Early vs. Late Endovascular Extension Following Frozen Elephant Trunk Procedure: Effects on Clinical Outcomes and Aortic Remodeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Follow-Up
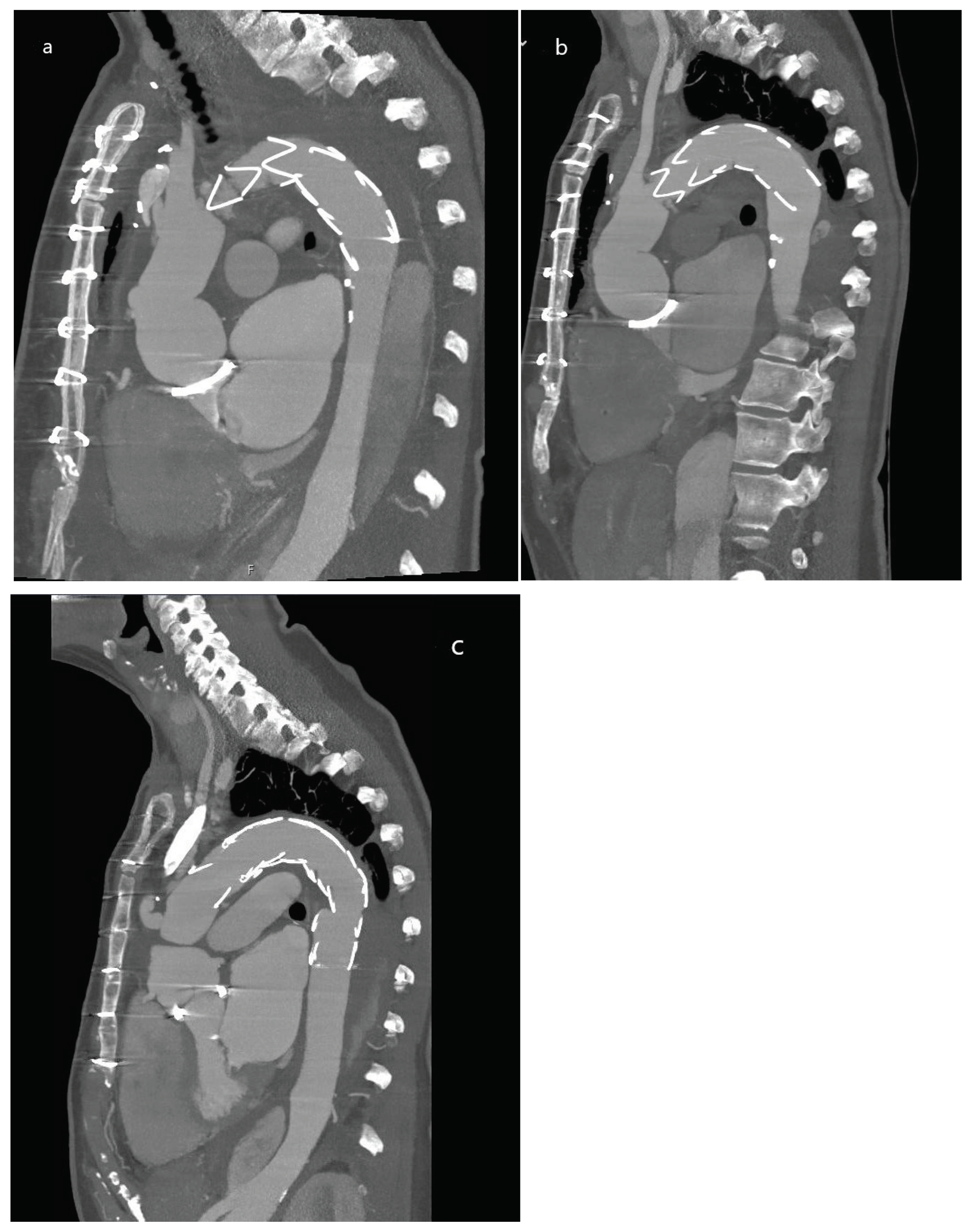
2.2. Imaging and Surgical Procedure
3. Results
3.1. Surgical Data
3.2. Early Follow-Up and Outcomes
3.3. Mid-Term Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal fluid |
| CTA | Computed tomography angiography |
| DOAJ | Directory of open access journals |
| dSINE | Distal stent-induced new entry |
| DTA | Descending thoracic aorta |
| EC | Early completion |
| FET | Frozen elephant trunk |
| LC | Late completion |
| LSA | Left subclavian artery |
| MDPI | Multidisciplinary Digital Publishing Institute |
| TEVAR | Thoracic endovascular aortic repair |
References
- Borst, H.G.; Walterbusch, G.; Schaps, D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac. Cardiovasc. Surg. 1983, 31, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Dohle, D.-S.; El Beyrouti, H.; Brendel, L.; Pfeiffer, P.; El-Mehsen, M.; Vahl, C.-F. Survival and reinterventions after isolated proximal aortic repair in acute type A aortic dissection. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 981–988. [Google Scholar] [CrossRef]
- Leeuwerke, S.J.G.; Bohan, P.; Saleem, B.R.; Clucas, J.; Reijnen, M.M.P.J.; Zeebregts, C.J. Frozen Elephant Trunk Completion: Endovascular Extension in The Distal Thoracic Aorta. Surg. Technol. Int. 2022, 40, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Suto, Y.; Yasuda, K.; Shiiya, N.; Murashita, T.; Kawasaki, M.; Imamura, M.; Takigami, K.; Sasaki, S.; Matsui, Y.; Sakuma, M. Stented elephant trunk procedure for an extensive aneurysm involving distal aortic arch and descending aorta. J. Thorac. Cardiovasc. Surg. 1996, 112, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Grabenwöger, M.; Berger, T.; Aboyans, V.; Della Corte, A.; Chen, E.P.; Desai, N.D.; Dumfarth, J.; Elefteriades, J.A.; Etz, C.D.; et al. EACTS/STS Guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezad426. [Google Scholar] [CrossRef]
- Bashir, M.; Mohammed, I.; Al-Tawil, M.; Jubouri, M.; Agbobu, T.; Chen, E.P. Frozen elephant trunk: The gold standard. Cardiovasc. Diagn. Ther. 2023, 13, 623–627. [Google Scholar] [CrossRef]
- Jakob, H.; Moughal, S.; Bashir, M. Frozen elephant trunk with straight vascular prosthesis: Single-center experience with a review of current trends. J. Cardiovasc. Surg. 2020, 61, 301–307. [Google Scholar] [CrossRef]
- Hostalrich, A.; Porterie, J.; Boisroux, T.; Marcheix, B.; Ricco, J.B.; Chaufour, X. Outcomes of Secondary Endovascular Aortic Repair After Frozen Elephant Trunk. J. Endovasc. Ther. 2023, 32, 15266028231169172. [Google Scholar] [CrossRef]
- Kreibich, M.; Berger, T.; Rylski, B.; Chen, Z.; Beyersdorf, F.; Siepe, M.; Czerny, M. Aortic reinterventions after the frozen elephant trunk procedure. J. Thorac. Cardiovasc. Surg. 2020, 159, 392–399.e1. [Google Scholar] [CrossRef]
- Berger, T.; Graap, M.; Rylski, B.; Fagu, A.; Gottardi, R.; Walter, T.; Discher, P.; Hagar, M.T.; Kondov, S.; Czerny, M.; et al. Distal Aortic Failure Following the Frozen Elephant Trunk Procedure for Aortic Dissection. Front. Cardiovasc. Med. 2022, 9, 911548. [Google Scholar] [CrossRef]
- Gombert, A.; Ketting, S.; Rückbeil, M.V.; Hundertmark, A.-K.; Barbati, M.; Keschenau, P.; Pedersoli, F.; Schurink, G.W.; Mees, B.; Kotelis, D.; et al. Perioperative and long-term outcome after ascending aortic and arch repair with elephant trunk and open thoracoabdominal aortic aneurysm repair. J. Vasc. Surg. 2022, 75, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Ali-Hasan-Al-Saegh, S.; Halloum, N.; Scali, S.; Kriege, M.; Abualia, M.; Stamenovic, D.; Bashar Izzat, M.; Bohan, P.; Kloeckner, R.; Oezkur, M.; et al. A systematic review and meta-analysis of retrograde type A aortic dissection after thoracic endovascular aortic repair in patients with type B aortic dissection. Medicine 2023, 102, e32944. [Google Scholar] [CrossRef]
- El Beyrouti, H.; Lescan, M.; Doemland, M.; Mustafi, M.; Jungmann, F.; Jorg, T.; Halloum, N.; Dorweiler, B. Early results of a low-profile stent-graft for thoracic endovascular aortic repair. PLoS ONE 2020, 15, e0240560. [Google Scholar] [CrossRef] [PubMed]
- Halloum, N.; Meyer, A.-S.; Wenkel, M.; Dohle, D.-S.; Youssef, M.; Dorweiler, B.; Treede, H.; El Beyrouti, H. Aortic Remodeling After False Lumen Embolization in Aortic Dissection. J. Clin. Med. 2025, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Czerny, M.; Schmidli, J.; Adler, S.; van den Berg, J.C.; Bertoglio, L.; Carrel, T.; Chiesa, R.; Clough, R.E.; Eberle, B.; Etz, C.; et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: An expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur. J. Cardio-Thorac. Surg. 2019, 55, 133–162. [Google Scholar] [CrossRef]
- Leone, A.; Beckmann, E.; Martens, A.; Di Marco, L.; Pantaleo, A.; Reggiani, L.B.; Haverich, A.; Di Bartolomeo, R.; Pacini, D.; Shrestha, M. Total aortic arch replacement with frozen elephant trunk technique: Results from two European institutes. J. Thorac. Cardiovasc. Surg. 2020, 159, 1201–1211. [Google Scholar] [CrossRef]
- Mylonas, S.N.; Mammadov, R.; Dorweiler, B. Complementary Thoracic Endovascular Aortic Repair (TEVAR) After Frozen Elephant Trunk for Residual Type A Aortic Dissection: Perioperative and Mid-Term Outcomes. J. Clin. Med. 2024, 13, 3007. [Google Scholar] [CrossRef]
- Rustum, S.; Beckmann, E.; Wilhelmi, M.; Krueger, H.; Kaufeld, T.; Umminger, J.; Haverich, A.; Martens, A.; Shrestha, M. Is the frozen elephant trunk procedure superior to the conventional elephant trunk procedure for completion of the second stage? Eur. J. Cardio-Thorac. Surg. 2017, 52, 725–732. [Google Scholar] [CrossRef]
- Takagi, H.; Umemoto, T.; ALICE Group. A Meta-Analysis of Total Arch Replacement with Frozen Elephant Trunk in Acute Type A Aortic Dissection. Vasc. Endovasc. Surg. 2016, 50, 33–46. [Google Scholar] [CrossRef]
- Liebrich, M.; Charitos, E.I.; Schlereth, S.; Meißner, H.; Trabold, T.; Geisbüsch, P.; Hemmer, W.; Seeburger, J.; Voth, V. The zone 2 concept and distal stent graft positioning in TH 2-3 are associated with high rates of secondary aortic interventions in frozen elephant trunk surgery. Eur. J. Cardio-Thorac. Surg. 2021, 60, 343–351. [Google Scholar] [CrossRef]
- Fortin, W.; Gautier, C.-H.; Escande, R.; Bel, A.; Sutter, W.; El Batti, S.; Julia, P.; Achouh, P.; Alsac, J.-M. Thoracic Endovascular Repair After Total Aortic Arch Replacement with Frozen Elephant Trunk for Type a Aortic Dissection. Ann. Vasc. Surg. 2024, 99, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Meisenbacher, K.; Osswald, A.; Bischoff, M.S.; Böckler, D.; Karck, M.; Ruhparwar, A.; Geisbüsch, P. TEVAR Following FET: Current Outcomes of Rendezvous Procedures in Clinical Practice. Thorac. Cardiovasc. Surg. 2022, 70, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.; Gudbjartsson, T.; Ahlsson, A.; Fuglsang, S.; Geirsson, A.; Hansson, E.C.; Hjortdal, V.; Jeppsson, A.; Järvelä, K.; Mennander, A.; et al. Low rate of reoperations after acute type A aortic dissection repair from The Nordic Consortium Registry. J. Thorac. Cardiovasc. Surg. 2018, 156, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira Santos, Á.; Cruz Tomás, A.; Varela-Afonso, D.; Pinheiro Santos, J.; Fragata, J. Elective 2nd Stage TEVAR to Complete Frozen Elephant Trunk in the Surgical Correction of Complex Multisegmental Pathology of the Thoracic Aorta. Rev. Port. Cir. Cardio-Torac. Vasc. Orgao Of. Soc. Port. Cir. Cardio-Torac. Vasc. 2017, 24, 164. [Google Scholar]
- Uchida, N.; Kodama, H.; Katayama, K.; Takasaki, T.; Katayama, A.; Takahashi, S.; Sueda, T. Endovascular aortic repair as second-stage surgery after hybrid open arch repair by the frozen elephant trunk technique for extended thoracic aneurysm. Ann. Thorac. Cardiovasc. Surg. 2013, 19, 257–261. [Google Scholar] [CrossRef]
- Brisard, L.; El Batti, S.; Borghese, O.; Maurel, B. Risk Factors for Spinal Cord Injury During Endovascular Repair of Thoracoabdominal Aneurysm: Review of the Literature and Proposal of a Prognostic Score. J. Clin. Med. 2023, 12, 7520. [Google Scholar] [CrossRef]
- Rinaldi, E.; Melloni, A.; Gallitto, E.; Fargion, A.; Isernia, G.; Kahlberg, A.; Bertoglio, L.; Faggioli, G.; Lenti, M.; Pratesi, C.; et al. Spinal Cord Ischemia After Thoracoabdominal Aortic Aneurysms Endovascular Repair: From the Italian Multicenter Fenestrated/Branched Endovascular Aneurysm Repair Registry. J. Endovasc. Ther. 2023, 30, 281–288. [Google Scholar] [CrossRef]
- Aucoin, V.J.; Motyl, C.M.; Novak, Z.; Eagleton, M.J.; Farber, M.A.; Gasper, W.; Oderich, G.S.; Mendes, B.; Schanzer, A.; Tenorio, E.; et al. Predictors and outcomes of spinal cord injury following complex branched/fenestrated endovascular aortic repair in the US Aortic Research Consortium. J. Vasc. Surg. 2023, 77, 1578–1587. [Google Scholar] [CrossRef]



| All | Early Completion | Late Completion | p-Value | |
|---|---|---|---|---|
| n | 37 | 14 (37.8%) | 23 (62.2%) | |
| Male | 24 (64.9%) | 11 (78.6%) | 13 (56.5%) | |
| Age at FET (years) | 62.3 ± 10.5 | 62.8 ± 12.1 | 62.1 ± 9.3 | 0.860 |
| Age at TEVAR (years) | 63.3 ± 10.3 | 62.8 ± 12.1 | 63.6 ± 9.0 | 0.834 |
| BMI (kg/m2) | 25.8 ± 4.6 | 27.2 ± 3.2 | 24.9 ± 5.0 | 0.135 |
| Comorbidities | ||||
| Arterial hypertension | 31 (83.3%) | 10 (71.4%) | 21 (91.3%) | 0.118 |
| Diabetes | 1 (2.7%) | 0 | 1 (4.3%) | 0.443 |
| CAD | 4 (10.8%) | 1 (7.1%) | 3 (13.0%) | 0.587 |
| COPD | 1 (2.7%) | 1 (71%) | 0 | 0.336 |
| CKD | 4 (10.8%) | 2 (14.3%) | 3 (13.0%) | 0.629 |
| Stroke | 6 (16.2%) | 3 (21.4%) | 3 (13.0%) | 0.539 |
| Smoking history | ||||
| Former or current | 14 (37.8%) | 5 (35.7%) | 9 (39.1%) | 0.841 |
| Atrial fibrillation | 3 (8.1%) | 1 (7.1%) | 2 (8.7%) | 0.871 |
| Anticoagulants and platelet inhibitors | ||||
| DOAC or coumadin | 7 (18.9%) | 2 (14.3%) | 5 (21.7%) | 0.573 |
| Platelet inhibitors | 24 (64.9%) | 3 (21.4%) | 21 (91.3%) | <0.001 |
| Abdominal aneurysm | 5 (13.5%) | 1 (7.1%) | 4 (17.4%) | 0.349 |
| Previous vascular or endovascular surgery | 4 (10.8%) | 0 | 4 (17.4%) | 0.104 |
| ASA I | 0 | 0 | 0 | |
| ASA II | 4 (10.8%) | 2 (14.3%) | 2 (8.7%) | 0.607 |
| ASA III | 14 (37.8%) | 0 | 14 (60.9%) | 0.206 |
| ASA IV | 19 (51.4%) | 12 (85.7%) | 7 (30.4%) | 0.145 |
| All | Early Completion | Late Completion | p-Value | |
|---|---|---|---|---|
| Diameter post-FET (mm) | ||||
| Aneurysm diameter/Aorta (Total) | 39.9 ± 9.7 | 38.4 ± 7.8 | 40.6 ± 10.3 | |
| True lumen | 20.7 ± 8.5 | 17.3 ± 7.2 | 23.3 ± 8.6 | |
| False lumen | 20.8 ± 11.5 | 21.1 ±10.4 | 20.5 ± 12.2 | |
| Diameter pre-TEVAR (mm) | ||||
| Aneurysm diameter/aorta (total) | 45.9 ± 14.1 | 46.2 ± 18.8 | 45.8 ± 11.0 | |
| True lumen | 24.7 ± 14.7 | 17.3 ± 7.2 | 30.1 ± 16.3 | |
| False lumen | 19.6 ± 14.7 | 21.1 ± 10.4 | 18.5 ± 17.1 | |
| Diameter post-TEVAR (mm) | ||||
| Aneurysm diameter/aorta (total) | 39.6 ± 10.9 | 31.6 ± 7.7 | 42.9 ± 10.3 | |
| True lumen | 23.7 ± 9.7 | 26.0 ± 12.4 | 22.1 ± 6.6 | |
| False lumen | 13.8 ± 10.9 | 12.1 ± 11.2 | 15.1 ± 10.6 | |
| Diameter follow-up (mm) | ||||
| Aneurysm diameter/aorta (total) | 39.7 ± 8.3 | 38.3 ± 9.9 | 40.2 ± 7.6 | |
| True lumen | 24.2 ± 4.9 | 24.5 ± 3.5 | 24.0 ± 5.5 | |
| False lumen | 16.4 ± 12.6 | 14.8 ± 11.7 | 17.2 ± 13.0 | |
| Angulation post-FET | 20.9° ± 33 | 20.0° ± 33 | 21.4° ± 33 | 0.909 |
| Angulation pre-TEVAR | 30.7° ± 35 | 20.0° ± 33 | 36.7° ± 35 | 0.183 |
| All | Early Completion | Late Completion | p-Value | |
|---|---|---|---|---|
| Interval between surgeries | 11.4 ± 16.7 months | 4.8 ± 5.2 days | 18.4 ± 18.0 months | |
| Urgency | ||||
| Emergency | 12 (32.4%) | 8 (57.1%) | 4 (17.4%) | 0.277 |
| Urgent | 9 (24.3%) | 5 (35.7%) | 4 (17.4%) | 0.757 |
| Elective | 16 (43.2%) | 1 (7.1%) | 15 (65.2%) | 0.193 |
| Indication | ||||
| Intended 2-stage | 6 (16.2%) | 3 (21.4%) | 3 (13.0%) | |
| Endoleak | 8 (21.6%) | 3 (21.4%) | 5 (21.7%) | |
| dSINE | 6 (16.2%) | 1 (7.1%) | 5 (21.7%) | |
| Rupture | 2 (5.4%) | 1 (7.1%) | 1 (4.3%) | |
| Aneurysm growth | 1 (2.7%) | 0 | 1 (4.3%) | |
| Malperfusion | 6 (16.2%) | 4 (28.6%) | 2 (8.7%) | |
| Endoleak + dSINE | 3 (8.1%) | 0 | 3 (13.0%) | |
| Endoleak + aneurysm growth | 3 (8.1%) | 2 (14.3%) | 1 (4.3%) | |
| Other | 2 (5.4%) | 0 | 2 (8.7%) | |
| Duration of surgery (min) | 118 ± 60 | 146 ± 110 | 108 ± 110 | 0.068 |
| Contrast used (mL) | 117 ± 70 | 132 ± 100 | 101 ± 100 | 0.383 |
| Radiation dose (cGy*cm2) | 3596 ± 3261 | 4105 ± 2552 | 3286 ± 2552 | 0.506 |
| TEVAR diameter (mm) | 31 (24–44) | 31 (28–42) | 36 (24–44) | 0.758 |
| TEVAR length (mm) | 164 (94–209) | 164 (94–209) | 164 (104–209) | 0.576 |
| Number of TEVAR devices used | ||||
| 1 device | 23 (62.2%) | 11 (78.6%) | 12 (52.2%) | 0.114 |
| 2 devices | 13 (35.1%) | 3 (21.4%) | 10 (43.5%) | 0.183 |
| 3 devices | 1 (2.7%) | 0 | 1 (4.3%) | 0.443 |
| Tapered TEVAR | 8 (21.6%) | 2 (14.3%) | 6 (26.1%) | 0.412 |
| Vascular access | ||||
| Cut-down | 24 (64.9%) | 11 (78.6%) | 13 (56.5%) | 0.183 |
| Percutaneous | 13 (35.1%) | 3 (21.4%) | 10 (43.5%) | 0.004 |
| CSF drainage | 4 (10.8%) | 1 (7.1%) | 3 (13.0%) | 0.294 |
| Technical success | 37 (100%) | 14 (100%) | 23 (100%) | |
| Conversion | 0 | 0 | 0 | |
| Concomitant procedure | 6 (16.2%) | 5 (35.7%) | 1 (4.3%) | 0.040 |
| All | Early Completion | Late Completion | p-Value | |
|---|---|---|---|---|
| Mortality | ||||
| 30-day mortality (n = 37) | 1 (2.7%) | 1 (7.1%) | 0 | 0.336 |
| Late mortality (n = 33) | 3 (9.1%) | 3 (23.1%) | 0 | 0.038 |
| Intensive care (days) | 3.9 ± 6.5 | 10.7 ± 6.9 | 0.1 ± 0.3 | <0.001 |
| Hospitalization (days) | 13.5 ± 11.9 | 22.4 ± 14 | 8.1 ± 5.6 | 0.003 |
| Follow-up (months) | 22.0 ± 22.9 | 24.7 ± 24.6 | 20.5 ± 21.8 | 0.497 |
| 30-day complications | ||||
| Myocardial infarction | 0 | 0 | 0 | |
| Stroke | 1 (2.7%) | 1 (7.1%) | 0 | 0.336 |
| Bleeding | 2 (5.4%) | 1 (7.1%) | 1 (4.3%) | 0.741 |
| Respiratory failure | 7 (18.9%) | 7 (50.0%) | 0 | 0.003 |
| Acute kidney failure | 4 (10.8%) | 4 (28.6%) | 0 | 0.040 |
| Spinal cord injury | 1 (2.7%) | 1 (7.1%) | 0 | 0.336 |
| Surgical site infection | 0 | 0 | 0 | |
| Sepsis | 2 (5.4%) | 2 (14.3%) | 0 | 0.165 |
| Pseudoaneurysm | 1 (2.7%) | 0 | 1 (4.3%) | 0.328 |
| Complications during mid-term follow-up (n = 33) | ||||
| Myocardial infarction | 0 | 0 | 0 | |
| Stroke | 1 (3.1%) | 0 | 1 (4.3%) | 0.329 |
| Aortic rupture | 1 (3.1%) | 0 | 1 (4.3%) | 0.329 |
| Graft migration | 0 | 0 | 0 | |
| Graft infection | 0 | 0 | 0 | |
| Reintervention | 4 (12.5%) | 1 (7.1%) | 3 (13.0%) | 0.805 |
| All | Early Completion | Late Completion | p-Value | |
|---|---|---|---|---|
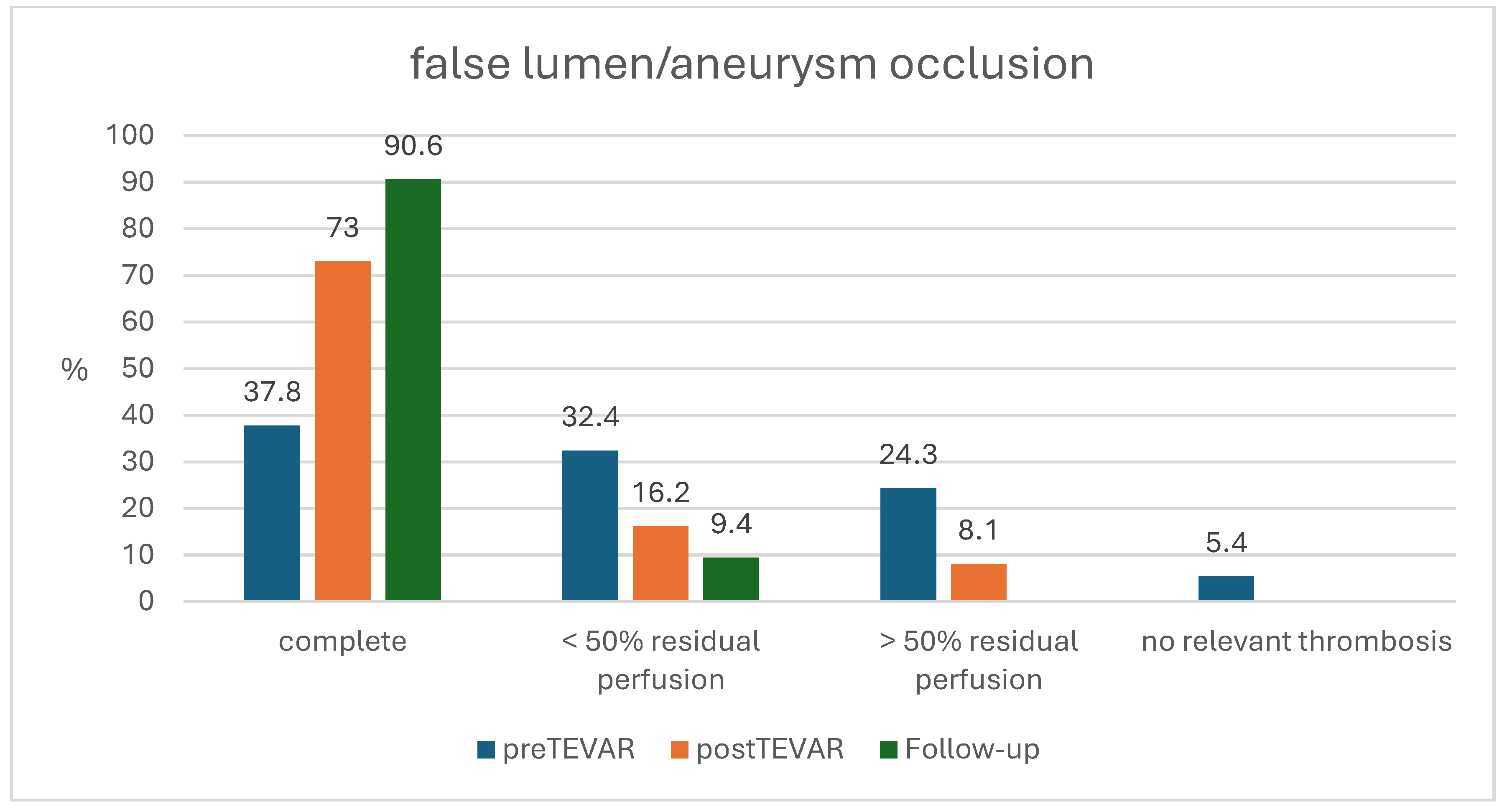
| Pre-TEVAR CTA | n = 37 | n = 14 | n = 23 | |
| Complete occlusion | 14 (37.8%) | 6 (42.5%) | 8 (34.8%) | 0.635 |
| <50% residual perfusion | 12 (32.4%) | 3 (21.4%) | 9 (39.1%) | 0.277 |
| >50% residual perfusion | 9 (24.3%) | 3 (21.4%) | 6 (26.1%) | 0.757 |
| No relevant thrombosis | 2 (5.4%) | 2 (14.3%) | 0 | 0.065 |
| Post-TEVAR CTA | n = 36 | n = 13 | n = 23 | |
| Complete occlusion | 27 (73.0%) | 12 (92.3%) | 15 (65.2%) | 0.170 |
| <50% residual perfusion | 6 (16.2%) | 0 | 6 (26.1%) | 0.065 |
| >50% residual perfusion | 3 (8.1%) | 1 (7.7%) | 2 (8.7%) | 0.920 |
| No relevant thrombosis | 0 | 0 | 0 | |
| Endoleaks | ||||
| Type Ib | 4 (10.8%) | 1 (7.1%) | 3 (13.0%) | 0.564 |
| Type II | 3 (8.1%) | 1 (7.1%) | 2 (8.7%) | 0.871 |
| Type III | 0 | 0 | 0 | |
| dSINE | 0 | 0 | 0 | |
| CTA at Follow-up | n = 32 | n = 10 | n = 22 | |
| Complete occlusion | 29 (90.6%) | 8 (80.0%) | 21 (95.5%) | 0.920 |
| <50% residual perfusion | 3 (9.4%) | 2 (20.0%) | 1 (4.5%) | 0.175 |
| >50% residual perfusion | 0 | 0 | 0 | |
| No relevant thrombosis | 0 | 0 | 0 | |
| Endoleak | ||||
| Type Ib | 4 (10.8%) | 2 (20.0%) | 2 (9.1%) | 0.429 |
| Type II | 1 (2.7%) | 0 | 1 (4.5%) | 0.329 |
| Type III | 1 (2.7%) | 1 (10.0%) | 0 | 0.347 |
| dSINE | 1 (2.7%) | 0 | 1 (4.5%) | 0.329 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenkel, M.; Halloum, N.; Neufang, A.; Doemland, M.; Pfeiffer, P.; Ghazy, A.; Probst, C.; Dohle, D.-S.; Treede, H.; El Beyrouti, H. Early vs. Late Endovascular Extension Following Frozen Elephant Trunk Procedure: Effects on Clinical Outcomes and Aortic Remodeling. J. Cardiovasc. Dev. Dis. 2025, 12, 99. https://doi.org/10.3390/jcdd12030099
Wenkel M, Halloum N, Neufang A, Doemland M, Pfeiffer P, Ghazy A, Probst C, Dohle D-S, Treede H, El Beyrouti H. Early vs. Late Endovascular Extension Following Frozen Elephant Trunk Procedure: Effects on Clinical Outcomes and Aortic Remodeling. Journal of Cardiovascular Development and Disease. 2025; 12(3):99. https://doi.org/10.3390/jcdd12030099
Chicago/Turabian StyleWenkel, Martin, Nancy Halloum, Achim Neufang, Marco Doemland, Philipp Pfeiffer, Ahmad Ghazy, Chris Probst, Daniel-Sebastian Dohle, Hendrik Treede, and Hazem El Beyrouti. 2025. "Early vs. Late Endovascular Extension Following Frozen Elephant Trunk Procedure: Effects on Clinical Outcomes and Aortic Remodeling" Journal of Cardiovascular Development and Disease 12, no. 3: 99. https://doi.org/10.3390/jcdd12030099
APA StyleWenkel, M., Halloum, N., Neufang, A., Doemland, M., Pfeiffer, P., Ghazy, A., Probst, C., Dohle, D.-S., Treede, H., & El Beyrouti, H. (2025). Early vs. Late Endovascular Extension Following Frozen Elephant Trunk Procedure: Effects on Clinical Outcomes and Aortic Remodeling. Journal of Cardiovascular Development and Disease, 12(3), 99. https://doi.org/10.3390/jcdd12030099






