ventricular ejection fraction |
| LVESV | Left ventricular end-systolic volume |
| LVH | Left ventricular hypertrophy |
| LVPW | Left ventricular posterior wall |
| METs | Metabolic equivalents of task (plural) |
| NO | Nitric oxide |
| PAD | Peripheral artery disease |
| PES | End-systolic pressure |
| PWV | Pulse wave velocity |
| ROC | Receiver operating characteristic |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| SpO2 | Peripheral oxygen saturation |
| SV | Stroke volume |
| VAC | Ventricular–arterial coupling |
| VES | End-systolic volume |
References
- Hooglugt, A.; Klatt, O.; Huveneers, S. Vascular stiffening and endothelial dysfunction in atherosclerosis. Curr. Opin. Lipidol. 2022, 1, 353–363. [Google Scholar] [CrossRef]
- Benetos, A.; Waeber, B.; Izzo, J.; Mitchell, G.; Resnick, L.; Asmar, R.; Safar, M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: Clinical applications. Am. J. Hypertens. 2002, 15, 1101–1108. [Google Scholar] [CrossRef]
- Chauhan, D.; Vande Walle, L.; Lamkanfi, M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 2020, 297, 123–138. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 14, 849–867. [Google Scholar] [CrossRef]
- Humphrey, J.D. Mechanisms of Vascular Remodeling in Hypertension. Am. J. Hypertens. 2021, 22, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef]
- Avolio, A.P.; Van Bortel, L.M.; Boutouyrie, P.; Cockcroft, J.R.; McEniery, C.M.; Protogerou, A.D.; Roman, M.J.; Safar, M.E.; Segers, P.; Smulyan, H. Role of pulse pressure amplification in arterial hypertension: Experts’ opinion and review of the data. Hypertension 2009, 54, 375–383. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Bhattacharya, P.; Kumar, A.; Proto, E.; Konda, P.; Segers, P.; Akers, S.R.; Townsend, R.R.; Zamani, P. Impact of Diabetes Mellitus on Ventricular Structure, Arterial Stiffness, and Pulsatile Hemodynamics in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2019, 19, 011457. [Google Scholar] [CrossRef]
- Collins, B.N.; Nair, U.S.; Hovell, M.F.; DiSantis, K.I.; Jaffe, K.; Tolley, N.M.; Wileyto, E.P.; Audrain-McGovern, J. Reducing underserved children’s exposure to tobacco smoke: A randomized counseling trial with maternal smokers. Am. J. Prev. Med. 2015, 49, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Goksu, K.; Vural, A.; Kahraman, A.N.; Aslan, I.K. Evaluation of common carotid artery wall stiffness by shear wave elastography in smokers and non-smokers. Tob. Induc. Dis. 2024, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Boutouyrie, P.; Laurent, S. Vascular aging: A tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 2009, 54, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Boutouyrie, P.; Cunha, P.G.; Lacolley, P.; Nilsson, P.M. Concept of Extremes in Vascular Aging. Hypertension 2019, 74, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arter. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef]
- Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- Tang, C.J.; Lee, P.Y.; Chuang, Y.H.; Huang, C.C. Measurement of local pulse wave velocity for carotid artery by using an ultrasound-based method. Ultrasonics 2020, 102, 106064. [Google Scholar] [CrossRef] [PubMed]
- González Falcón, D.; Gómez-Sánchez, L.; Gómez-Sánchez, M.; Rodríguez-Sánchez, E.; Tamayo-Morales, O.; Lugones-Sánchez, C.; González-Sánchez, S.; Diaz, M.; Gómez-Marcos, M.A.; on behalf of the EVA Study Investigators. Evolution of arterial stiffness and association with cardiovascular risk factors in the Spanish population. Follow-Up EVA. Med. Clin. 2025, 164, 461–469. [Google Scholar] [CrossRef]
- Banegas, J.R.; Townsend, R.R. Arterial stiffness and reference values. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef]
- Kullo, I.J.; Rooke, T.W. Clinical Practice. Peripheral artery disease. N. Engl. J. Med. 2016, 374, 861–871. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Rosamond, W.D.; Chambless, L.E.; Nieto, F.J.; Barnes, R.W.; Hutchinson, R.G.; Tyroler, H.A.; Heiss, G.; ARIC Investigators. Lower extremity arterial disease assessed by ankle-brachial index in a middle-aged population of African Americans and whites: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Prev. Med. 2005, 29 (Suppl. S1), 42–49. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M. Sex Differences in the Ankle Brachial Index Measurement and Interpreting Findings of Sex Differences in Peripheral Artery Disease Burden. Circ. Cardiovasc. Qual. Outcomes 2016, 9 (Suppl. S1), S5–S7. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitão, C.; Gouveia, M.; Figueiredo, D.; Alves, A.J.; Polonia, J.; Oliveira, J.; et al. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J. Hypertens. 2021, 1, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.A.; Brinks, J.; Berra, K.; Lavie, C.J.; Gordon, N.F.; Sperling, L.S. Using Metabolic Equivalents in Clinical Practice. Am. J. Cardiol. 2018, 1, 382–387. [Google Scholar] [CrossRef]
- Ross, R.; Blair, S.N.; Arena, R.; Church, T.S.; Després, J.P.; Franklin, B.A.; Haskell, W.L.; Kaminsky, L.A.; Levine, B.D.; Lavie, C.J.; et al. American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: A Case for Fitness as a Clinical Vital Sign: A Scientific Statement From the American Heart Association. Circulation 2016, 13, 653–699. [Google Scholar] [CrossRef]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Katsanos, S.; Triantafyllidi, H.; Parissis, J.; Tzortzis, S.; Pavlidis, G.; Trivilou, P.; Makavos, G.; Varoudi, M.; Frogoudaki, A.; et al. Pulse wave velocity to global longitudinal strain ratio in hypertension. Eur. J. Clin. Investig. 2019, 49, 13049. [Google Scholar] [CrossRef]
- Tanaka, H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: Special focus on speckle-tracking longitudinal strain. J. Echocardiogr. 2021, 19, 71–79. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Gherbesi, E.; Gianstefani, S.; Angeli, F.; Ryabenko, K.; Bergamaschi, L.; Armillotta, M.; Guerra, E.; Tuttolomondo, D.; Gaibazzi, N.; Squeri, A.; et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: From physics to clinical practice. Echocardiography 2024, 41, e15753. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Holm, H.; Nilsson, P.M. Ventricular-arterial coupling: Definition, pathophysiology and therapeutic targets in cardiovascular disease. Expert Rev. Cardiovasc. Ther. 2021, 19, 753–761. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef]
- Chantler, P.D. Arterial Ventricular Uncoupling with Age and Disease and Recoupling with Exercise. Exerc. Sport Sci. Rev. 2017, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Monge Garcia, M.I.; Santos, A. Understanding ventriculo-arterial coupling. Ann. Transl. Med. 2020, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Fadl Elmula, F.M.; Antonini-Canterin, F. Noninvasive Assessment of Ventricular-Arterial Coupling in Heart Failure. Heart Fail. Clin. 2021, 17, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.R.; Ding, P.A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034. [Google Scholar] [CrossRef]
- Galderisi, M.; Lomoriello, V.S.; Santoro, A.; Esposito, R.; Olibet, M.; Raia, R.; Di Minno, M.N.; Guerra, G.; Mele, D.; Lombardi, G. Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: A speckle-tracking echocardiography study. J. Am. Soc. Echocardiogr. 2010, 23, 1190–1198. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, 498–504. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Ichihashi, S.; Desormais, I.; Hashimoto, T.; Magne, J.; Kichikawa, K.; Aboyans, V. Accuracy and Reliability of the Ankle Brachial Index Measurement Using a Multicuff Oscillometric Device Versus the Doppler Method. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 462–468. [Google Scholar] [CrossRef]
- Hageman, D.; van den Houten, M.M.L.; Pesser, N.; Gommans, L.N.M.; Scheltinga, M.R.M.; Teijink, J.A.W. Diagnostic accuracy of automated oscillometric determination of the ankle-brachial index in peripheral artery disease. J. Vasc. Surg. 2021, 73, 652–660. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, C.A. Age-related vascular stiffening: Causes and consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef]
- Bohn, L.; Ramoa, A.; Silva, G.; Silva, N.; Abreu, S.M.; Ribeiro, F.; Boutouyrie, P.; Laurent, S.; Oliveira, J. Sedentary Behavior and Arterial Stiffness in Adults with and without Metabolic Syndrome. Int. J. Sports Med. 2017, 38, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Topouchian, J.; Labat, C.; Gautier, S.; Bäck, M.; Achimastos, A.; Blacher, J.; Cwynar, M.; Sierra, A.; Pall, D.; Fantin, F.; et al. Effects of metabolic syndrome on arterial function in different age groups: The Advanced Approach to Arterial Stiffness study. J. Hypertens. 2018, 36, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.B.; Donley, D.A.; Bonner, D.E.; Devallance, E.; Olfert, I.M.; Chantler, P.D. Improved arterial-ventricular coupling in metabolic syndrome after exercise training: A pilot study. Med. Sci. Sports Exerc. 2015, 47, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Abell, B.; Glasziou, P.; Hoffmann, T. The Contribution of Individual Exercise Training Components to Clinical Outcomes in Randomised Controlled Trials of Cardiac Rehabilitation: A Systematic Review and Meta-regression. Sports Med. Open 2017, 3, 19. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.H.; Cruickshank, J.K.; et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, S.H.; Kang, S.H.; Kim, H.J.; Yoon, C.H.; Youn, T.J.; Chae, I.H. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur. Heart J. 2019, 14, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Fredericks, S.; Jones, I.; Neubeck, L.; Sanders, J.; De Stoutz, N.; Thompson, D.R.; Wadhwa, D.N.; Grace, S.L. Global perspectives on heart disease rehabilitation and secondary prevention: A scientific statement from the Association of Cardiovascular Nursing and Allied Professions, European Association of Preventive Cardiology, and International Council of Cardiovascular Prevention and Rehabilitation. Eur. Heart J. 2023, 44, 2515–2525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holm, H.; Magnusson, M.; Jujić, A.; Pugliese, N.R.; Bozec, E.; Lamiral, Z.; Huttin, O.; Zannad, F.; Rossignol, P.; Girerd, N. Ventricular-arterial coupling (VAC) in a population-based cohort of middle-aged individuals: The STANISLAS cohort. Atherosclerosis 2023, 374, 11–20. [Google Scholar] [CrossRef]
- Stoichescu-Hogea, G.; Buleu, F.N.; Nicusor Pop, G.; Duda-Seiman, D.; Ember, A.; Tudor, A.; Baneu, P.; Kundnani, N.R.; Christodorescu, R.; Drăgan, S. Ventricular-arterial coupling assessed by PWV/GLS ratio in hypertensive patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7024–7035. [Google Scholar] [CrossRef]
- Murray, J.; Bennett, H.; Bezak, E.; Perry, R.; Boyle, T. The effect of exercise on left ventricular global longitudinal strain. Eur. J. Appl. Physiol. 2022, 122, 1397–1408. [Google Scholar] [CrossRef]
- Matsuo, R.; Ikeda, A.; Tomooka, K.; Naito, Y.; Uesugi, Y.; Maruyama, K.; Kawamura, R.; Takata, Y.; Osawa, H.; Saito, I.; et al. Longitudinal Changes in Arterial Stiffness Associated with Physical Activity Intensity: The Toon Health Study. J. Atheroscler. Thromb. 2024, 31, 171–179. [Google Scholar] [CrossRef]
- Duman, O.O.; Goldeli, O.; Gursul, E.; Baris, N.; Ozpelit, E.; Simsek, M.A. The value of aortic pulse wave velocity in predicting coronary artery disease diagnosis and severity. Acta Cardiol. 2015, 70, 315–322. [Google Scholar] [CrossRef]
- Hofmann, B.; Riemer, M.; Erbs, C.; Plehn, A.; Navarrete, S.A.; Wienke, A.; Silber, R.E.; Simm, A. Carotid to femoral pulse wave velocity reflects the extent of coronary artery disease. J. Clin. Hypertens. 2014, 16, 629–633. [Google Scholar] [CrossRef]
- De Silva, D.A.; Woon, F.P.; Gan, H.Y.; Chen, C.P.; Chang, H.M.; Koh, T.H.; Kingwell, B.A.; Cameron, J.D.; Wong, M.C. Arterial stiffness is associated with intracranial large artery disease among ethnic Chinese and South Asian ischemic stroke patients. J. Hypertens. 2009, 27, 1453–1458. [Google Scholar] [CrossRef]
- Lind, L.; Sundström, J.; Ärnlöv, J.; Risérus, U.; Lampa, E. A longitudinal study over 40 years to study the metabolic syndrome as a risk factor for cardiovascular diseases. Sci. Rep. 2021, 11, 2978–2987. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, S.; Marais, L.; Khettab, H.; Poli, F.; Li, Y.; Segers, P.; Aasmul, S.; de Melis, M.; Baets, R.; Greenwald, S.; et al. Clinical Validation of Carotid-Femoral Pulse Wave Velocity Measurement Using a Multi-Beam Laser Vibrometer: The CARDIS Study. Hypertension 2024, 81, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, L.; Iancu, D.G.; Marusteri, M.S.; Tilea, I.; Varga, A. Proposed Novel Heart Failure Biomarkers and Their Association with Length of Hospital Stay and Mortality: A Retrospective Observational Pilot Study. Diagnostics 2025, 15, 589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varga, A.; Cristescu, L.; Marusteri, M.S.; Mares, R.G.; Iancu, D.G.; Suteu, R.A.; Tilinca, R.M.; Tilea, I. Prognostic Value of the Red Cell Distribution Width-to-eGFR Ratio (RGR) Across Chronic Heart Failure Phenotypes: A Retrospective Observational Pilot Study. J. Clin. Med. 2025, 14, 2852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Characteristics | Total (n = 81) | Sedentary (n = 39) | Physical Active (n = 42) | p Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 50.27 ± 12.93 | 57.46 ± 11.83 | 43.60 ± 10.09 | <0.001 |
| Female (n, %) | 49 (60.5) | 26 (66.67) | 23 (54.76) | ns |
| Urban (n, %) | 57 (70.37) | 26 (66.67) | 31 (73.81) | ns |
| Baseline risk factors | ||||
| Abdominal circumference, cm (mean ± SD) | 101.50 ± 14.43 | 106.30 ± 14.48 | 94.12 ± 10.89 | <0.001 |
| BMI, kg/m2 (mean ± SD) | 28.41 ± 5.78 | 30.19 ± 6.09 | 25.56 ± 3.82 | <0.001 |
| BSA, m2 (mean ± SD) | 1.92 ± 0.24 | 1.95 ± 0.24 | 1.88 ± 0.24 | ns |
| HTN (mmHg, %) | 38 (46.91) | 26 (66.67) | 12 (28.57) | 0.001 |
| DM (n, %) | 6 (7.41) | 6 (15.38) | 0 (0) | 0.030 |
| Active smoking (n, %) | 19 (23.46) | 8 (20.51) | 11 (26.19) | ns |
| Cardiovascular parameters | ||||
| SBP, mmHg (mean ± SD) | 124.36 ± 15.66 | 128.69 ± 16.77 | 120.98 ± 14.02 | 0.036 |
| DBP, mmHg (mean ± SD) | 76.57 ± 9.77 | 76.60 ± 9.42 | 76.51 ± 10.40 | ns |
| SpO2 (mean ± SD) | 0.97 ± 0.02 | 0.96 ± 0.03 | 0.98 ± 0.01 | <0.001 |
| cfPWV, m/s (mean ± SD) | 8.75 ± 1.70 | 9.30 ± 1.82 | 8.13 ± 1.31 | 0.005 |
| LVPW, mm (median, IQR) | 9.0 (8.0–10.0) | 9.0 (9.0–10.0) | 9.0 (8.0–10.0) | ns |
| LVESV, mL (mean ± SD) | 41.76 ± 13.60 | 42.92 ± 14.45 | 40.85 ± 13.01 | ns |
| SV, mL (mean ± SD) | 54.90 ± 15.80 | 51.61 ± 14.42 | 57.46 ± 16.52 | ns |
| CO, L/min (median, IQR) | 3.7 (3.1–4.4) | 3.6 (2.8–4.4) | 3.9 (3.3–4.5) | ns |
| Ea (median, IQR) | 2.24 (1.91–2.85) | 2.71 (2.04–3.13) | 2.17 (1.85–2.49) | 0.013 |
| Ees (median, IQR) | 3.10 (2.52–3.93) | 3.22 (2.44–4.00) | 3.08 (2.53–3.83) | ns |
| GLS A3C (mean ± SD) | 18.92 ± 3.18 | 18.19 ± 3.37 | 19.45 ± 2.95 | ns |
| GLS A4C (mean ± SD) | 18.11 ± 2.98 | 17.61 ± 3.25 | 18.47 ± 2.74 | ns |
| GLS A2C (mean ± SD) | 19.77 ± 3.18 | 18.13 ± 2.98 | 21.01 ± 2.76 | <0.001 |
| GLS, % (mean ± SD) | 17.52 ± 4.75 | 15.63 ± 5.50 | 19.54 ± 2.59 | <0.001 |
| (mean ± SD) | 0.77 ± 0.20 | 0.85 ± 0.24 | 0.72 ± 0.13 | 0.007 |
| VAC (cfPWV/GLS ratio) (median, IQR) | 0.43 (0.38–0.53) | 0.49 (0.40–0.62) | 0.40 (0.35–0.45) | 0.001 |
| Characteristics | Total Physical Active Patients (n = 42) | G2 (n = 2) | G3 (n = 23) | G4 (n = 17) | p Value |
|---|---|---|---|---|---|
| Age, years (mean ± SD) | 43.60 ± 10.09 | 46.00 ± 25.46 | 45.26 ± 8.63 | 41.06 ± 10.32 | ns |
| Female (n, %) | 23 (54.76) | 1 (50.00) | 16 (69.57) | 6 (35.29) | <0.001 |
| Urban (n, %) | 31 (73.81) | 2 (100.00) | 15 (65.22) | 14 (82.35) | ns |
| Baseline risk factors | |||||
| Abdominal circumference, cm (mean ± SD) | 93.73 ± 10.26 | 85.75 ± 6.72 | 93.85 ± 9.84 | 94.50 ± 11.20 | ns |
| BMI, kg/m2 (mean ± SD) | 25.43 ± 3.50 | 24.00 ± 2.12 | 24.68 ± 3.62 | 26.61 ± 3.25 | ns |
| BSA, m2 (mean ± SD) | 1.87 ± 0.24 | 1.80 ± 0.24 | 1.80 ± 0.21 | 1.98 ± 0.25 | 0.049 |
| HTN (mmHg, %) | 12 (28.57) | 1 (50.00) | 6 (26.09) | 5 (29.41) | ns |
| Current smoking (n, %) | 8 (19.05) | 1 (50.00) | 5 (21.74) | 2 (11.76) | ns |
| Cardiovascular parameters | |||||
| SBP, mmHg (mean ± SD) | 120.93 ± 13.86 | 136.00 ± 28.28 | 116.91 ± 13.02 | 124.59 ± 11.95 | ns |
| DBP, mmHg (mean ± SD) | 77.00 ± 9.90 | 80.00 ± 19.80 | 76.57 ± 10.26 | 77.24 ± 8.98 | ns |
| SpO2 (mean ± SD) | 0.98 ± 0.01 | 0.98 ± 0.02 | 0.98 ± 0.01 | 0.98 ± 0.01 | ns |
| cfPWV, m/s (mean ± SD) | 8.11 ± 1.29 | 8.00 ± 0.85 | 8.26 ± 1.41 | 7.93 ± 1.20 | ns |
| LVPW, mm (mean ± SD) | 9.14 ± 1.34 | 8.00 ± 1.41 | 8.96 ± 1.22 | 9.53 ± 1.42 | ns |
| LVESV, mL (median, IQR) | 40.85 ± 13.01 | 42.50 ± 19.09 | 36.61 ± 10.92 | 46.75 ± 13.68 | ns |
| SV, mL (mean ± SD) | 57.90 ± 16.49 | 61.50 ± 13.44 | 50.82 ± 13.80 | 67.19 ± 16.15 | 0.006 |
| CO, L/min (median, IQR) | 3.96 ± 1.09 | 4.10 ± 0.14 | 3.65 ± 1.01 | 4.38 ± 1.16 | ns |
| Ea (mean ± SD) | 2.25 ± 0.62 | 2.21 ± 0.02 | 2.45 ± 0.62 | 1.97 ± 0.58 | 0.05 |
| Ees (mean ± SD) | 3.21 ± 0.91 | 3.39 ± 0.86 | 3.42 ± 0.92 | 2.88 ± 0.87 | ns |
| GLS A3C (mean ± SD) | 19.45 ± 2.95 | 18.65 ± 4.31 | 19.32 ± 2.69 | 19.73 ± 3.32 | ns |
| GLS A4C (mean ± SD) | 18.47 ± 2.74 | 17.30 ± 2.83 | 18.67 ± 2.64 | 18.35 ± 2.99 | ns |
| GLS A2C (mean ± SD) | 21.01 ± 2.76 | 19.95 ± 2.19 | 21.05 ± 2.86 | 21.09 ± 2.81 | ns |
| GLS, % (mean ± SD) | 19.77 ± 2.40 | 18.65 ± 3.04 | 19.91 ± 2.23 | 19.73 ± 2.65 | ns |
| (mean ± SD) | 0.72 ± 0.13 | 0.67 ± 0.16 | 0.74 ± 0.15 | 0.69 ± 0.10 | ns |
| VAC (cfPWV/GLS ratio) (mean ± SD) | 0.42 ± 0.10 | 0.44 ± 0.12 | 0.42 ± 0.10 | 0.41 ± 0.11 | ns |
| Parameter | G1 (n = 39) | G2 (n = 2) | G3 (n = 23) | G4 (n = 17) | p Value |
|---|---|---|---|---|---|
| GLS A3C (mean ± SD) | 18.19 ± 3.37 | 18.65 ± 4.31 | 19.32 ± 2.69 | 19.73 ± 3.32 | ns * |
| GLS A4C (mean ± SD) | 17.61 ± 3.25 | 17.3 ± 2.83 | 18.67 ± 2.64 | 18.35 ± 2.99 | ns * |
| GLS A2C (mean ± SD) | 18.13 ± 2.98 | 19.95 ± 2.19 | 21.05 ± 2.86 | 21.09 ± 2.81 | 0.001 * |
| GLS average (mean ± SD) | 17.99 ± 2.64 | 18.65 ± 3.04 | 19.91 ± 2.23 | 19.73 ± 2.65 | 0.035 * |
| LVEF, % (median, IQR) | 55.00 (52.00–57.50) | 60.00 (58.00–62.00) | 59.50 (56.00–61.00) | 58.00 (56.25–62.75) | 0.002 ** |
| cfPWV (m/s) (median, IQR) | 9.00 (8.10–9.80) | 8.00 (7.70–8.30) | 7.90 (7.15–9.25) | 7.65 (7.32–8.92) | 0.021 ** |
| , (median, IQR) | 0.85 (0.69–0.94) | 0.67 (0.62–0.73) | 0.73 (0.64–0.80) | 0.71 (0.62–0.79) | 0.027 ** |
| VAC (cfPWV/GLS ratio), (median, IQR) | 0.49 (0.40–0.62) | 0.43 (0.39–0.47) | 0.40 (0.35–0.45) | 0.39 (0.34–0.43) | 0.013 ** |
| Dunn’s Multiple Comparison Test | Mean Rank Difference | Adjusted p Value |
|---|---|---|
| G1 vs. G2 | 11.060 | 0.999 |
| G1 vs. G3 | 15.520 | 0.046 |
| G1 vs. G4 | 17.390 | 0.031 |
| G2 vs. G3 | 4.452 | >0.999 |
| G2 vs. G4 | 6.324 | >0.999 |
| G3 vs. G4 | 1.871 | >0.999 |
| cfPWV/GLS Ratio | Estimated Coefficient | p Value |
|---|---|---|
| Intercept (β0) | 0.809 | ns |
| Sex (female) (β1) | −0.016 | ns |
| Right ABI (β2) | −0.281 | 0.049 |
| Left ABI (β3) | 0.393 | 0.009 |
| DBP (mmHg) (β4) | 0.002 | ns |
| Arterial age (years) (β5) | 0.006 | <0.001 |
| SpO2 (β6) | −0.010 | 0.027 |
| HTN (β7) | −0.052 | 0.003 |
| DM (β8) | 0.095 | 0.032 |
| BMI (β9) | 0.003 | ns |
| LVPW (β10) | 0.002 | 0.002 |
| CO (β11) | −0.001 | ns |
| r2 | 0.81 | |
| Parameters | VAC (Ea/Ees) | VAC (cfPWV/GLS) | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Age | 0.38 | 0.005 | 0.52 | <0.001 |
| Abdominal circumference | 0.36 | 0.003 | 0.32 | 0.008 |
| BMI | 0.34 | 0.004 | 0.28 | 0.018 |
| BSA | 0.19 | 0.110 | 0.24 | 0.040 |
| METs | −0.37 | 0.002 | −0.34 | 0.004 |
| Variable | Coefficient | Standard Error | Odds Ratio | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|---|---|
| Intercept | −50.673 | 25.953 | 0.000 | 0.000 | 1.215 | 0.051 |
| Female sex | −1.573 | 0.762 | 0.207 | 0.047 | 0.922 | 0.039 |
| Age | 0.127 | 0.041 | 1.136 | 1.048 | 1.232 | 0.002 |
| Smoker | 0.277 | 0.714 | 1.319 | 0.325 | 5.345 | 0.699 |
| Treated hypertension | −0.520 | 0.803 | 0.595 | 0.123 | 2.869 | 0.517 |
| Treated DM | −0.243 | 1.351 | 0.784 | 0.052 | 11.769 | 0.857 |
| BMI | 0.140 | 0.122 | 1.150 | 0.904 | 1.462 | 0.250 |
| SpO2 | 0.087 | 0.109 | 1.091 | 0.878 | 1.357 | 0.424 |
| Parameters | VAC (Ea/Ees) | VAC (cfPWV/GLS) | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| LAESD | 0.24 | ns | 0.19 | ns |
| LAA | 0.18 | ns | 0.07 | ns |
| LAV | 0.26 | 0.041 | −0.02 | ns |
| E/A ratio | −0.41 | 0.001 | −0.61 | <0.001 |
| Maximum E velocity | −0.37 | 0.002 | −0.44 | <0.001 |
| Maximum e’ velocity (septal) | −0.55 | <0.001 | −0.71 | <0.001 |
| E/e’ (septal) | 0.33 | 0.008 | 0.39 | 0.002 |
| Maximum e’ velocity (lateral) | −0.33 | 0.008 | −0.49 | <0.001 |
| Lateral E/e’ | 0.07 | ns | 0.17 | ns |
| Maximum e’ velocity (mean) | −0.46 | <0.001 | −0.61 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulea, P.-A.; Tilea, I.; Stoica, F.; Cristescu, L.; Moldovan, D.-A.; Tatar, R.; Tilinca, R.-M.; Mares, R.G.; Varga, A. Higher Physical Activity Is Associated with Improved Ventricular–Arterial Coupling: Assessment Using the cfPWV/GLS Ratio in Primary Care—A Pilot Study. J. Cardiovasc. Dev. Dis. 2025, 12, 208. https://doi.org/10.3390/jcdd12060208
Sulea P-A, Tilea I, Stoica F, Cristescu L, Moldovan D-A, Tatar R, Tilinca R-M, Mares RG, Varga A. Higher Physical Activity Is Associated with Improved Ventricular–Arterial Coupling: Assessment Using the cfPWV/GLS Ratio in Primary Care—A Pilot Study. Journal of Cardiovascular Development and Disease. 2025; 12(6):208. https://doi.org/10.3390/jcdd12060208
Chicago/Turabian StyleSulea, Paula-Anca, Ioan Tilea, Florin Stoica, Liviu Cristescu, Diana-Andreea Moldovan, Radu Tatar, Raluca-Maria Tilinca, Razvan Gheorghita Mares, and Andreea Varga. 2025. "Higher Physical Activity Is Associated with Improved Ventricular–Arterial Coupling: Assessment Using the cfPWV/GLS Ratio in Primary Care—A Pilot Study" Journal of Cardiovascular Development and Disease 12, no. 6: 208. https://doi.org/10.3390/jcdd12060208
APA StyleSulea, P.-A., Tilea, I., Stoica, F., Cristescu, L., Moldovan, D.-A., Tatar, R., Tilinca, R.-M., Mares, R. G., & Varga, A. (2025). Higher Physical Activity Is Associated with Improved Ventricular–Arterial Coupling: Assessment Using the cfPWV/GLS Ratio in Primary Care—A Pilot Study. Journal of Cardiovascular Development and Disease, 12(6), 208. https://doi.org/10.3390/jcdd12060208









