Decreased Endothelial Progenitor Cells Are Associated with Severe Coronary Artery Disease: Insights from a Clinical Study
Abstract
1. Introduction
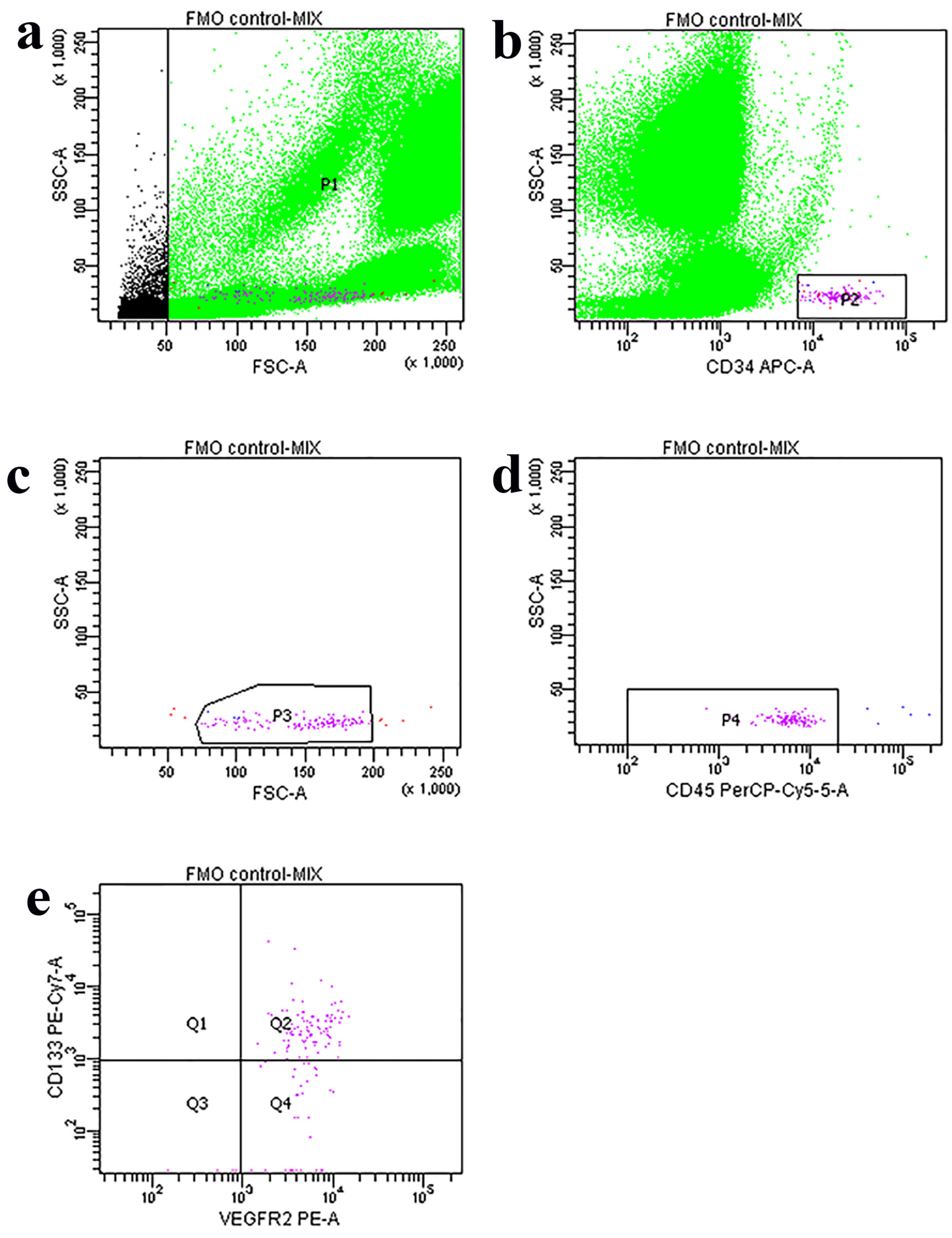
2. Materials and Methods
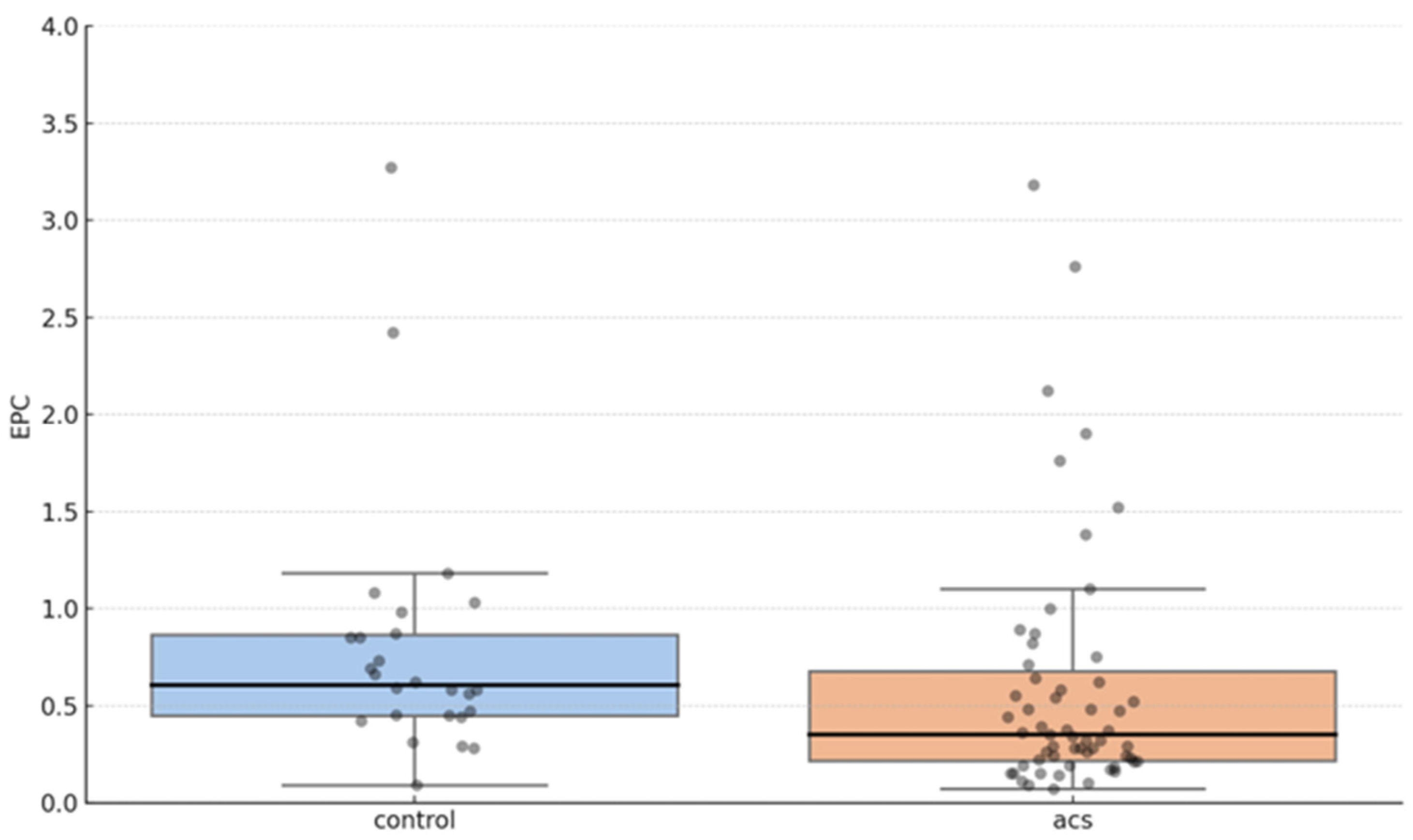
3. Results
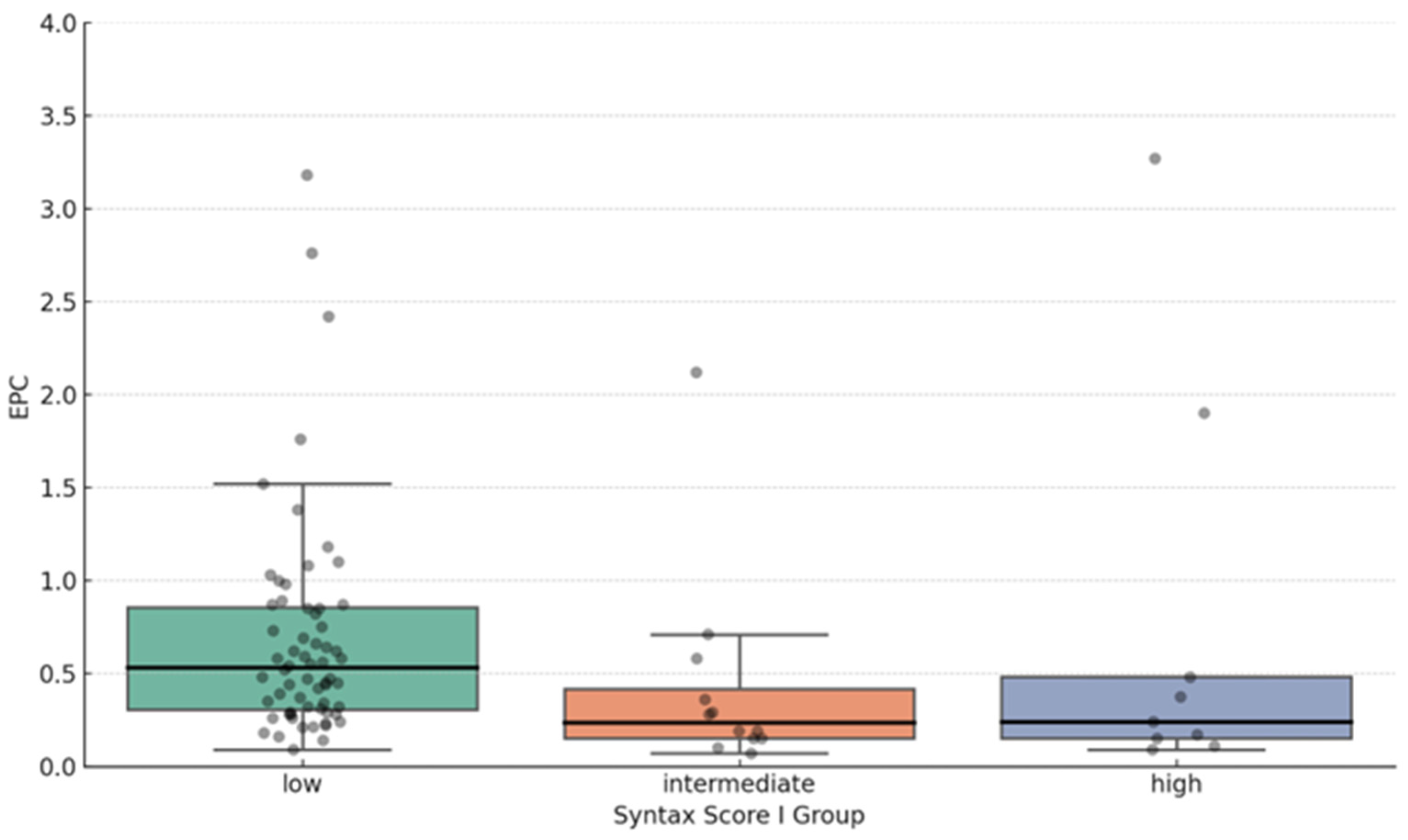
EPC Proportion and Syntax Score Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial dysfunction, inflammation and coronary artery disease: Potential biomarkers and promising therapeutical approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ganesh, M.R.; Sridhar, A.; Jena, A. Evaluation of endothelial and platelet-derived microparticles in patients with acute coronary syndrome. J. Clin. Diagn. Res. 2015, 9, OC09–OC13. [Google Scholar] [CrossRef]
- Leal, V.; Ribeiro, C.F.; Oliveiros, B.; António, N.; Silva, S. Intrinsic vascular repair by endothelial progenitor cells in acute coronary syndromes: An update overview. Stem Cell Rev Rep. 2019, 15, 35–47. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Lerman, A. Endothelial dysfunction and coronary artery disease: Assessment, prognosis, and treatment. Coron Artery Dis. 2014, 25, 713–724. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G.; Li, R.; Wei, L.; Yuan, Z.; Huang, W. Exercise training after myocardial infarction enhances endothelial progenitor cells function via NRG-1 signaling. Cardiovasc. Toxicol. 2025, 25, 411–426. [Google Scholar] [CrossRef]
- Heinisch, P.P.; Bello, C.; Emmert, M.Y.; Carrel, T.; Dreßen, M.; Hörer, J.; Winkler, B.; Luedi, M.M. Endothelial progenitor cells as biomarkers of cardiovascular pathologies: A narrative review. Cells 2022, 11, 1678. [Google Scholar] [CrossRef]
- Wojtacha, J.J.; Morawin, B.; Wawrzyniak-Gramacka, E.; Tylutka, A.; Freitas, A.K.E.d.; Zembron-Lacny, A. Endothelial dysfunction with aging: Does sex matter? Int. J. Mol. Sci. 2024, 25, 12203. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Böhm, M.; Nickenig, G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef]
- Samman Tahhan, A.; Hammadah, M.; Raad, M.; Almwaqqat, Z.; Alkhoder, A.; Sandesara, P.B.; Mohamed-Kelli, H.; Hayek, S.S.; Kim, J.H.; O’Neal, W.T.; et al. Progenitor cells and clinical outcomes in patients with acute coronary syndromes. Circ Res. 2018, 122, 421–429. [Google Scholar] [CrossRef]
- Wojakowski, W.; Kucia, M.; Kaźmierski, M.; Ratajczak, M.Z.; Tendera, M. Circulating progenitor cells in stable coronary heart disease and acute coronary syndromes: Relevant reparatory mechanism? Heart 2008, 94, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Jaffe, A.S. Caveats of high-sensitivity cardiac troponin rapid risk stratification protocols: One size does not fit all. Heart 2024, 110, 821–822. [Google Scholar] [CrossRef]
- Sianos, G.; Morel, M.A.; Kappetein, A.P.; Morice, M.C.; Colombo, A.; Dawkins, K.; van den Brand, M.; Van Dyck, N.; Russell, M.E.; Mohr, F.W.; et al. The SYNTAX Score: An angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005, 1, 219–227. [Google Scholar]
- Head, S.J.; Farooq, V.; Serruys, P.W.; Kappetein, A.P. The SYNTAX score and its clinical implications. Heart 2014, 100, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Schmitz, S.; Nauwelaers, F.; Weber, C. A flow cytometric protocol for enumeration of endothelial progenitor cells and monocyte subsets in human blood. J. Immunol. Methods 2012, 381, 9–13. [Google Scholar] [CrossRef]
- Řádek, M.; Babuňková, E.; Špaček, M.; Kvasnička, T.; Kvasnička, J. Determination of circulating endothelial cells and endothelial progenitor cells using multicolor flow cytometry in patients with thrombophilia. Acta Haematol. 2019, 142, 113–119. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial pro-genitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Dhindsa, D.S.; Desai, S.R.; Jin, Q.; Sandesara, P.B.; Mehta, A.; Liu, C.; Tahhan, A.S.; Nayak, A.; Ejaz, K.; Hooda, A.; et al. Circulating progenitor cells and outcomes in patients with coronary artery disease. Int. J. Cardiol. 2023, 373, 7–16. [Google Scholar] [CrossRef]
- Pelliccia, F.; Zimarino, M.; De Luca, G.; Viceconte, N.; Tanzilli, G.; De Caterina, R. Endothelial progenitor cells in coronary artery disease: From bench to bedside. Stem Cells Transl. Med. 2022, 11, 451–460. [Google Scholar] [CrossRef]
- Vlachogiannis, N.I.; Stellos, K. Circulating progenitor cells predict clinical outcomes in patients with coronary artery disease and renal insufficiency. JACC Basic Transl. Sci. 2020, 5, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009, 15, 3133–3145. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhou, J.; Gong, R.; Huang, X.; Pansuria, M.; Virtue, A.; Li, X.; Wang, H.; Yang, X.F. Endothelial progenitor cells in atherosclerosis. Front. Biosci. 2012, 17, 2327–2349. [Google Scholar] [CrossRef]
- Caller, T.; Rotem, I.; Shaihov-Teper, O.; Lendengolts, D.; Schary, Y.; Shai, R.; Glick-Saar, E.; Dominissini, D.; Motiei, M.; Katzir, I.; et al. Small extracellular vesicles from infarcted and failing heart accelerate tumor growth. Circulation 2024, 149, 1729–1748. [Google Scholar] [CrossRef]
- Xiao, S.T.; Kuang, C.Y. Endothelial progenitor cells and coronary artery disease: Current concepts and future research directions. World J. Clin. Cases 2021, 9, 8953–8966. [Google Scholar] [CrossRef]

| N = 85 Patients | |
|---|---|
| Sex | Male 77 (91%) Female 8 (9%) |
| Smokers | 34 (40%) |
| Acute coronary syndrome | 59 (69%) |
| STEMI NSTEMI UA Control group | 38 (45%) 13 (15%) 8 (9%) 26 (31%) |
| Age (yr) | 60 ± 10.7 |
| Height (cm) | 180.3 ± 8.3 |
| Weight (kg) | 92.7 ± 14.3 |
| BMI (kg/m2) Acetylsalicylic acid Clopidogrel Statins | 28.5 ± 4 63 (74%) 59 (69%) 67 (79%) |
| Systolic blood pressure (mmHg) | 128.1 ± 18 |
| Diastolic blood pressure (mmHg) | 78.1 ± 8.7 |
| Proportion of EPC × 10−2—median (IQR) | |
| All patients (N = 85) | 0.45 (0.26–0.82) |
| Syntax Score I groups | |
| Low | 0.53 (0.31–0.86) |
| Intermediate | 0.23 (0.15–0.42) |
| High | 0.24 (0.15–0.48) |
| Coronary artery disease groups | |
| ACS (N = 59) | 0.35 (0.22–0.68) |
| Control group (N = 26) | 0.61 (0.45–0.87) |
| Variable | Coefficient | Std. Error | t-Statistic | p-Value |
|---|---|---|---|---|
| Intercept | 11.28 | 1.87 | 6.03 | <0.001 |
| Log (EPC) | −3.54 | 1.59 | −2.22 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić, I.; Zeljko, I.; Brizić, I.; Šoljić, V.; Ivančić, I.; Tomić, M.; Ćurlin, M.; Tomić, D. Decreased Endothelial Progenitor Cells Are Associated with Severe Coronary Artery Disease: Insights from a Clinical Study. J. Cardiovasc. Dev. Dis. 2025, 12, 132. https://doi.org/10.3390/jcdd12040132
Tomić I, Zeljko I, Brizić I, Šoljić V, Ivančić I, Tomić M, Ćurlin M, Tomić D. Decreased Endothelial Progenitor Cells Are Associated with Severe Coronary Artery Disease: Insights from a Clinical Study. Journal of Cardiovascular Development and Disease. 2025; 12(4):132. https://doi.org/10.3390/jcdd12040132
Chicago/Turabian StyleTomić, Ivan, Ivan Zeljko, Ivica Brizić, Violeta Šoljić, Ivona Ivančić, Monika Tomić, Marina Ćurlin, and Domagoj Tomić. 2025. "Decreased Endothelial Progenitor Cells Are Associated with Severe Coronary Artery Disease: Insights from a Clinical Study" Journal of Cardiovascular Development and Disease 12, no. 4: 132. https://doi.org/10.3390/jcdd12040132
APA StyleTomić, I., Zeljko, I., Brizić, I., Šoljić, V., Ivančić, I., Tomić, M., Ćurlin, M., & Tomić, D. (2025). Decreased Endothelial Progenitor Cells Are Associated with Severe Coronary Artery Disease: Insights from a Clinical Study. Journal of Cardiovascular Development and Disease, 12(4), 132. https://doi.org/10.3390/jcdd12040132






