Left Ventricular Systolic Function in Asymptomatic Men Aged 65–75 Years, Relation to Insulin Resistance and Pre-Diabetes: A DANCAVAS Cross-Sectional Sub-Study
Abstract
1. Introduction
2. Methods
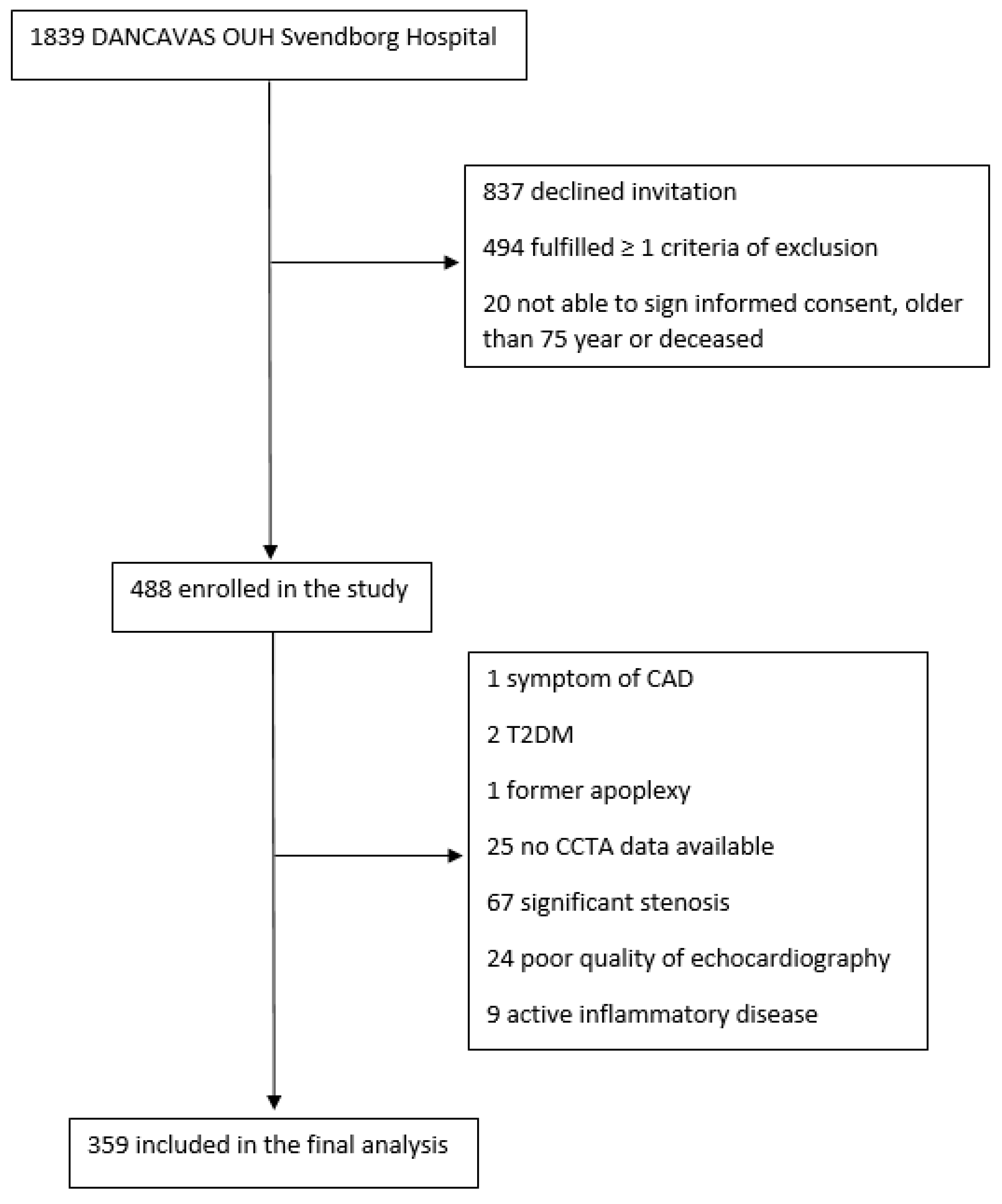
2.1. Study Design and Study Poulation
2.2. Echocardiography
2.3. Global Longitudinal Strain
2.4. Insulin Resistance, Oral Glucose Tolerance Test, and Blood Sample
2.5. Data Collection
2.6. Statistics
2.7. Ethics
3. Results
3.1. Clinical Characteristics
3.2. Echocardiography
3.3. Uni- and Multivariable Regression Analysis
3.4. Intra- and Inter-Observer Variability
4. Discussion
4.1. Insulin Resistance and Heart Failure
4.2. Pre-Diabetes and Heart Failure
4.3. Obesity and Heart Failure
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.A.; Gullion, C.M.; Koro, C.E.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004, 27, 1879–1884. [Google Scholar] [CrossRef]
- Iribarren, C.; Karter, A.J.; Go, A.S.; Ferrara, A.; Liu, J.Y.; Sidney, S.; Selby, J.V. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001, 103, 2668–2673. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Blecker, S.; Pazin-Filho, A.; Bertoni, A.; Chang, P.P.; Coresh, J.; Selvin, E. The association of hemoglobin a1c with incident heart failure among people without diabetes: The atherosclerosis risk in communities study. Diabetes 2010, 59, 2020–2026. [Google Scholar] [CrossRef]
- Banerjee, D.; Biggs, M.L.; Mercer, L.; Mukamal, K.; Kaplan, R.; Barzilay, J.; Kuller, L.; Kizer, J.R.; Djousse, L.; Tracy, R.; et al. Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 364–370. [Google Scholar] [CrossRef]
- Thrainsdottir, I.S.; Aspelund, T.; Thorgeirsson, G.; Gudnason, V.; Hardarson, T.; Malmberg, K.; Sigurdsson, G.; Rydén, L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care 2005, 28, 612–616. [Google Scholar] [CrossRef]
- Ingelsson, E.; Sundström, J.; Arnlöv, J.; Zethelius, B.; Lind, L. Insulin resistance and risk of congestive heart failure. Jama 2005, 294, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Gupta, D.K.; Claggett, B.; Burke, S.; Shah, A.; Loehr, L.; Rasmussen-Torvik, L.; Selvin, E.; Chang, P.P.; Aguilar, D.; et al. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities). JACC Heart Fail. 2013, 1, 531–536. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Georgiopoulou, V.; Harris, T.B.; Kritchevsky, S.B.; Bauer, D.C.; Smith, A.L.; Strotmeyer, E.; Newman, A.B.; Wilson, P.W.; Psaty, B.M.; et al. Glycemic status and incident heart failure in elderly without history of diabetes mellitus: The health, aging, and body composition study. J. Card. Fail. 2009, 15, 593–599. [Google Scholar] [CrossRef][Green Version]
- Chandra, S.; Skali, H.; Blankstein, R. Novel techniques for assessment of left ventricular systolic function. Heart Fail. Rev. 2011, 16, 327–337. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11 2 Pt 1, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Hoffmann, S.; Mogelvang, R.; Zeeberg Iversen, A.; Galatius, S.; Fritz-Hansen, T.; Bech, J.; Jensen, J.S. Myocardial strain analysis by 2-dimensional speckle tracking echocardiography improves diagnostics of coronary artery stenosis in stable angina pectoris. Circ. Cardiovasc. Imaging 2014, 7, 58–65. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Elkind, M.S.; Rundek, T.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur. J. Heart Fail. 2014, 16, 1301–1309. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Cauwenberghs, N.; Knez, J.; Yang, W.Y.; Herbots, L.; D’Hooge, J.; Haddad, F.; Thijs, L.; Voigt, J.U.; Staessen, J.A. Additive Prognostic Value of Left Ventricular Systolic Dysfunction in a Population-Based Cohort. Circ. Cardiovasc. Imaging 2016, 9, e004661. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Biggs, M.L.; Kizer, J.R.; Shah, S.J.; Psaty, B.; Carnethon, M.; Gottdiener, J.S.; Siscovick, D.; Mukamal, K.J. Glucose dysregulation and subclinical cardiac dysfunction in older adults: The Cardiovascular Health Study. Cardiovasc. Diabetol. 2022, 21, 112. [Google Scholar] [CrossRef]
- Hirose, K.; Nakanishi, K.; Daimon, M.; Sawada, N.; Yoshida, Y.; Iwama, K.; Yamamoto, Y.; Ishiwata, J.; Hirokawa, M.; Koyama, K.; et al. Impact of insulin resistance on subclinical left ventricular dysfunction in normal weight and overweight/obese japanese subjects in a general community. Cardiovasc. Diabetol. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Atici, A.; Asoglu, R.; Barman, H.A.; Sarikaya, R.; Arman, Y.; Tukek, T. Multilayer global longitudinal strain assessment of subclinical myocardial dysfunction related to insulin resistance. Int. J. Cardiovasc. Imaging 2021, 37, 539–546. [Google Scholar] [CrossRef]
- Lin, J.L.; Sung, K.T.; Su, C.H.; Chou, T.H.; Lo, C.I.; Tsai, J.P.; Chang, S.C.; Lai, Y.H.; Hu, K.C.; Liu, C.Y.; et al. Cardiac Structural Remodeling, Longitudinal Systolic Strain, and Torsional Mechanics in Lean and Nonlean Dysglycemic Chinese Adults. Circ. Cardiovasc. Imaging 2018, 11, e007047. [Google Scholar] [CrossRef]
- Skali, H.; Shah, A.; Gupta, D.K.; Cheng, S.; Claggett, B.; Liu, J.; Bello, N.; Aguilar, D.; Vardeny, O.; Matsushita, K.; et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: The Atherosclerosis Risk In the Community study. Circ. Heart Fail. 2015, 8, 448–454. [Google Scholar] [CrossRef]
- Ho, J.E.; McCabe, E.L.; Wang, T.J.; Larson, M.G.; Levy, D.; Tsao, C.; Aragam, J.; Mitchell, G.F.; Benjamin, E.J.; Vasan, R.S.; et al. Cardiometabolic Traits and Systolic Mechanics in the Community. Circ. Heart Fail. 2017, 10, e003536. [Google Scholar] [CrossRef]
- Lindholt, J.S.; Rasmussen, L.M.; Søgaard, R.; Lambrechtsen, J.; Steffensen, F.H.; Frost, L.; Egstrup, K.; Urbonaviciene, G.; Busk, M.; Olsen, M.H.; et al. Baseline findings of the population-based, randomized, multifaceted Danish cardiovascular screening trial (DANCAVAS) of men aged 65-74 years. Br. J. Surg. 2019, 106, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A.C.; Rasmussen, L.M.; Søgaard, R.; Lambrechtsen, J.; Steffensen, F.H.; Frost, L.; Egstrup, K.; Urbonaviciene, G.; Busk, M.; Olsen, M.H.; et al. The Danish Cardiovascular Screening Trial (DANCAVAS): Study protocol for a randomized controlled trial. Trials 2015, 16, 554. [Google Scholar] [CrossRef]
- Larsson, J.; Auscher, S.; Shamoun, A.; Pararajasingam, G.; Heinsen, L.J.; Andersen, T.R.; Lindholt, J.S.; Diederichsen, A.C.P.; Lambrechtsen, J.; Egstrup, K. Insulin resistance is associated with high-risk coronary artery plaque composition in asymptomatic men between 65 and 75 years and no diabetes: A DANCAVAS cross-sectional sub-study. Atherosclerosis 2023, 385, 117328. [Google Scholar] [CrossRef]
- de Graaf, M.A.; Broersen, A.; Kitslaar, P.H.; Roos, C.J.; Dijkstra, J.; Lelieveldt, B.P.F.; Jukema, J.W.; Schalij, M.J.; Delgado, V.; Bax, J.J.; et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: Cross-correlation with intravascular ultrasound virtual histology. Int. J. Cardiovasc. Imaging 2013, 29, 1177–1190. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Unger, G.; Benozzi, S.F.; Perruzza, F.; Pennacchiotti, G.L. Triglycerides and glucose index: A useful indicator of insulin resistance. Endocrinol. Nutr. 2014, 61, 533–540. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Wood, D.; De Backer, G.; Faergeman, O.; Graham, I.; Mancia, G.; Pyörälä, K. Prevention of coronary heart disease in clinical practice: Recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Atherosclerosis 1998, 140, 199–270. [Google Scholar] [CrossRef]
- Meier, U. A note on the power of Fisher’s least significant difference procedure. Pharm. Stat. 2006, 5, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging 2017, 10, e005521. [Google Scholar] [CrossRef]
- Ishikawa, H.; Otsuka, K.; Kono, Y.; Hojo, K.; Yamaura, H.; Hirata, K.; Kasayuki, N.; Izumiya, Y.; Fukuda, D. Extent of coronary atherosclerosis is associated with deterioration of left ventricular global longitudinal strain in patients with preserved ejection fraction undergoing coronary computed tomography angiography. Int. J. Cardiol. Heart Vasc. 2023, 44, 101176. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Prevedello, F.; Dolci, G.; Roos, C.J.; Djaberi, R.; Bertini, M.; Ewe, S.H.; Allman, C.; Leung, D.Y.; Marsan, N.A.; et al. Impact of Diabetes and Increasing Body Mass Index Category on Left Ventricular Systolic and Diastolic Function. J. Am. Soc. Echocardiogr. 2018, 31, 916–925. [Google Scholar] [CrossRef]
- Butt, J.H.; Petrie, M.C.; Jhund, P.S.; Sattar, N.; Desai, A.S.; Køber, L.; Rouleau, J.L.; Swedberg, K.; Zile, M.R.; Solomon, S.D.; et al. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: Revisiting the obesity paradox. Eur. Heart J. 2023, 44, 1136–1153. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; López-Acosta, O.; Barrios-Maya, M.A.; El-Hafidi, M. Cell Death and Heart Failure in Obesity: Role of Uncoupling Proteins. Oxid. Med. Cell Longev. 2016, 2016, 9340654. [Google Scholar] [CrossRef]
- Takatsu, M.; Nakashima, C.; Takahashi, K.; Murase, T.; Hattori, T.; Ito, H.; Murohara, T.; Nagata, K. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension 2013, 62, 957–965. [Google Scholar] [CrossRef]
- Świątkiewicz, I.; Wróblewski, M.; Nuszkiewicz, J.; Sutkowy, P.; Wróblewska, J.; Woźniak, A. The Role of Oxidative Stress Enhanced by Adiposity in Cardiometabolic Diseases. Int. J. Mol. Sci. 2023, 24, 6382. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Sanders, P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc. Med. 2015, 25, 119–126. [Google Scholar] [CrossRef]
- Kankaanpää, M.; Lehto, H.R.; Pärkkä, J.P.; Komu, M.; Viljanen, A.; Ferrannini, E.; Knuuti, J.; Nuutila, P.; Parkkola, R.; Iozzo, P. Myocardial triglyceride content and epicardial fat mass in human obesity: Relationship to left ventricular function and serum free fatty acid levels. J. Clin. Endocrinol. Metab. 2006, 91, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nakanishi, K.; Jin, Z.; Daimon, M.; Ishiwata, J.; Sawada, N.; Hirokawa, M.; Kaneko, H.; Nakao, T.; Mizuno, Y.; et al. Association Between Progression of Arterial Stiffness and Left Ventricular Remodeling in a Community-Based Cohort. JACC Adv. 2023, 2, 100409. [Google Scholar] [CrossRef]
- Petersen, K.S.; Blanch, N.; Keogh, J.B.; Clifton, P.M. Effect of weight loss on pulse wave velocity: Systematic review and meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Piko, N.; Bevc, S.; Hojs, R.; Petreski, T.; Ekart, R. Higher Body Mass Index is associated with increased arterial stiffness prior to target organ damage: A cross-sectional cohort study. BMC Cardiovasc. Disord. 2023, 23, 460. [Google Scholar] [CrossRef]
- Vasan, R.S. Cardiac function and obesity. Heart 2003, 89, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Bešević, J.; Conroy, M.; Omiyale, W.; Woodward, M.; Lacey, B.; Allen, N. Waist-to-height ratio and body fat percentage as risk factors for ischemic cardiovascular disease: A prospective cohort study from UK Biobank. Am. J. Clin. Nutr. 2024, 119, 1386–1396. [Google Scholar] [CrossRef]
- Moosaie, F.; Fatemi Abhari, S.M.; Deravi, N.; Karimi Behnagh, A.; Esteghamati, S.; Dehghani Firouzabadi, F.; Rabizadeh, S.; Nakhjavani, M.; Esteghamati, A. Waist-To-Height Ratio Is a More Accurate Tool for Predicting Hypertension Than Waist-To-Hip Circumference and BMI in Patients with Type 2 Diabetes: A Prospective Study. Front. Public Health 2021, 9, 726288. [Google Scholar] [CrossRef]
- Jamar, G.; Almeida, F.R.; Gagliardi, A.; Sobral, M.R.; Ping, C.T.; Sperandio, E.; Romiti, M.; Arantes, R.; Dourado, V.Z. Evaluation of waist-to-height ratio as a predictor of insulin resistance in non-diabetic obese individuals. A cross-sectional study. Sao Paulo Med. J. 2017, 135, 462–468. [Google Scholar] [CrossRef]
- Kurniawan, L.B. Triglyceride-Glucose Index As A Biomarker of Insulin Resistance, Diabetes Mellitus, Metabolic Syndrome, And Cardiovascular Disease: A Review. Ejifcc 2024, 35, 44–51. [Google Scholar]
| All (n = 359) | |
|---|---|
| Age, years | 70 ± 3 |
| Hypertension, n (%) | 116 (32) |
| Hypercholesterolemia, n (%) | 120 (33) |
| Smoking, n (%) | |
| Active | 39 (11) |
| Previous | 185 (52) |
| Never | 132 (37) |
| Pack-years | 15.9 ± 18.8 |
| Aspirin, n (%) | 95 (26) |
| ACEIs/ARBs, n (%) | 92 (26) |
| Beta-blocker, n (%) | 22 (6) |
| Statins, n (%) | 119 (33) |
| Duration statin treatment, months, median [IQR] | 17 [3; 60] |
| Systolic blood pressure, mmHg | 138 ± 18 |
| Diastolic blood pressure, mmHg | 81 ± 10 |
| BMI, kg/m2 | 27 ± 3 |
| Waist circumference, cm | 99 ± 10 |
| Hip, cm | 103 ± 6 |
| Waist-to-height-ratio | 0.56 ± 0.06 |
| HbA1c, mmol/mol | 36.8 ± 3.7 |
| HDL, mmol/L | 1.6 ± 0.4 |
| LDL, mmol/L | 2.8 ± 1.0 |
| Total cholesterols, mmol/L | 4.9 ± 1.0 |
| Triglycerides, mmol/L | 1.1 ± 0.6 |
| Prediabetes, n (%) | 165 (46) |
| HOMA-IR, * median [IQR] | 2.4 [1.7; 3.6] |
| FPG, mmol/L | 5.9 ± 0.6 |
| 2HPG, mmol/L | 7.0 ± 2.2 |
| TyG index | 8.4 ± 0.48 |
| hs-CRP, mg/L | 2.1 ± 2.6 |
| All (n = 359) | Lower HOMA-IR Tertile (n = 119) | Middle HOMA-IR Tertile (n = 124) | Higher HOMA-IR Tertile (n= 115) | p-Value | |
|---|---|---|---|---|---|
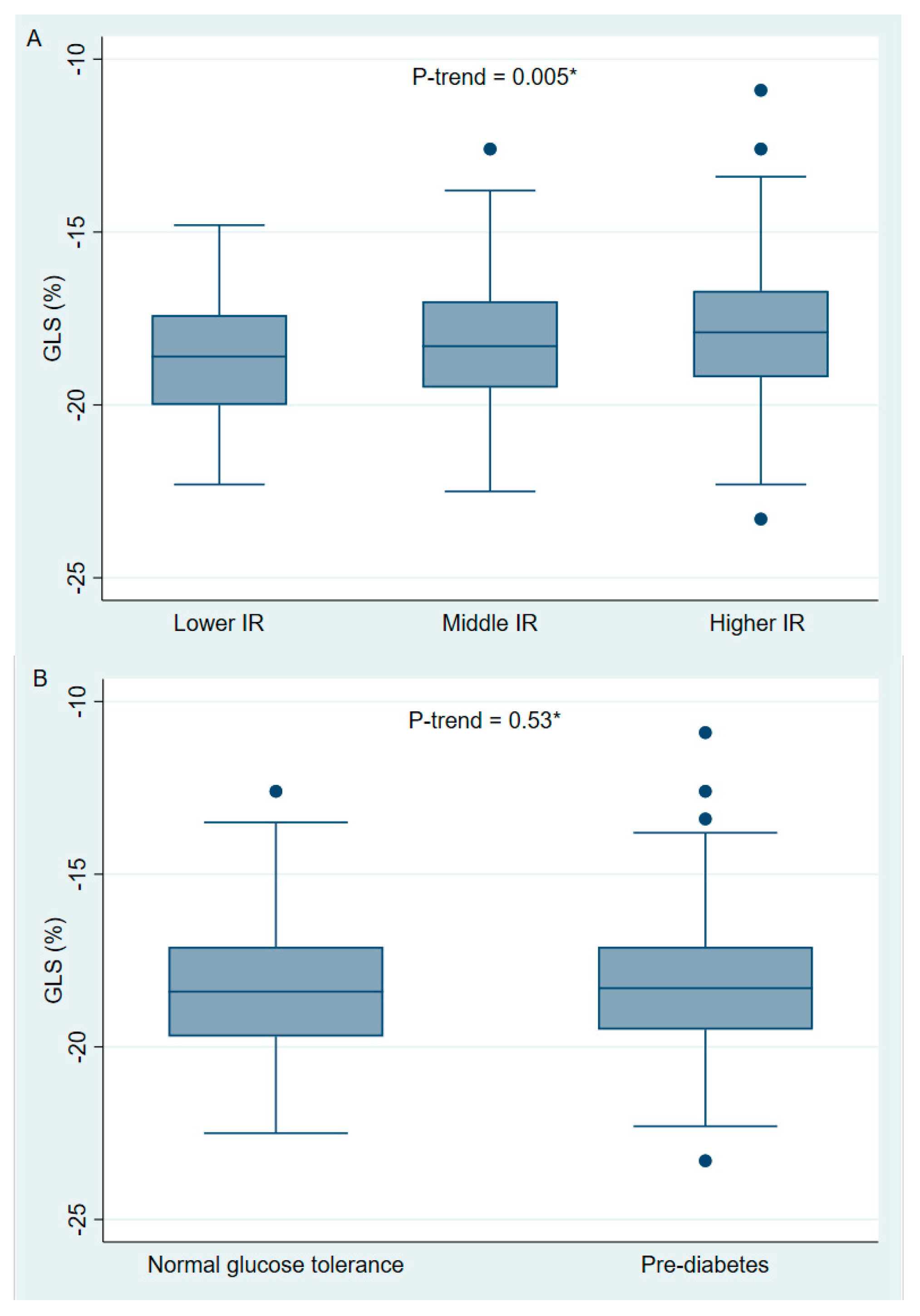
| GLS (%) | −18.3 ± 1.9 | −18.7 ± 1.7 | −18.2 ± 1.8 | −17.9 ± 2.1 ** | 0.02 |
| LVEF (%) † | 61.9 ± 5.2 | 62.2 ± 5.3 | 61.7 ± 5.2 | 61.7 ± 5.1 | 0.75 |
| LAVI (mL/m2) †† | 24.6 ± 7.8 | 25.2 ± 8.8 | 24.7 ± 7.2 | 23.6 ± 6.9 | 0.42 |
| LVMI (g/m2) ††† | 65.1 ± 14.8 | 64.9 ± 14.7 | 65.5 ± 15.5 | 64.9 ± 14.2 | 0.87 |
| E/e’ [IQR] | 8.24 [6.86; 9.85] | 8 [6.57; 9.23] | 8.17 [7.03; 9.82] | 8.40 [7.38; 10.46] | 0.08 |
| E/A [IQR] | 0.89 [0.78; 1.02] | 0.94 [0.8; 1.06] | 0.87 [0.75; 1.02] | 0.85 [0.76; 1] ** | 0.01 |
| Normal Glucose Tolerance (n = 194) | Pre-Diabetes (n = 165) | p-Value | |
|---|---|---|---|
| GLS (%) | −18.3 ± 1.8 | −18.2 ± 2.0 | 0.42 |
| LVEF (%) † | 62.3 ± 5.0 | 61.5 ± 5.3 | 0.15 |
| LAVI (mL/m2) †† | 24.0 ± 7.8 | 25.2 ± 7.8 | 0.15 |
| LVMI (g/m2) ††† | 64.0 ± 14.1 | 66.4 ± 15.4 | 0.13 |
| E/e’ [IQR] | 8.07 [6.75; 9.46] | 8.31 [7.13; 10.50] | 0.03 |
| E/A [IQR] | 0.91 [0.78; 1.04] | 0.87 [0.77; 1.01] | 0.11 |
| Univariable | Multivariable * | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| GLS | ||||||
| Pre-diabetes | 0.16 | −0.24–0.56 | 0.42 | |||
| HOMA IR tertile | ||||||
| Lower | ref. | |||||
| Middle | 0.48 | 0.005–0.96 | 0.048 | 0.09 | −0.42–0.61 | 0.73 |
| Higher | 0.74 | 0.26–1.2 | 0.003 | 0.11 | −0.47–0.69 | 0.72 |
| Statin use | 0.26 | −0.16–0.68 | 0.23 | |||
| Hypertension | 0.37 | −0.06–0.79 | 0.09 | |||
| Systolic blood pressure, mmHg | 0.01 | 0.003–0.03 | 0.01 | 0.01 | −0.0002–0.02 | 0.054 |
| Pack-years | −0.002 | −0.01–0.008 | 0.67 | |||
| WH | 7.9 | 4.6–11.3 | <0.001 | 7.1 | 3.1–11.1 | 0.001 |
| Age | 0.0008 | −0.07–0.07 | 0.98 | |||
| HbA1c | 0.05 | −0.002–0.11 | 0.06 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsson, J.; Auscher, S.; Madsen, F.S.; Overgaard, K.S.; Pararajasingam, G.; Heinsen, L.J.; Andersen, T.R.; Lindholt, J.S.; Lambrechtsen, J.; Egstrup, K. Left Ventricular Systolic Function in Asymptomatic Men Aged 65–75 Years, Relation to Insulin Resistance and Pre-Diabetes: A DANCAVAS Cross-Sectional Sub-Study. J. Cardiovasc. Dev. Dis. 2025, 12, 180. https://doi.org/10.3390/jcdd12050180
Larsson J, Auscher S, Madsen FS, Overgaard KS, Pararajasingam G, Heinsen LJ, Andersen TR, Lindholt JS, Lambrechtsen J, Egstrup K. Left Ventricular Systolic Function in Asymptomatic Men Aged 65–75 Years, Relation to Insulin Resistance and Pre-Diabetes: A DANCAVAS Cross-Sectional Sub-Study. Journal of Cardiovascular Development and Disease. 2025; 12(5):180. https://doi.org/10.3390/jcdd12050180
Chicago/Turabian StyleLarsson, Johanna, Søren Auscher, Freja Sønder Madsen, Katrine Schultz Overgaard, Gokulan Pararajasingam, Laurits Juhl Heinsen, Thomas Rueskov Andersen, Jes Sanddal Lindholt, Jess Lambrechtsen, and Kenneth Egstrup. 2025. "Left Ventricular Systolic Function in Asymptomatic Men Aged 65–75 Years, Relation to Insulin Resistance and Pre-Diabetes: A DANCAVAS Cross-Sectional Sub-Study" Journal of Cardiovascular Development and Disease 12, no. 5: 180. https://doi.org/10.3390/jcdd12050180
APA StyleLarsson, J., Auscher, S., Madsen, F. S., Overgaard, K. S., Pararajasingam, G., Heinsen, L. J., Andersen, T. R., Lindholt, J. S., Lambrechtsen, J., & Egstrup, K. (2025). Left Ventricular Systolic Function in Asymptomatic Men Aged 65–75 Years, Relation to Insulin Resistance and Pre-Diabetes: A DANCAVAS Cross-Sectional Sub-Study. Journal of Cardiovascular Development and Disease, 12(5), 180. https://doi.org/10.3390/jcdd12050180