Isolated Rapid Deployment Aortic Valve Replacement in Patients with Aortic Stenosis: Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Surgical Procedure
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgery and Postoperative Outcomes
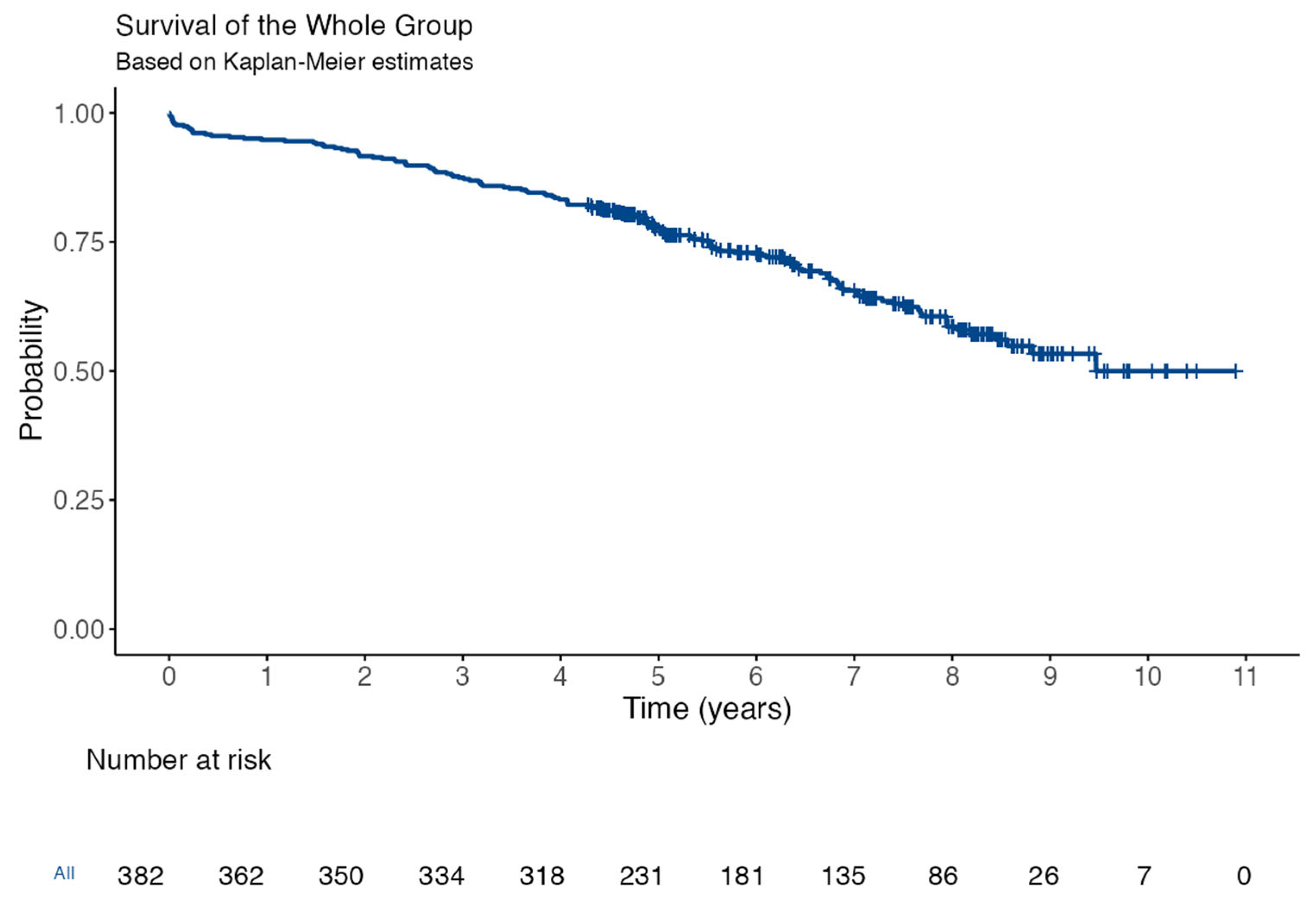
3.3. Follow-Up
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ancona’, ’Roberta; Pinto’, ’Salvatore Comenale Epidemiology of Aortic Valve Stenosis (AS) and of Aortic Valve Incompetence (AI): Is the Prevalence of AS/AI Similar in Different Parts of the World? Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-18/epidemiology-of-aortic-valve-stenosis-as-and-of-aortic-valve-incompetence-ai (accessed on 16 August 2024).
- Sohn, B.; Choi, J.W.; Hwang, H.Y.; Kim, K.H.; Kim, K.-B. Aortic Valve Replacement for Aortic Stenosis in Elderly Patients (75 Years or Older). Korean J. Thorac. Cardiovasc. Surg. 2018, 51, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Velho, T.R.; Pereira, R.M.; Pedroso, D.; Draiblate, B.; Constantino, S.; Nobre, Â.; Almeida, A.G.; Moita, L.F.; Pinto, F. Growth Differentiation Factor 15 as a Biomarker for Risk Stratification in the Cardiothoracic Surgery Intensive Care Unit. Biomolecules 2024, 14, 1593. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.; Wallner, S.; Habertheuer, A.; Rath, C.; Schauperl, M.; Binder, T.; Beitzke, D.; Rosenhek, R.; Loewe, C.; Wiedemann, D.; et al. Conventional versus rapid-deployment aortic valve replacement: A single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 799–805. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Salizzoni, S.; Filippini, C.; Tessari, C.; Bagozzi, L.; Messina, A.; Troise, G.; Tomba, M.D.; Rambaldini, M.; Dalén, M.; et al. Surgical aortic valve replacement with new-generation bioprostheses: Sutureless versus rapid-deployment. J. Thorac. Cardiovasc. Surg. 2020, 159, 432–442.e1. [Google Scholar] [CrossRef]
- Sakata, T.; De La Pena, C.; Ohira, S. Rapid-Deployment Aortic Valve Replacement: Patient Selection and Special Considerations. Vasc. Health Risk Manag. 2023, 19, 169–180. [Google Scholar] [CrossRef]
- Ferreira, R.; Rua, N.; Sena, A.; Velho, T.R.; Gonçalves, J.; Junqueira, N.; Almeida, A.G.; Nobre, A.; Pinto, F. Sutureless bioprosthesis for aortic valve replacement: Surgical and clinical outcomes. J. Card. Surg. 2022, 37, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, R.; Borger, M.A.; Briffa, N.P.; Jahangiri, M. Sutureless aortic valve prostheses. Heart 2019, 105, S16–S20. [Google Scholar] [CrossRef]
- 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Valvular-Heart-Disease-Guidelines (accessed on 14 December 2024).
- Pibarot, P.; Dumesnil, J.G. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart 2006, 92, 1022–1029. [Google Scholar] [CrossRef]
- Hahn, R.T.; Pibarot, P. Prosthesis-patient mismatch in transcatheter and surgical aortic valve replacement. Ann. Cardiothorac. Surg. 2024, 13, 21123–21223. [Google Scholar] [CrossRef]
- Committee, V.-W.; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Aladwan, H.; Alshoubaki, W.; Alqaisi, A.I.; Abuamereh, H.A.; Alatoum, L.M.; Mohd, A.F. Impact of duration of cardiopulmonary bypass on recovery after open heart surgery. Int. J. Adv. Med. 2024, 11, 185–188. [Google Scholar] [CrossRef]
- Barnhart, G.R.; Accola, K.D.; Grossi, E.A.; Woo, Y.J.; Mumtaz, M.A.; Sabik, J.F.; Slachman, F.N.; Patel, H.J.; Borger, M.A.; Garrett, H.E.; et al. TRANSFORM (Multicenter Experience With Rapid Deployment Edwards INTUITY Valve System for Aortic Valve Replacement) US clinical trial: Performance of a rapid deployment aortic valve. J. Thorac. Cardiovasc. Surg. 2017, 153, 241–251.e2. [Google Scholar] [CrossRef]
- Micovic, S.; Nobre, A.; Choi, J.W.; Solinas, M.; Shehada, S.-E.; Torella, M.; Baeza, C.; Parrino, E.; Pollari, F.; Troise, G.; et al. Early outcomes of aortic valve replacement with Perceval PLUS sutureless valve: Results of the prospective multicentric MANTRA study. J. Cardiothorac. Surg. 2024, 19, 340. [Google Scholar] [CrossRef]
- Fischlein, T.; Folliguet, T.; Meuris, B.; Shrestha, M.L.; Roselli, E.E.; McGlothlin, A.; Kappert, U.; Pfeiffer, S.; Corbi, P.; Lorusso, R.; et al. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2021, 161, 920–932. [Google Scholar] [CrossRef]
- Glauber, M.; Miceli, A.; Di Bacco, L. Sutureless and rapid deployment valves: Implantation technique from A to Z—The INTUITY Elite valve. Ann. Cardiothorac. Surg. 2020, 9, 417–423. [Google Scholar] [CrossRef]
- Di Bacco, L.; D’Alonzo, M.; Baudo, M.; Montisci, A.; Di Eusanio, M.; Folliguet, T.; Solinas, M.; Miceli, A.; Fischlein, T.; Rosati, F.; et al. Reliability of EuroSCORE II on Prediction of Thirty-Day Mortality and Long-Term Results in Patients Treated with Sutureless Valves. J. Clin. Med. 2024, 13, 3986. [Google Scholar] [CrossRef]
- Liu, D.; Liu, B.; Liang, Z.; Yang, Z.; Ma, F.; Yang, Y.; Hu, W. Acute Kidney Injury following Cardiopulmonary Bypass: A Challenging Picture. Oxid. Med. Cell Longev. 2021, 2021, 8873581. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.; Doyle, M.; Hong, R.; Manganas, C.; Peeceeyen, S. Octogenarians and aortic valve surgery: Surgical outcomes in the geriatric population. Indian. J. Thorac. Cardiovasc. Surg. 2020, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.W.; Lancaster, T.S.; Schuessler, R.B.; Melby, S.J. Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur. J. Cardio-Thorac. Surg. 2017, 52, 665–672. [Google Scholar] [CrossRef]
- Ullah, W.; Zahid, S.; Zaidi, S.R.; Sarvepalli, D.; Haq, S.; Roomi, S.; Mukhtar, M.; Khan, M.A.; Gowda, S.N.; Ruggiero, N.; et al. Predictors of Permanent Pacemaker Implantation in Patients Undergoing Transcatheter Aortic Valve Replacement—A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e020906. [Google Scholar] [CrossRef]
- Fabre, O.; Radutoiu, M.; Carjaliu, I.; Rebet, O.; Gautier, L.; Hysi, I. Recent improvement in operative techniques lead to lower pacemaker rate after Perceval implant. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac182. [Google Scholar] [CrossRef] [PubMed]
- Rück, A.; Saleh, N.; Glaser, N. Outcomes Following Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement: SWEDEHEART Observational Study. JACC Cardiovasc. Interv. 2021, 14, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- 2015 ESC Guidelines for the Management of Infective Endocarditis | European Heart Journal | Oxford Academic. Available online: https://academic.oup.com/eurheartj/article/36/44/3075/2293384 (accessed on 20 October 2024).
- García, E.B.; Camazón, N.V.; Pruñonosa, L.M.; Carrasco, S.L.; Bayés-Genís, A.; Guijosa, C.M. Endocarditis on a Perceval S sutureless prosthesis. A new valve with a new form of clinical presentation. Rev. Española De Cardiol. (Engl. Ed.) 2021, 74, 635–637. [Google Scholar] [CrossRef]
- Gargiulo, G.; Sannino, A.; Capodanno, D.; Barbanti, M.; Buccheri, S.; Perrino, C.; Capranzano, P.; Indolfi, C.; Trimarco, B.; Tamburino, C.; et al. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 165, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Talanas, G.; Laconi, A.; Kereiakes, D.J.; Merella, P.; Reardon, M.J.; Spano, A.; Petretto, G.; Lauriola, F.; Casula, M.; Micheluzzi, V.; et al. Long-Term Outcomes of Transcatheter vs Surgical Aortic Valve Replacement: Meta-analysis of Randomized Trials. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102143. [Google Scholar] [CrossRef]
- Avvedimento, M.; Tang, G.H.L. Commissural alignment in TAVI: A new frontier to facilitate coronary reaccess and Redo TAVI. Mini-Invasive Surg. 2022, 6, 24. [Google Scholar] [CrossRef]
- Ferrara, J.; Deharo, P.; Resseguier, N.; Porto, A.; Jaussaud, N.; Morera, P.; Lavagna, F.; Amanatiou, C.; Lambert, M.; Gariboldi, V.; et al. Rapid deployment aortic-valve replacement versus trans-catheter aortic-valve replacement in intermediate-risk patients: A propensity score analysis. Arch. Cardiovasc. Dis. Suppl. 2020, 12, 90. [Google Scholar] [CrossRef]
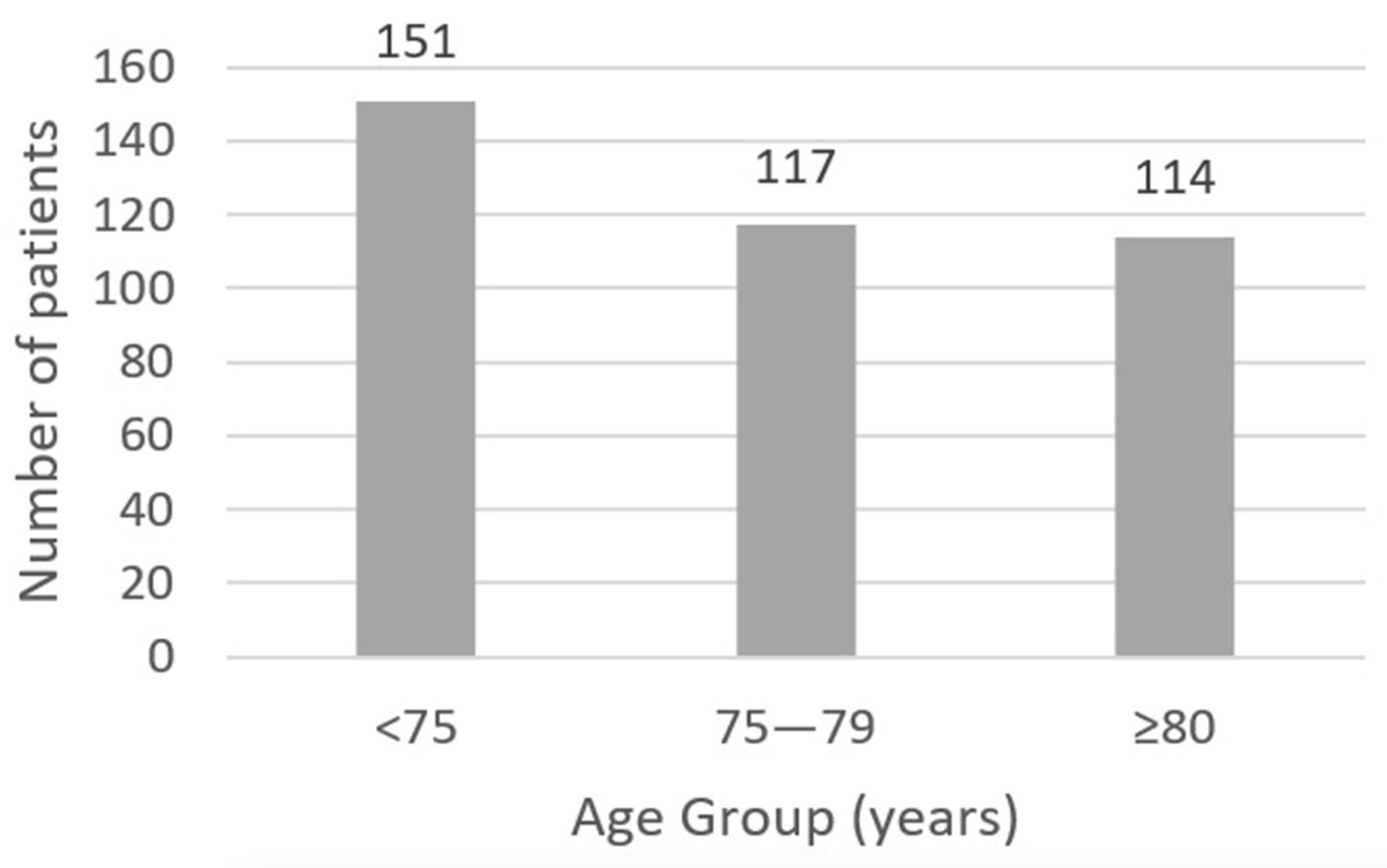
| N | 382 |
|---|---|
| Age (years), mean ± SD | 75.6 ± 5.9 |
| Male Sex, n (%) | 190 (50.0) |
| Euroscore II (%), mean ± SD | 2.3 ± 1.5 |
| Diabetes Mellitus (DM), n (%) | 146 (38.2) |
| Insulin-Treated DM, n (%) | 17 (4.4) |
| Atrial Fibrillation, n (%) | 83 (21.7) |
| Previous Pacemaker, n (%) | 15 (3.9) |
| Impaired Kidney Function, n (%) | 313 (81.9) |
| Hemodialysis, n (%) | 8 (2.1) |
| Respiratory Disease *, n (%) | 84 (21.9) |
| Smoker|Ex-Smoker, n (%) | 96 (25.1)|86 (22.5) |
| Carotid Disease **, n (%) | 39 (10.2) |
| Cerebrovascular Disease ***, n (%) | 29 (7.5) |
| LVEF > 50%, n (%) | 314 (82.1) |
| Postoperative Outcomes | N = 382 |
|---|---|
| Length of Stay | |
| ICU Stay (days), median [IQR] (mean ± SD) | 3 [2–4] (3.0 ± 2.5) |
| Total Hospital Stay (days), median [IQR] (mean ± SD) | 6 [4–8] (6.6 ± 3.8) |
| Postoperative Complications | |
| Hemodynamic Support > 24 h †, n (%) | 93 (24.3) |
| Acute Renal Injury (AKI, any stage), n (%) | 204 (53.4) |
| AKI (AKIN ≥ 2), n (%) | 35 (9.2) |
| Renal Replacement Therapy, n (%) | 9 (2.3) |
| De Novo Atrial Fibrillation, n (%) | 92/299 (31.9) |
| Definitive Pacemaker Implantation, n (%) | 36/367 (9.8) |
| Stroke, n (%) | 2 (0.5) |
| Seizures, n (%) | 8 (2.0) |
| Infection ‡, n (%) | 26 (6.8) |
| Significative Bleeding §, n (%) | 57 (14.9) |
| Reoperation for Bleeding, n (%) | 11 (2.8) |
| Mortality | |
| In-Hospital Mortality, n (%) | 4 (1.02) |
| 30-Day Mortality, n (%) | 9 (2.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.; Velho, T.R.; Gonçalves, J.; Sena, A.; Draiblate, B.; Almeida, A.G.; Nobre, Â.; Pinto, F. Isolated Rapid Deployment Aortic Valve Replacement in Patients with Aortic Stenosis: Single-Center Retrospective Study. J. Cardiovasc. Dev. Dis. 2025, 12, 191. https://doi.org/10.3390/jcdd12050191
Ferreira R, Velho TR, Gonçalves J, Sena A, Draiblate B, Almeida AG, Nobre Â, Pinto F. Isolated Rapid Deployment Aortic Valve Replacement in Patients with Aortic Stenosis: Single-Center Retrospective Study. Journal of Cardiovascular Development and Disease. 2025; 12(5):191. https://doi.org/10.3390/jcdd12050191
Chicago/Turabian StyleFerreira, Ricardo, Tiago R. Velho, João Gonçalves, André Sena, Beatriz Draiblate, Ana G. Almeida, Ângelo Nobre, and Fausto Pinto. 2025. "Isolated Rapid Deployment Aortic Valve Replacement in Patients with Aortic Stenosis: Single-Center Retrospective Study" Journal of Cardiovascular Development and Disease 12, no. 5: 191. https://doi.org/10.3390/jcdd12050191
APA StyleFerreira, R., Velho, T. R., Gonçalves, J., Sena, A., Draiblate, B., Almeida, A. G., Nobre, Â., & Pinto, F. (2025). Isolated Rapid Deployment Aortic Valve Replacement in Patients with Aortic Stenosis: Single-Center Retrospective Study. Journal of Cardiovascular Development and Disease, 12(5), 191. https://doi.org/10.3390/jcdd12050191







