The Role of Cardiac Magnetic Resonance Imaging in the Management of Hypertrophic Cardiomyopathy
Abstract
1. Introduction
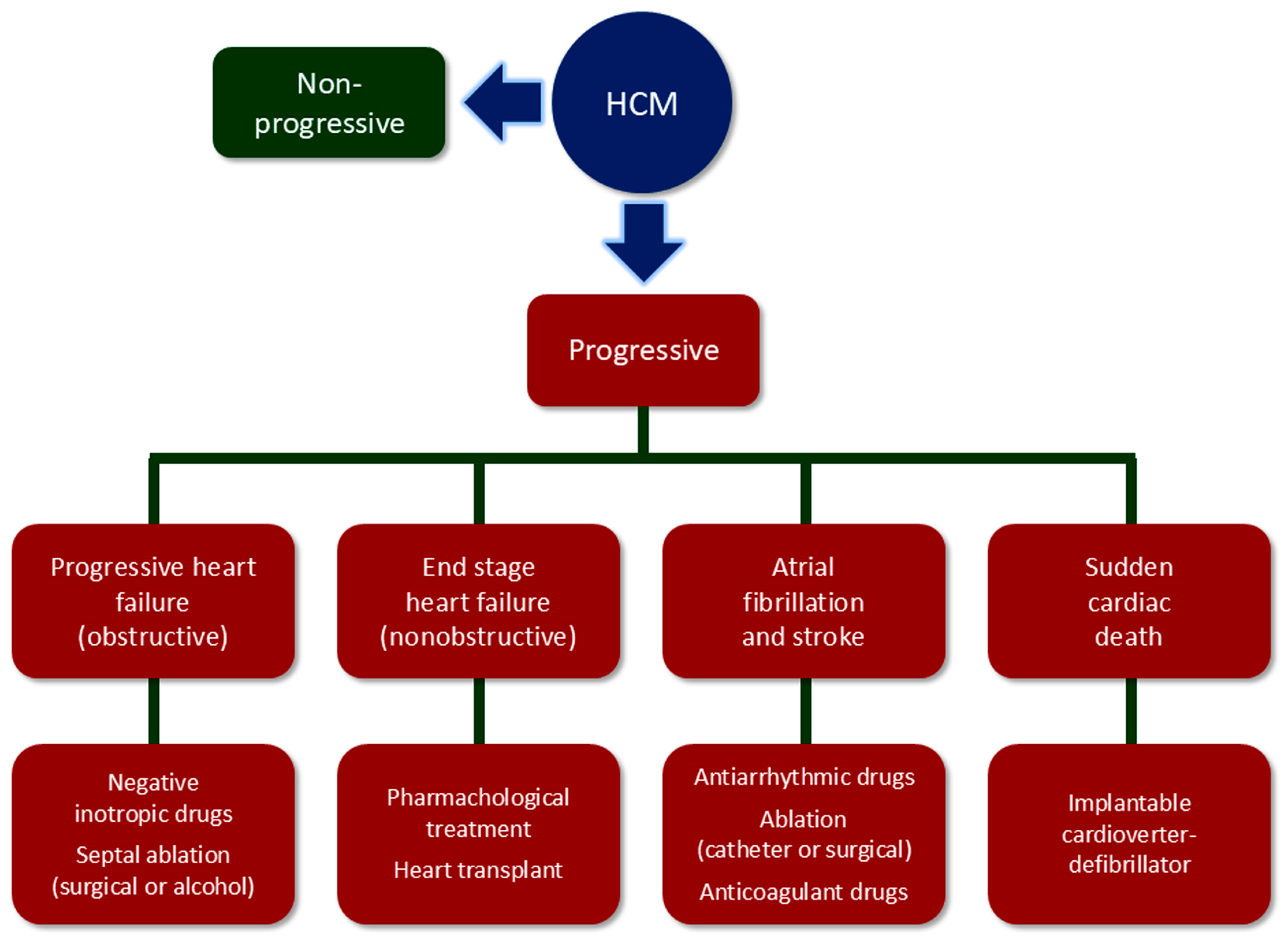
2. Multifaceted Nature of Hypertrophic Cardiomyopathy (HCM)
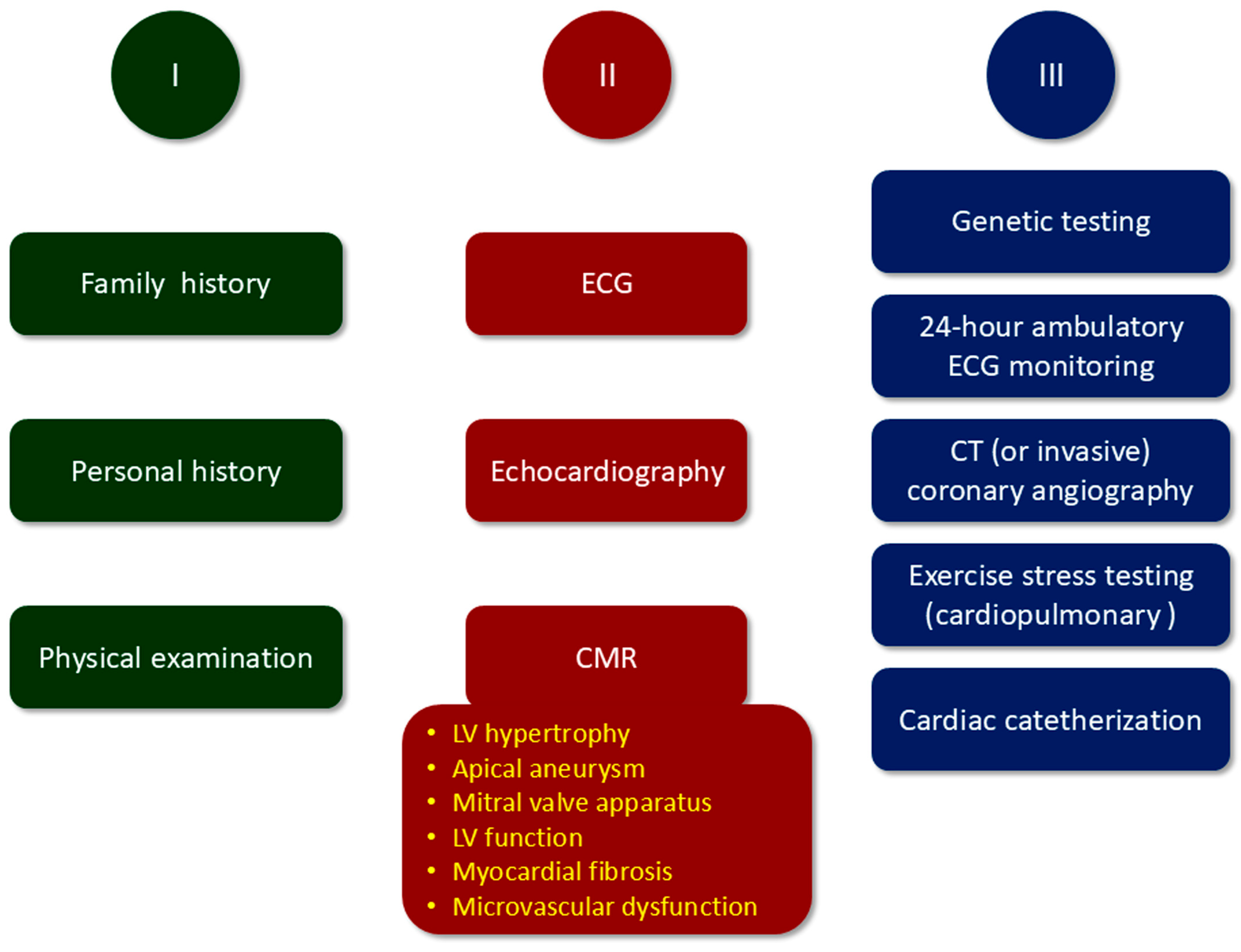
3. Diagnosis, Risk Stratification, and Follow-Up of HCM
4. Cardiac Magnetic Resonance (CMR) Features of HCM
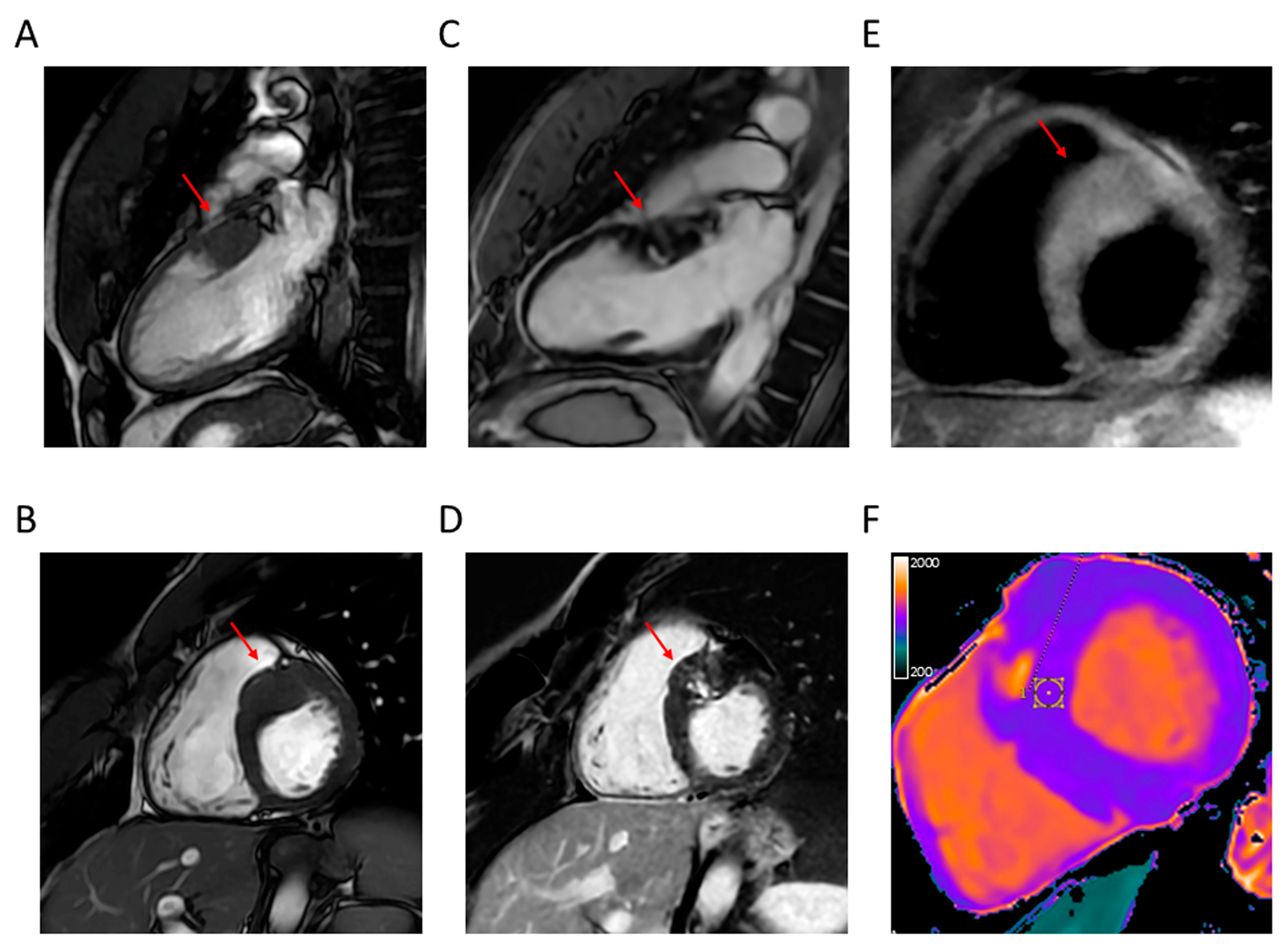
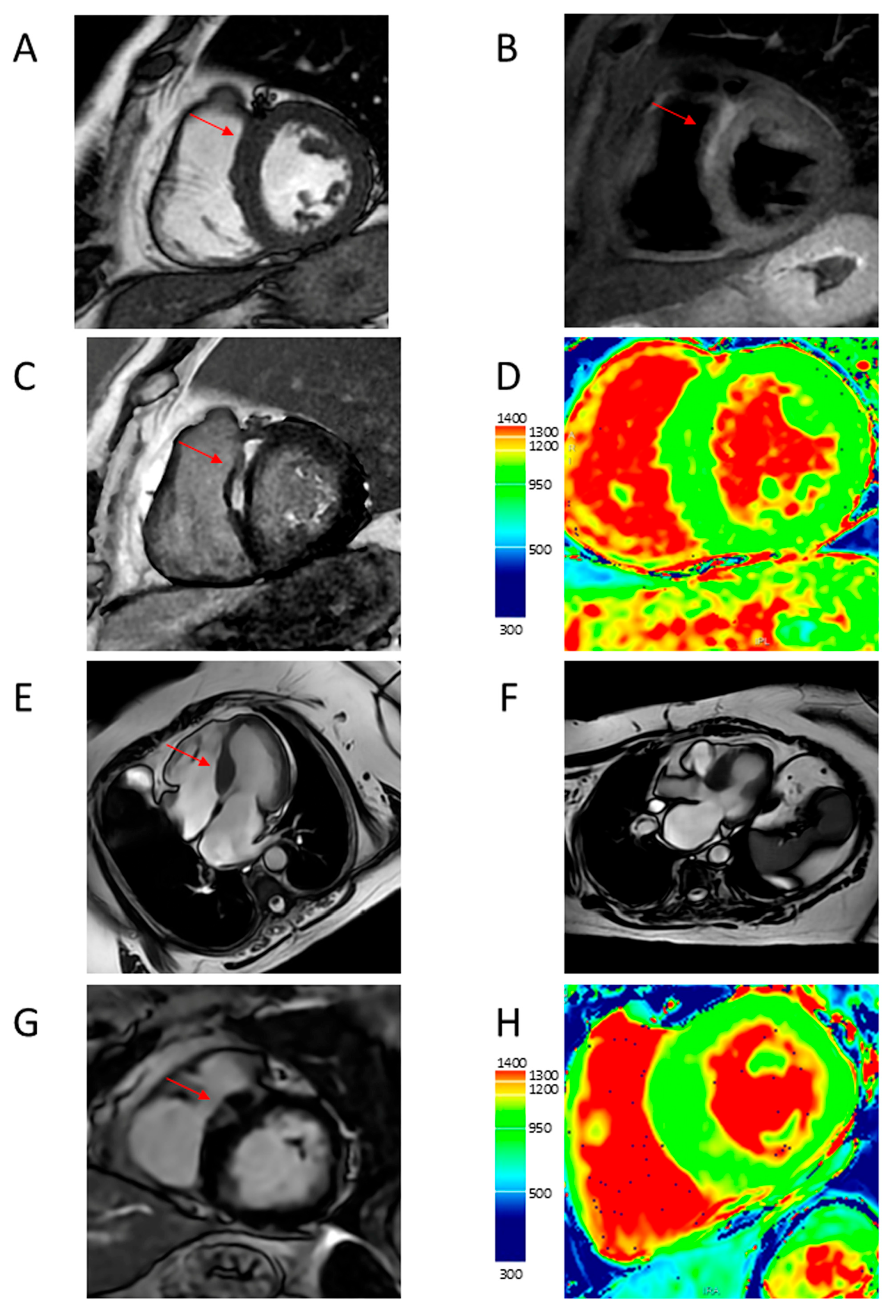
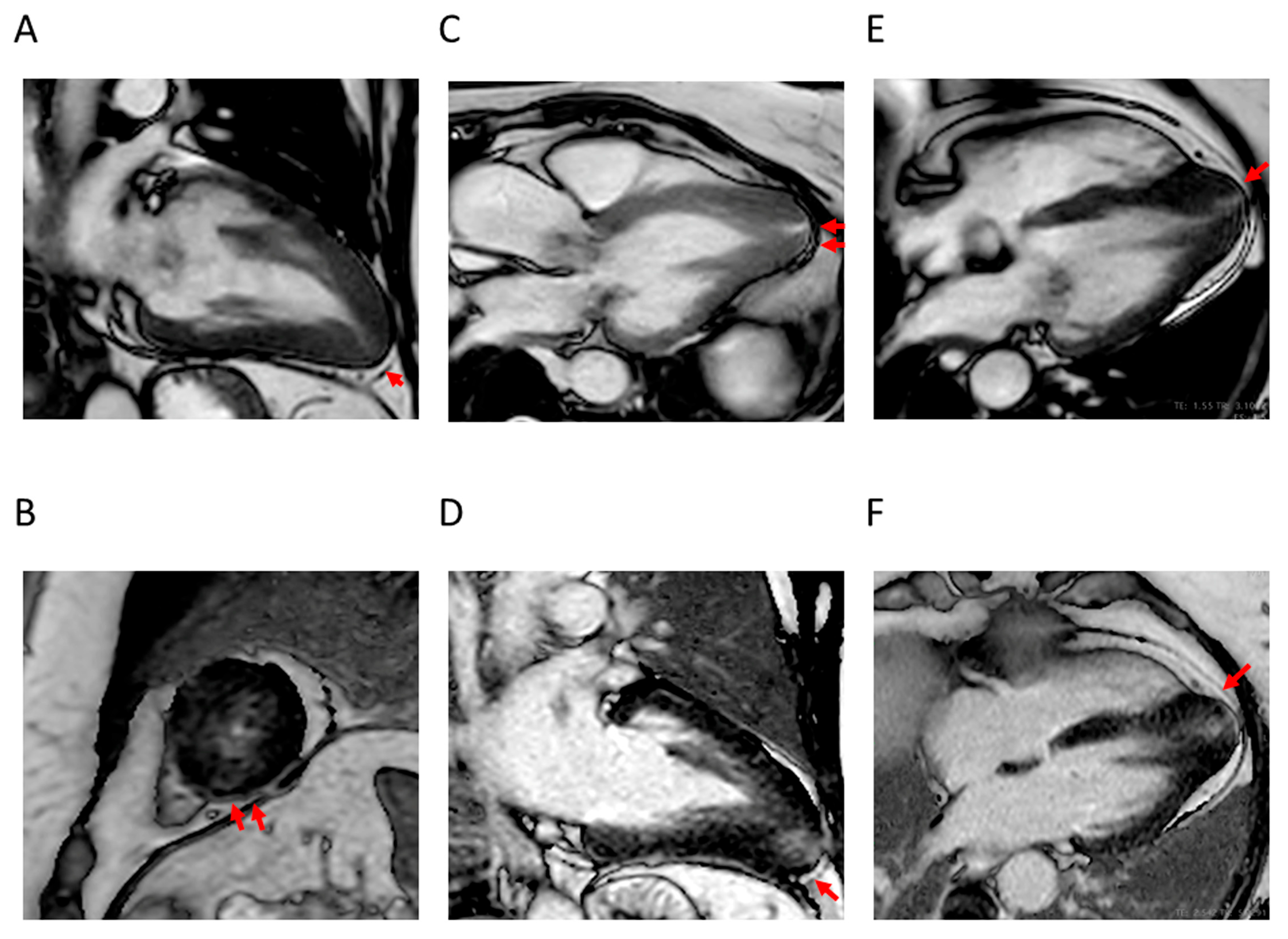
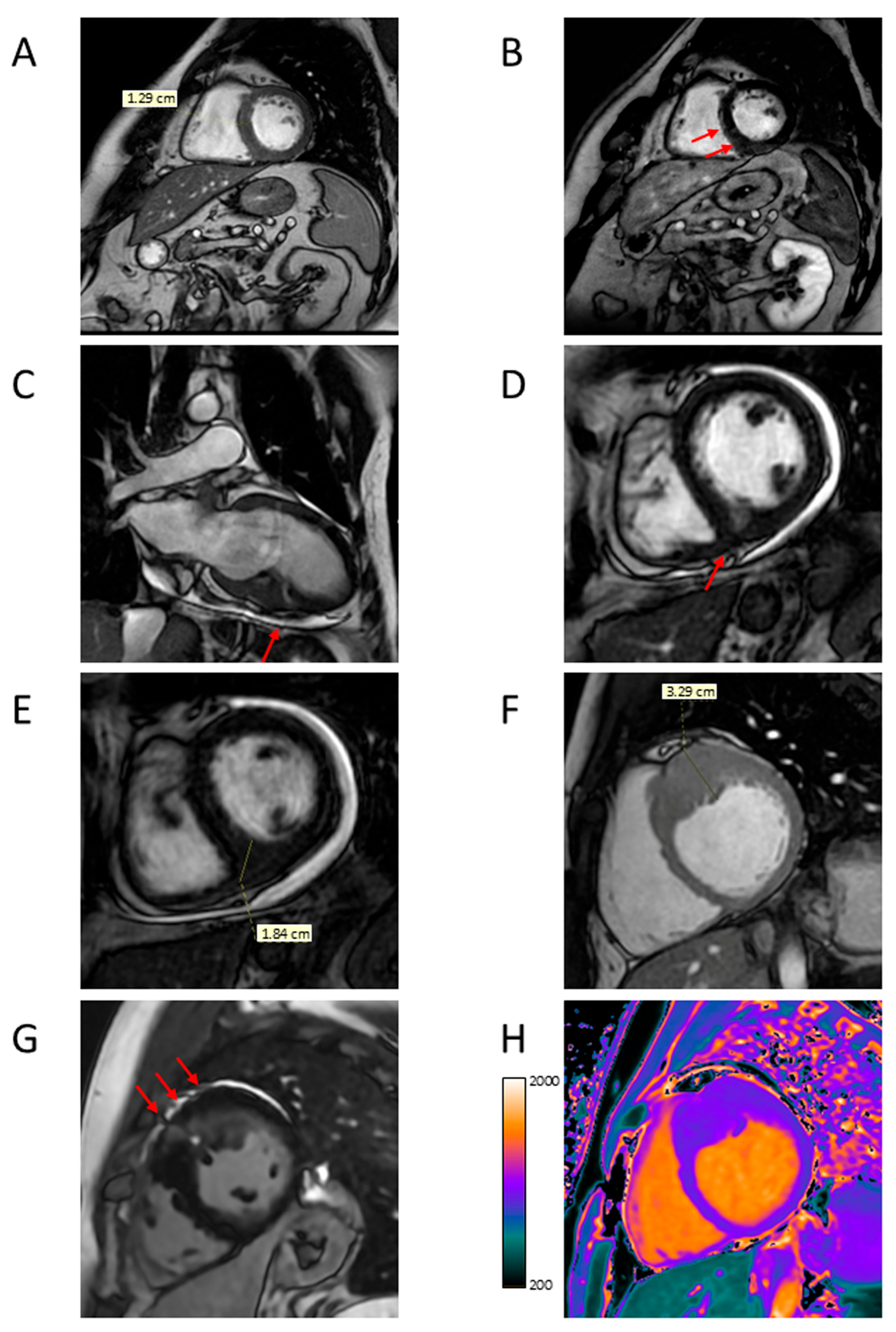
4.1. LV Hypertrophy
4.2. Apical Aneurysm
4.3. LVOTO
4.4. LV Function
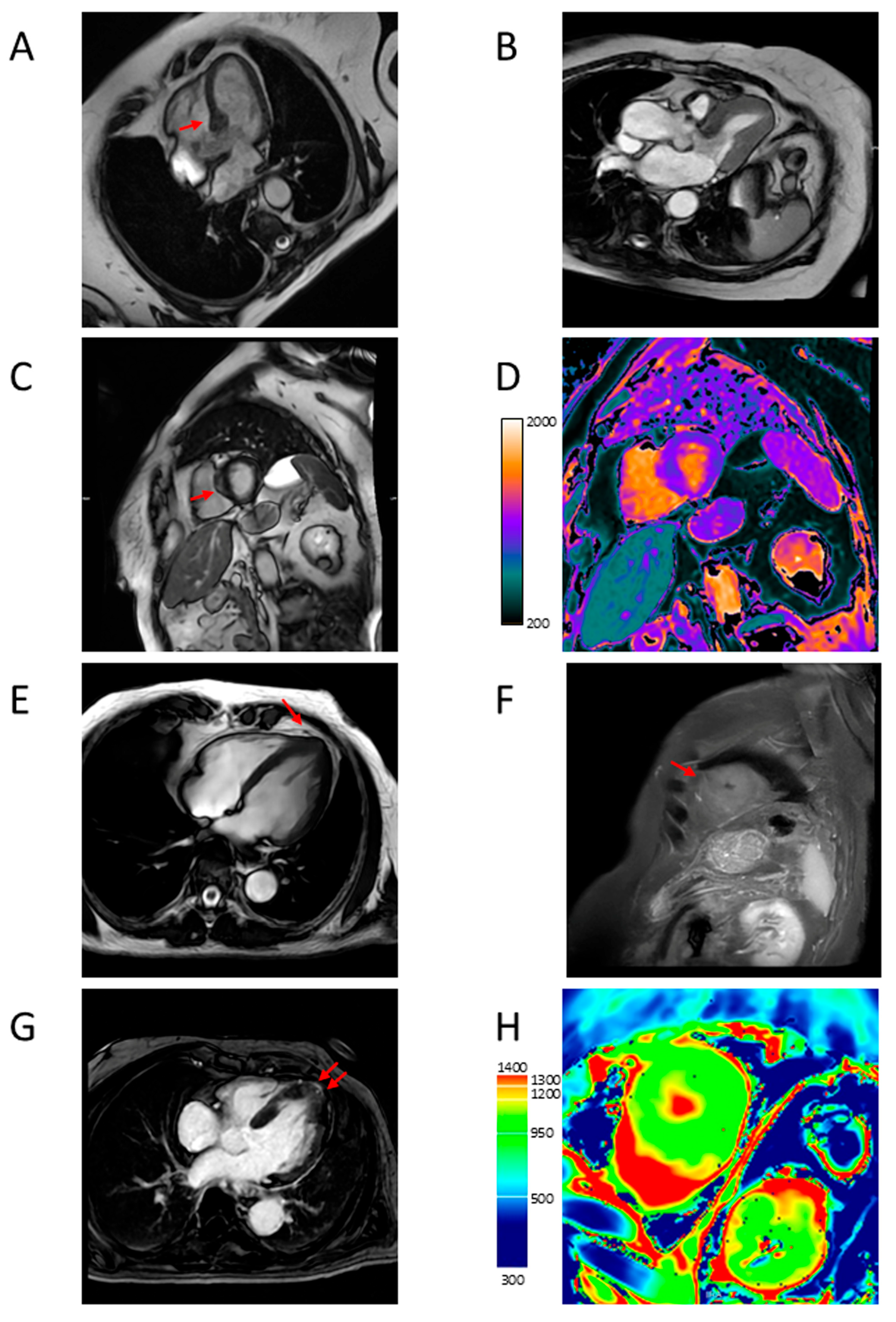
4.5. Myocardial Fibrosis
4.6. Microvascular Dysfunction
5. Role of CMR Imaging in HCM Management
5.1. Diagnosis and Differentiation from Phenotypic Mimics
5.2. Characterization of HCM Phenotypes and Risk Stratification
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCM | hypertrophic cardiomyopathy |
| LV | left ventricular |
| CMR | cardiac magnetic resonance |
| SCD | sudden cardiac death |
| AF | atrial fibrillation |
| RV | right ventricle |
| LVOTO | left ventricular outflow tract obstruction |
| SAM | systolic anterior motion |
| ICD | implantable cardioverter-defibrillator |
| ECG | electrocardiogram |
| 2D | two-dimension |
| CT | computed tomography |
| SSFP | steady-state free precession |
| LGE | late gadolinium enhancement |
| MOLLI | Modified Look-Locker Inversion Recovery |
| ECV | extracellular volume |
References
- Maron, B.J.; Rowin, E.J.; Maron, M.S. Hypertrophic Cardiomyopathy: New Concepts and Therapies. Annu. Rev. Med. 2022, 73, 363–375. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease with Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 2024, 45, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J.; Goldstein, J.; Thaxton, C.; Caleshu, C.; Corty, E.W.; Crowley, S.B.; Dougherty, K.; Harrison, S.M.; McGlaughon, J.; Milko, L.V.; et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ. Genom. Precis. Med. 2019, 12, e002460. [Google Scholar] [CrossRef]
- Maron, B.J.; Maron, M.S.; Maron, B.A.; Loscalzo, J. Moving Beyond the Sarcomere to Explain Heterogeneity in Hypertrophic Cardiomyopathy: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1978–1986. [Google Scholar] [CrossRef]
- Allouba, M.; Walsh, R.; Afify, A.; Hosny, M.; Halawa, S.; Galal, A.; Fathy, M.; Theotokis, P.I.; Boraey, A.; Ellithy, A.; et al. Ethnicity, consanguinity, and genetic architecture of hypertrophic cardiomyopathy. Eur. Heart J. 2023, 44, 5146–5158. [Google Scholar] [CrossRef]
- Marian, A.J.; Braunwald, E. Hypertrophic Cardiomyopathy: Genetics; Pathogenesis; Clinical Manifestations; Diagnosis; and Therapy. Circ. Res. 2017, 121, 749–770. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Davies, M.J.; McKenna, W.J. Hypertrophic cardiomyopathy--pathology and pathogenesis. Histopathology 1995, 26, 493–500. [Google Scholar] [CrossRef]
- Goldie, F.C.; Lee, M.M.Y.; Coats, C.J.; Nordin, S. Advances in Multi-Modality Imaging in Hypertrophic Cardiomyopathy. J. Clin. Med. 2024, 13, 842. [Google Scholar] [CrossRef]
- Neubauer, S.; Kolm, P.; Ho, C.Y.; Kwong, R.Y.; Desai, M.Y.; Dolman, S.F.; Appelbaum, E.; Desvigne-Nickens, P.; DiMarco, J.P.; Friedrich, M.G.; et al. Distinct Subgroups in Hypertrophic Cardiomyopathy in the NHLBI HCM Registry. J. Am. Coll. Cardiol. 2019, 74, 2333–2345. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Hypertrophic Cardiomyopathy: A Brief Overview. Am. J. Cardiol. 2024, 212S, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Zenovich, A.G.; Link, M.S.; Pandian, N.G.; Kuvin, J.T.; Nistri, S.; Cecchi, F.; Udelson, J.E.; Maron, B.J. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006, 114, 2232–2239. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Chokshi, A.; Kannappan, M.; Arkun, K.; Wang, W.; Rastegar, H.; Maron, M.S. Clinical Spectrum and Management Implications of Left Ventricular Outflow Obstruction with Mild Ventricular Septal Thickness in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 122, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., 3rd; Rosing, D.R.; Maron, B.J.; Leon, M.B.; Bonow, R.O.; Watson, R.M.; Epstein, S.E. Myocardial ischemia in patients with hypertrophic cardiomyopathy: Contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation 1985, 71, 234–243. [Google Scholar] [CrossRef]
- Sorajja, P.; Ommen, S.R.; Nishimura, R.A.; Gersh, B.J.; Berger, P.B.; Tajik, A.J. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation 2003, 108, 2342–2348. [Google Scholar] [CrossRef]
- Raphael, C.E.; Cooper, R.; Parker, K.H.; Collinson, J.; Vassiliou, V.; Pennell, D.J.; de Silva, R.; Hsu, L.Y.; Greve, A.M.; Nijjer, S.; et al. Mechanisms of Myocardial Ischemia in Hypertrophic Cardiomyopathy: Insights from Wave Intensity Analysis and Magnetic Resonance. J. Am. Coll. Cardiol. 2016, 68, 1651–1660. [Google Scholar] [CrossRef]
- Aguiar Rosa, S.; Rocha Lopes, L.; Fiarresga, A.; Ferreira, R.C.; Mota Carmo, M. Coronary microvascular dysfunction in hypertrophic cardiomyopathy: Pathophysiology; assessment; and clinical impact. Microcirculation 2021, 28, e12656. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S.; Chan, R.H.; Hausvater, A.; Wang, W.; Rastegar, H.; Maron, B.J. Interaction of Adverse Disease Related Pathways in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2017, 120, 2256–2264. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S.; Rowin, E.J. Perspectives on the Overall Risks of Living with Hypertrophic Cardiomyopathy. Circulation 2017, 135, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Maron, M.S. Paradigm of Sudden Death Prevention in Hypertrophic Cardiomyopathy. Circ. Res. 2019, 125, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Vanderlaan, R.D.; Woo, A.; Ralph-Edwards, A. Isolated septal myectomy for hypertrophic obstructive cardiomyopathy: An update on the Toronto General Hospital experience. Ann. Cardiothorac. Surg. 2017, 6, 364–368. [Google Scholar] [CrossRef]
- Hodges, K.; Rivas, C.G.; Aguilera, J.; Borden, R.; Alashi, A.; Blackstone, E.H.; Desai, M.Y.; Smedira, N.G. Surgical management of left ventricular outflow tract obstruction in a specialized hypertrophic obstructive cardiomyopathy center. J. Thorac. Cardiovasc. Surg. 2019, 157, 2289–2299. [Google Scholar] [CrossRef]
- Sorajja, P. Alcohol Septal Ablation for Obstructive Hypertrophic Cardiomyopathy: A Word of Balance. J. Am. Coll. Cardiol. 2017, 70, 489–494. [Google Scholar] [CrossRef]
- Carrick, R.T.; Maron, M.S.; Adler, A.; Wessler, B.; Hoss, S.; Chan, R.H.; Sridharan, A.; Huang, D.; Cooper, C.; Drummond, J.; et al. Development and Validation of a Clinical Predictive Model for Identifying Hypertrophic Cardiomyopathy Patients at Risk for Atrial Fibrillation: The HCM-AF Score. Circ. Arrhythm. Electrophysiol. 2021, 14, e009796. [Google Scholar] [CrossRef]
- Boll, G.; Rowin, E.J.; Maron, B.J.; Wang, W.; Rastegar, H.; Maron, M.S. Efficacy of Combined Cox-Maze IV and Ventricular Septal Myectomy for Treatment of Atrial Fibrillation in Patients with Obstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2020, 125, 120–126. [Google Scholar] [CrossRef]
- Maron, B.J.; Olivotto, I.; Bellone, P.; Conte, M.R.; Cecchi, F.; Flygenring, B.P.; Casey, S.A.; Gohman, T.E.; Bongioanni, S.; Spirito, P. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 301–307. [Google Scholar] [CrossRef]
- Argirò, A.; Zampieri, M.; Marchi, A.; Cappelli, F.; Del Franco, A.; Mazzoni, C.; Cecchi, F.; Olivotto, I. Stage-specific therapy for hypertrophic cardiomyopathy. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C155–C161. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; McNally, E.M.; Ackerman, M.J.; Baty, L.C.; Day, S.M.; Kullo, I.J.; Madueme, P.C.; Maron, M.S.; Martinez, M.W.; Salberg, L.; et al. Establishment of Specialized Clinical Cardiovascular Genetics Programs: Recognizing the Need and Meeting Standards: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2019, 12, e000054. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.C.; Bernal, J.; Bax, J.J.; Abraham, T.P. Echocardiography in hypertrophic cardiomyopathy: The role of conventional and emerging technologies. JACC Cardiovasc. Imaging 2008, 1, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.R.; Abraham, T.P. Role of Imaging in the Diagnosis; Evaluation; and Management of Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2024, 212S, S14–S32. [Google Scholar] [CrossRef]
- Joshi, S.; Patel, U.K.; Yao, S.S.; Castenada, V.; Isambert, A.; Winson, G.; Chaudhry, F.A.; Sherrid, M.V. Standing and exercise Doppler echocardiography in obstructive hypertrophic cardiomyopathy: The range of gradients with upright activity. J. Am. Soc. Echocardiogr. 2011, 24, 75–82. [Google Scholar] [CrossRef]
- Hansen, M.W.; Merchant, N. MRI of hypertrophic cardiomyopathy: Part I.; MRI appearances. AJR Am. J. Roentgenol. 2007, 189, 1335–1343. [Google Scholar] [CrossRef]
- Hundley, W.G.; Bluemke, D.A.; Bogaert, J.; Flamm, S.D.; Fontana, M.; Friedrich, M.G.; Grosse-Wortmann, L.; Karamitsos, T.D.; Kramer, C.M.; Kwong, R.Y.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) guidelines for reporting cardiovascular magnetic resonance examinations. J. Cardiovasc. Magn. Reson. 2022, 24, 29. [Google Scholar] [CrossRef]
- Fahmy, A.S.; Rowin, E.J.; Arafati, A.; Al-Otaibi, T.; Maron, M.S.; Nezafat, R. Radiomics and deep learning for myocardial scar screening in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2022, 24, 40. [Google Scholar] [CrossRef]
- Zhou, H.; Li, L.; Liu, Z.; Zhao, K.; Chen, X.; Lu, M.; Yin, G.; Song, L.; Zhao, S.; Zheng, H.; et al. Deep learning algorithm to improve hypertrophic cardiomyopathy mutation prediction using cardiac cine images. Eur. Radiol. 2021, 31, 3931–3940. [Google Scholar] [CrossRef]
- Amano, Y.; Kitamura, M.; Takano, H.; Yanagisawa, F.; Tachi, M.; Suzuki, Y.; Kumita, S.; Takayama, M. Cardiac MR Imaging of Hypertrophic Cardiomyopathy: Techniques; Findings; and Clinical Relevance. Magn. Reson. Med. Sci. 2018, 17, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.C.; Simonetti, O.; Bundy, J.; Li, D.; Pereles, S.; Finn, J.P. Cine MR angiography of the heart with segmented true fast imaging with steady-state precession. Radiology 2001, 219, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Lima, J.A. Cardiac MRI: A central prognostic tool in myocardial fibrosis. Nat. Rev. Cardiol. 2015, 12, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, O.P.; Finn, J.P.; White, R.D.; Laub, G.; Henry, D.A. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996, 199, 49–57. [Google Scholar] [CrossRef]
- Rickers, C.; Wilke, N.M.; Jerosch-Herold, M.; Casey, S.A.; Panse, P.; Panse, N.; Weil, J.; Zenovich, A.G.; Maron, B.J. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005, 112, 855–861. [Google Scholar] [CrossRef]
- Maron, M.S. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2012, 14, 13. [Google Scholar] [CrossRef]
- Moon, J.C.; Fisher, N.G.; McKenna, W.J.; Pennell, D.J. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004, 90, 645–649. [Google Scholar] [CrossRef]
- Suzuki, J.; Shimamoto, R.; Nishikawa, J.; Yamazaki, T.; Tsuji, T.; Nakamura, F.; Shin, W.S.; Nakajima, T.; Toyo-Oka, T.; Ohotomo, K. Morphological onset and early diagnosis in apical hypertrophic cardiomyopathy: A long term analysis with nuclear magnetic resonance imaging. J. Am. Coll. Cardiol. 1999, 33, 146–151. [Google Scholar] [CrossRef]
- Shuroog, J.; Canakis, J.; Khan, F.J.; Suryanarayana, P.; Soherwardi, S. A Rare Case of Mass-Like Hypertrophic Cardiomyopathy. Cureus 2021, 13, e12787. [Google Scholar] [CrossRef]
- Deva, D.P.; Williams, L.K.; Care, M.; Siminovitch, K.A.; Moshonov, H.; Wintersperger, B.J.; Rakowski, H.; Crean, A.M. Deep basal inferoseptal crypts occur more commonly in patients with hypertrophic cardiomyopathy due to disease-causing myofilament mutations. Radiology 2013, 269, 68–76. [Google Scholar] [CrossRef]
- Kebed, K.Y.; Al Adham, R.I.; Bishu, K.; Askew, J.W.; Klarich, K.W.; Oh, J.K.; Julsrud, P.R.; Foley, T.A.; Glockner, J.F.; Nishimura, R.A.; et al. Evaluation of apical pouches in hypertrophic cardiomyopathy using cardiac MRI. Int. J. Cardiovasc. Imaging 2014, 30, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Tarkiainen, M.; Sipola, P.; Jalanko, M.; Heliö, T.; Laine, M.; Järvinen, V.; Häyrinen, K.; Lauerma, K.; Kuusisto, J. Cardiovascular magnetic resonance of mitral valve length in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2016, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Abbasi, S.A.; Neilan, T.G.; Shah, R.V.; Chen, Y.; Heydari, B.; Cirino, A.L.; Lakdawala, N.K.; Orav, E.J.; González, A.; et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ. Cardiovasc. Imaging 2013, 6, 415–422. [Google Scholar] [CrossRef]
- Yang, K.; Song, Y.Y.; Chen, X.Y.; Wang, J.X.; Li, L.; Yin, G.; Zheng, Y.C.; Wei, M.D.; Lu, M.J.; Zhao, S.H. Apical hypertrophic cardiomyopathy with left ventricular apical aneurysm: Prevalence, cardiac magnetic resonance characteristics, and prognosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1341–1350. [Google Scholar] [CrossRef]
- Minami, Y.; Kajimoto, K.; Terajima, Y.; Yashiro, B.; Okayama, D.; Haruki, S.; Nakajima, T.; Kawashiro, N.; Kawana, M.; Hagiwara, N. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 2346–2355. [Google Scholar] [CrossRef]
- Maron, M.S.; Finley, J.J.; Bos, J.M.; Hauser, T.H.; Manning, W.J.; Haas, T.S.; Lesser, J.R.; Udelson, J.E.; Ackerman, M.J.; Maron, B.J. Prevalence; clinical significance; and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation 2008, 118, 1541–1549. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, M.S. The Role of Cardiac MRI in the Diagnosis and Risk Stratification of Hypertrophic Cardiomyopathy. Arrhythm. Electrophysiol. Rev. 2016, 5, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Dhillon, A.; Popovic, Z.B.; Smedira, N.G.; Rizzo, J.; Thamilarasan, M.; Agler, D.; Lytle, B.W.; Leverm, H.M.; Desai, M.Y. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardiography. Circ. Cardiovasc. Imaging 2015, 8, e003132. [Google Scholar] [CrossRef]
- Martin, R.; Lairez, O.; Boudou, N.; Méjean, S.; Lhermusier, T.; Dumonteil, N.; Berry, M.; Cognet, T.; Massabuau, P.; Elbaz, M.; et al. Relation between left ventricular outflow tract obstruction and left ventricular shape in patients with hypertrophic cardiomyopathy: A cardiac magnetic resonance imaging study. Arch. Cardiovasc. Dis. 2013, 106, 440–447. [Google Scholar] [CrossRef]
- Martínez-Vives, P.; Cecconi, A.; Vera, A.; López-Melgar, B.; Sanz-García, A.; Viliani, D.; Nogales-Romo, M.T.; Muñiz, S.H.; Olivera, M.J.; Caballero, P.; et al. Tissue tracking analysis and left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Magn. Reson. Imaging 2025, 119, 110363. [Google Scholar] [CrossRef]
- Proctor, R.D.; Shambrook, J.S.; McParland, P.; Peebles, C.R.; Brown, I.W.; Harden, S.P. Imaging hypertrophic heart diseases with cardiovascular MR. Clin. Radiol. 2011, 66, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, B.J.; Lesser, J.R.; Rastegar, H.; Maron, M.S. Papillary muscle insertion directly into the anterior mitral leaflet in hypertrophic cardiomyopathy; its identification and cause of outflow obstruction by cardiac magnetic resonance imaging; and its surgical management. Am. J. Cardiol. 2013, 111, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Gommans, D.H.; Bakker, J.; Cramer, G.E.; Verheugt, F.W.; Brouwer, M.A.; Kofflard, M.J. Impact of the papillary muscles on cardiac magnetic resonance image analysis of important left ventricular parameters in hypertrophic cardiomyopathy. Neth Heart J. 2016, 24, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Fulton, N.L.; Bolen, M. Magnetic resonance imaging of the papillary muscles of the left ventricle: Normal anatomy; variants; and abnormalities. Insights Imaging 2019, 10, 83. [Google Scholar] [CrossRef]
- Ünlü, S.; Özden Tok, Ö.; Avcı Demir, F.; Papadopoulos, K.; Monaghan, M.J. Differential diagnosis of apical hypertrophic cardiomyopathy and apical displacement of the papillary muscles: A multimodality imaging point of view. Echocardiography 2021, 38, 103–113. [Google Scholar] [CrossRef]
- Filomena, D.; Vandenberk, B.; Dresselaers, T.; Willems, R.; Van Cleemput, J.; Olivotto, I.; Robyns, T.; Bogaert, J. Apical papillary muscle displacement is a prevalent feature and a phenotypic precursor of apical hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1009–1016. [Google Scholar] [CrossRef]
- Grothues, F.; Smith, G.C.; Moon, J.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Hudsmith, L.E.; Petersen, S.E.; Francis, J.M.; Robson, M.D.; Neubauer, S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 2005, 7, 775–782. [Google Scholar] [CrossRef]
- Harris, K.M.; Spirito, P.; Maron, M.S.; Zenovich, A.G.; Formisano, F.; Lesser, J.R.; Mackey-Bojack, S.; Manning, W.J.; Udelson, J.E.; Maron, B.J. Prevalence; clinical profile; and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 2006, 114, 216–225. [Google Scholar] [CrossRef]
- Choudhury, L.; Mahrholdt, H.; Wagner, A.; Choi, K.M.; Elliott, M.D.; Klocke, F.J.; Bonow, R.O.; Judd, R.M.; Kim, R.J. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 2156–2164. [Google Scholar] [CrossRef]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef]
- Park, Y.J.; Park, S.J.; Kim, E.K.; Park, K.M.; Lee, S.C.; On, Y.K.; Kim, J.S. Semi-quantitative versus quantitative assessments of late gadolinium enhancement extent for predicting spontaneous ventricular tachyarrhythmia events in patients with hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 2920. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Appelbaum, E.; Harrigan, C.J.; Buros, J.; Gibson, C.M.; Hanna, C.; Lesser, J.R.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ. Heart Fail. 2008, 1, 184–191. [Google Scholar] [CrossRef]
- Spiewak, M.; Malek, L.A.; Misko, J.; Chojnowska, L.; Milosz, B.; Klopotowski, M.; Petryka, J.; Dabrowski, M.; Kepka, C.; Ruzyllo, W. Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur. J. Radiol. 2010, 74, e149–e153. [Google Scholar] [CrossRef]
- Aquaro, G.D.; Positano, V.; Pingitore, A.; Strata, E.; Di Bella, G.; Formisano, F.; Spirito, P.; Lombardi, M. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2010, 12, 21. [Google Scholar] [CrossRef]
- Mikami, Y.; Kolman, L.; Joncas, S.X.; Stirrat, J.; Scholl, D.; Rajchl, M.; Lydell, C.P.; Weeks, S.G.; Howarth, A.G.; White, J.A. Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2014, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Kiaos, A.; Daskalopoulos, G.N.; Kamperidis, V.; Ziakas, A.; Efthimiadis, G.; Karamitsos, T.D. Quantitative Late Gadolinium Enhancement Cardiac Magnetic Resonance and Sudden Death in Hypertrophic Cardiomyopathy: A Meta-Analysis. JACC Cardiovasc. Imaging 2024, 17, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients with Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Peker, E.; Chandrashekhar, Y.; Nagel, E. T1 Mapping in Characterizing Myocardial Disease: A Comprehensive Review. Circ. Res. 2016, 119, 277–299. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Lee, S.C.; Chang, S.A.; Jang, S.Y.; Kim, S.M.; Park, S.J.; Choi, J.O.; Park, S.W.; Jeon, E.S.; Choe, Y.H. Prevalence and clinical significance of cardiovascular magnetic resonance adenosine stress-induced myocardial perfusion defect in hypertrophic cardiomyopathy. J. Cardiovasc. Magn. Reson. 2020, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F.; Hsu, L.Y.; Greve, A.M.; Gonçalves, C.; Jabbour, A.; Gulati, A.; Hewins, B.; Mistry, N.; Wage, R.; Roughton, M.; et al. Coronary microvascular ischemia in hypertrophic cardiomyopathy—A pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J. Cardiovasc. Magn. Reson. 2014, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, C.; Knott, K.D.; Augusto, J.B.; Seraphim, A.; Rosmini, S.; Ricci, F.; Boubertakh, R.; Xue, H.; Hughes, R.; Captur, G.; et al. Inline perfusion mapping provides insights into the disease mechanism in hypertrophic cardiomyopathy. Heart 2020, 106, 824–829. [Google Scholar] [CrossRef]
- Yin, L.; Xu, H.Y.; Zheng, S.S.; Zhu, Y.; Xiao, J.X.; Zhou, W.; Yu, S.S.; Gong, L.G. 3.0 T magnetic resonance myocardial perfusion imaging for semi-quantitative evaluation of coronary microvascular dysfunction in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2017, 33, 1949–1959. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Kramer, C.M.; Neubauer, S.; Nagueh, S.F.; Desai, M.Y. Multimodality Imaging in Hypertrophic Cardiomyopathy for Risk Stratification. Circ. Cardiovasc. Imaging 2020, 13, e009026. [Google Scholar] [CrossRef]
- Soler, R.; Méndez, C.; Rodríguez, E.; Barriales, R.; Ochoa, J.P.; Monserrat, L. Phenotypes of hypertrophic cardiomyopathy. An illustrative review of MRI findings. Insights Imaging 2018, 9, 1007–1020. [Google Scholar] [CrossRef]
- Mushtaq, S.; Chiesa, M.; Novelli, V.; Sommariva, E.; Biondi, M.L.; Manzoni, M.; Florio, A.; Lampus, M.L.; Avallone, C.; Zocchi, C.; et al. Role of advanced CMR features in identifying a positive genotype of hypertrophic cardiomyopathy. Int. J. Cardiol. 2024, 417, 132554. [Google Scholar] [CrossRef]
- Hansen, M.W.; Merchant, N. MRI of hypertrophic cardiomyopathy: Part 2; Differential diagnosis; risk stratification; and posttreatment MRI appearances. AJR Am. J. Roentgenol. 2007, 189, 1344–1352. [Google Scholar] [CrossRef]
- Angelini, F.; Bocchino, P.P.; Dusi, V.; Pidello, S.; De Ferrari, G.M.; Raineri, C. From thick walls to clear answers: Approaches to diagnosing hypertrophic cardiomyopathy and its mimics. Eur. Heart. J. Suppl. 2025, 27 (Suppl. S1), i40–i46. [Google Scholar] [CrossRef]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J. Clin. Med. 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; De Gaetano, F.; Campisi, M.; Losi, V.; Zito, C.; Tamburino, C.; Di Bella, G.; Monte, I.P. Myocardial Work Appraisal in Transthyretin Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2023, 208, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Maron, M.S.; Urbano-Moral, J.A.; Pandian, N.G.; Maron, B.J.; Pelliccia, A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2014, 114, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Selvanayagam, J.B.; Francis, J.M.; Myerson, S.G.; Wiesmann, F.; Robson, M.D.; Ostman-Smith, I.; Casadei, B.; Watkins, H.; Neubauer, S. Differentiation of athlete’s heart from pathological forms of cardiac hypertrophy by means of geometric indices derived from cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2005, 7, 551–558. [Google Scholar] [CrossRef]
- Fogante, M.; Agliata, G.; Basile, M.C.; Compagnucci, P.; Volpato, G.; Falanga, U.; Stronati, G.; Guerra, F.; Vignale, D.; Esposito, A.; et al. Cardiac Imaging in Athlete’s Heart: The Role of the Radiologist. Medicina 2021, 57, 455. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Plein, S.; Milivojevic, I.G.; Wang, D.W.; Grassi, G.; Mancia, G. Comprehensive assessment of hypertensive heart disease: Cardiac magnetic resonance in focus. Heart Fail. Rev. 2021, 26, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, M.; Klasnja, S.; Popovic, M.; Djuran, P.; Mrda, D.; Ivankovic, T.; Manojlovic, A.; Koracevic, G.; Lovic, D.; Popadic, V. Cardiac Magnetic Resonance in Hypertensive Heart Disease: Time for a New Chapter. Diagnostics 2022, 13, 137. [Google Scholar] [CrossRef]
- Child, N.; Muhr, T.; Sammut, E.; Dabir, D.; Ucar, E.A.; Bueser, T.; Gill, J.; Carr-White, G.; Nagel, E.; Puntmann, V.O. Prevalence of myocardial crypts in a large retrospective cohort study by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 66. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Rohan, S.; Ghosh Dastidar, A.; Harries, I.; Lawton, C.B.; Ratcliffe, L.E.; Burchell, A.E.; Hart, E.C.; Hamilton, M.C.; Paton, J.F.; et al. Hypertensive heart disease versus hypertrophic cardiomyopathy: Multi-parametric cardiovascular magnetic resonance discriminators when end-diastolic wall thickness ≥15 mm. Eur. Radiol. 2017, 27, 1125–1135. [Google Scholar] [CrossRef]
- Arcari, L.; Hinojar, R.; Engel, J.; Freiwald, T.; Platschek, S.; Zainal, H.; Zhou, H.; Vasquez, M.; Keller, T.; Rolf, A.; et al. Native T1 and T2 provide distinctive signatures in hypertrophic cardiac conditions—Comparison of uremic; hypertensive and hypertrophic cardiomyopathy. Int. J. Cardiol. 2020, 306, 102–108. [Google Scholar] [CrossRef]
- Fattori, R.; Rocchi, G.; Celletti, F.; Bertaccini, P.; Rapezzi, C.; Gavelli, G. Contribution of magnetic resonance imaging in the differential diagnosis of cardiac amyloidosis and symmetric hypertrophic cardiomyopathy. Am. Heart J. 1998, 136, 824–830. [Google Scholar] [CrossRef]
- Maceira, A.M.; Joshi, J.; Prasad, S.K.; Moon, J.C.; Perugini, E.; Harding, I.; Sheppard, M.N.; Poole-Wilson, P.A.; Hawkins, P.N.; Pennell, D.J. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005, 111, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Baksi, A.J.; Hawkins, P.N.; Fontana, M. Diagnostic imaging of cardiac amyloidosis. Nat. Rev. Cardiol. 2020, 17, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Fong, H.K.; Birati, E.Y.; Han, Y. Cardiac Sarcoidosis. Am. J. Cardiol. 2019, 123, 513–522. [Google Scholar] [CrossRef]
- Markatis, E.; Afthinos, A.; Antonakis, E.; Papanikolaou, I.C. Cardiac sarcoidosis: Diagnosis and management. Rev. Cardiovasc. Med. 2020, 21, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S.; Bittencourt, M.S.; Murthy, V.L.; Kwong, R.; Di Carli, M.F.; et al. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients with Suspected Cardiac Sarcoidosis Is Associated with Adverse Cardiovascular Prognosis: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2016, 9, e005001. [Google Scholar] [CrossRef]
- De Cobelli, F.; Esposito, A.; Belloni, E.; Pieroni, M.; Perseghin, G.; Chimenti, C.; Frustaci, A.; Del Maschio, A. Delayed-enhanced cardiac MRI for differentiation of Fabry’s disease from symmetric hypertrophic cardiomyopathy. AJR Am. J. Roentgenol. 2009, 192, W97–W102. [Google Scholar] [CrossRef]
- Perry, R.; Shah, R.; Saiedi, M.; Patil, S.; Ganesan, A.; Linhart, A.; Selvanayagam, J.B. The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1230–1242. [Google Scholar] [CrossRef]
- Pica, S.; Sado, D.M.; Maestrini, V.; Fontana, M.; White, S.K.; Treibel, T.; Captur, G.; Anderson, S.; Piechnik, S.K.; Robson, M.D.; et al. Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 99. [Google Scholar] [CrossRef]
- O’Hara, R.P.; Prakosa, A.; Binka, E.; Lacy, A.; Trayanova, N.A. Arrhythmia in hypertrophic cardiomyopathy: Risk prediction using contrast enhanced MRI.; T1 mapping; and personalized virtual heart technology. J. Electrocardiol. 2022, 74, 122–127. [Google Scholar] [CrossRef]
- Maron, M.S.; Lesser, J.R.; Maron, B.J. Management implications of massive left ventricular hypertrophy in hypertrophic cardiomyopathy significantly underestimated by echocardiography but identified by cardiovascular magnetic resonance. Am. J. Cardiol. 2010, 105, 1842–1843. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Carrick, R.T.; Patel, P.P.; Koethe, B.; Wells, S.; Maron, M.S. Outcomes in Patients with Hypertrophic Cardiomyopathy and Left Ventricular Systolic Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 3033–3043. [Google Scholar] [CrossRef]
- Hanneman, K.; Crean, A.M.; Williams, L.; Moshonov, H.; James, S.; Jiménez-Juan, L.; Gruner, C.; Sparrow, P.; Rakowski, H.; Nguyen, E.T. Cardiac magnetic resonance imaging findings predict major adverse events in apical hypertrophic cardiomyopathy. J. Thorac. Imaging 2014, 29, 331–339. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophic Cardiomyopathy with Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Z.J.; Montazeri, M.; Bataiosu, R.; Hoss, S.; Adler, A.; Nguyen, E.T.; Rakowski, H.; Chan, R.H. Clinical Characteristics and Prognostic Importance of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Kamp, N.J.; Chery, G.; Kosinski, A.S.; Desai, M.Y.; Wazni, O.; Schmidler, G.S.; Patel, M.; Lopes, R.D.; Morin, D.P.; Al-Khatib, S.M. Risk stratification using late gadolinium enhancement on cardiac magnetic resonance imaging in patients with hypertrophic cardiomyopathy: A systematic review and meta-analysis. Prog. Cardiovasc. Dis. 2021, 66, 10–16. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2021, 162, e23–e106. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
| Acquisition Protocol | |
| 1. Anatomical features a. Pattern and severity of hypertrophy
| 2. Functional features a. Ventricular
|
| 3. Tissue characterization a. LGE
| |
| Sequences | |
| 1. Cine SSFP | 6. Early gadolinium enhancement |
| 2. Tagged and other strain-encoding cine | 7. Late gadolinium enhancement |
| 3. T1- and T2-weighted imaging | 8. Velocity-encoded/phase contrast |
| 4. Quantitative T1 and T2 mapping | 9. MR angiography |
| 5. T2* mapping | 10. Myocardial perfusion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, L.; Luciano, A.; Chiocchi, M. The Role of Cardiac Magnetic Resonance Imaging in the Management of Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2025, 12, 189. https://doi.org/10.3390/jcdd12050189
Pugliese L, Luciano A, Chiocchi M. The Role of Cardiac Magnetic Resonance Imaging in the Management of Hypertrophic Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2025; 12(5):189. https://doi.org/10.3390/jcdd12050189
Chicago/Turabian StylePugliese, Luca, Alessandra Luciano, and Marcello Chiocchi. 2025. "The Role of Cardiac Magnetic Resonance Imaging in the Management of Hypertrophic Cardiomyopathy" Journal of Cardiovascular Development and Disease 12, no. 5: 189. https://doi.org/10.3390/jcdd12050189
APA StylePugliese, L., Luciano, A., & Chiocchi, M. (2025). The Role of Cardiac Magnetic Resonance Imaging in the Management of Hypertrophic Cardiomyopathy. Journal of Cardiovascular Development and Disease, 12(5), 189. https://doi.org/10.3390/jcdd12050189







