Gender Differences for His Bundle Pacing Long-Term Performance in the Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
2.3. Pacing Procedure
2.4. Follow-Up
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Procedural Characteristics
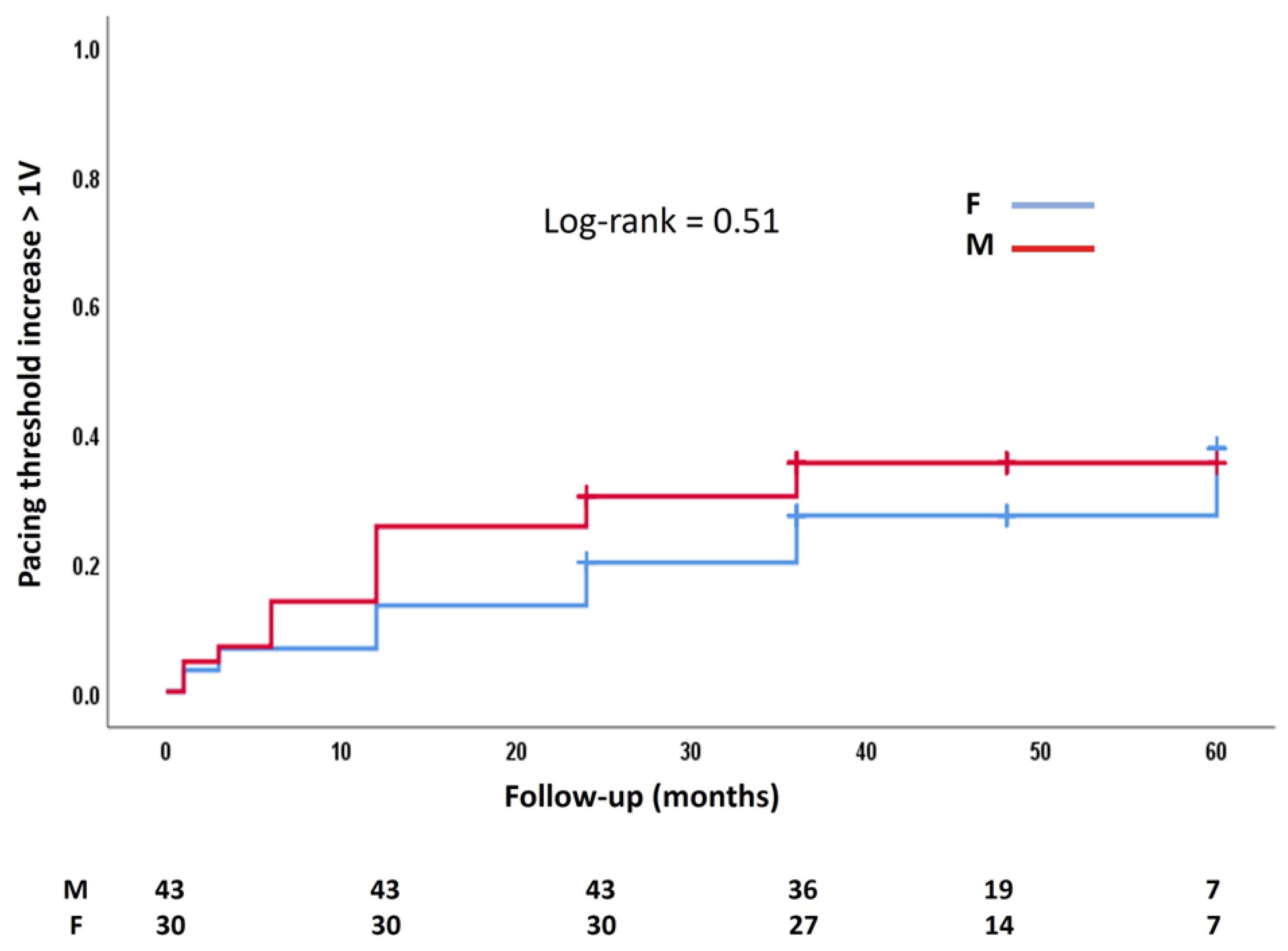
3.2. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Vries, L.M.; Dijk, W.A.; Hooijschuur, C.A.M.; Leening, M.J.G.; Stricker, B.H.C.; van Hemel, N.M. Utilisation of cardiac pacemakers over a 20-year period: Results from a nationwide pacemaker registry. Neth. Hear. J. 2016, 25, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Sumiyoshi, M.; Kimura, A.; Minami-Takano, A.; Maruyama, K.; Kimura, Y.; Tabuchi, H.; Hayashi, H.; Odagiri, F.; Sekita, G.; et al. Trend in Age at the Initial Pacemaker Implantation in Patients With Bradyarrhythmia—A 50-Year Analysis (1970–2019) in Japan. Circ. J. 2022, 86, 1292–1297. [Google Scholar] [CrossRef]
- Pyatt, J.R.; Somauroo, J.D.; Jackson, M.; Grayson, A.D.; Osula, S.; Aggarwal, R.K.; Charles, R.G.; Connelly, D.T. Long-term survival after permanent pacemaker implantation: Analysis of predictors for increased mortality. EP Europace 2002, 4, 113–119. [Google Scholar] [CrossRef]
- Nowak, B.; Misselwitz, B.; Erdogan, A.; Funck, R.; Irnich, W.; Israel, C.; Olbrich, H.-G.; Schmidt, H.; Sperzel, J.; Zegelman, M. Do gender differences exist in pacemaker implantation?—results of an obligatory external quality control program. EP Europace 2009, 12, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Makki, T.; Kumar, R.; Gumber, D.; Kwon, D.H.; Rickard, J.W.; Kanj, M.; Wazni, O.M.; Saliba, W.I.; Varma, N.; et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Hear. Rhythm. O2 2016, 13, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.A.; Razminia, P.; Tung, R. His-bundle pacing is the best approach to physiological pacing. Hear. Rhythm. O2 2020, 1, 68–75. [Google Scholar] [CrossRef]
- Keene, D.; Anselme, F.; Burri, H.; Pérez, Ó.C.; Čurila, K.; Derndorfer, M.; Foley, P.; Gellér, L.; Glikson, M.; Huybrechts, W.; et al. Conduction system pacing, a European survey: Insights from clinical practice. EP Europace 2023, 25, 1–9. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Subzposh, F.A.; Beer, D.; Durr, B.; Naperkowski, A.; Sun, H.; Oren, J.W.; Dandamudi, G.; Vijayaraman, P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018, 71, 2319–2330. [Google Scholar] [CrossRef]
- Vinther, M.; Risum, N.; Svendsen, J.H.; Møgelvang, R.; Philbert, B.T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients With Left Bundle Branch Block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Pestrea, C.; Cicala, E.; Gherghina, A.; Ortan, F.; Pop, D. Feasibility of Permanent His Bundle Pacing in the Elderly vs the Very Elderly. A Single-Center Mid-Term Follow-Up Study. Clin. Interv. Aging 2023, 18, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Burri, H.; Jastrzebski, M.; Cano, Ó.; Čurila, K.; de Pooter, J.; Huang, W.; Israel, C.; Joza, J.; Romero, J.; Vernooy, K.; et al. EHRA clinical consensus statement on conduction system pacing implantation: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). EP Europace 2023, 25, 1208–1236. [Google Scholar] [CrossRef]
- Tokavanich, N.; Prasitlumkum, N.; Mongkonsritragoon, W.; Cheungpasitporn, W.; Thongprayoon, C.; Vallabhajosyula, S.; Chokesuwattanaskul, R. A network meta-analysis and systematic review of change in QRS duration after left bundle branch pacing, His bundle pacing, biventricular pacing, or right ventricular pacing in patients requiring permanent pacemaker. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Oren, J.W.; et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: A secondary analysis of the His-SYNC Pilot Trial. Hear. Rhythm. O2 2019, 16, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Abdin, A.; Aktaa, S.; Vukadinović, D.; Arbelo, E.; Burri, H.; Glikson, M.; Meyer, C.; Munyombwe, T.; Nielsen, J.C.; Ukena, C.; et al. Outcomes of conduction system pacing compared to right ventricular pacing as a primary strategy for treating bradyarrhythmia: Systematic review and meta-analysis. Clin. Res. Cardiol. 2021, 111, 1198–1209. [Google Scholar] [CrossRef]
- Kawashima, T.; Sasaki, H. A macroscopic anatomical investigation of atrioventricular bundle locational variation relative to the membranous part of the ventricular septum in elderly human hearts. Surg. Radiol. Anat. 2005, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Keene, D.; Arnold, A.D.; Jastrzębski, M.; Burri, H.; Zweibel, S.; Crespo, E.; Chandrasekaran, B.; Bassi, S.; Joghetaei, N.; Swift, M.; et al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J. Cardiovasc. Electrophysiol. 2019, 30, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Patel, N.; Colburn, S.; Beer, D.; Naperkowski, A.; Subzposh, F.A. His-Purkinje Conduction System Pacing in Atrioventricular Block: New Insights into Site of Conduction Block. JACC Clin. Electrophysiol. 2022, 8, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Zanon, F.; Abdelrahman, M.; Marcantoni, L.; Naperkowski, A.; A Subzposh, F.; Pastore, G.; Baracca, E.; Boaretto, G.; Raffagnato, P.; Tiribello, A.; et al. Long term performance and safety of His bundle pacing: A multicenter experience. J. Cardiovasc. Electrophysiol. 2019, 30, 1594–1601. [Google Scholar] [CrossRef]
- Beer, D.; A Subzposh, F.; Colburn, S.; Naperkowski, A.; Vijayaraman, P. His bundle pacing capture threshold stability during long-term follow-up and correlation with lead slack. EP Europace 2020, 23, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shang, W.; Ye, Y.; Su, L.; Wang, S.; Cai, M.; Wang, D.; He, Y.; Zheng, R.; Fu, G.; et al. Sex differences outcomes in conduction system pacing for patients with typical left bundle branch block. Int. J. Cardiol. 2024, 415, 132475. [Google Scholar] [CrossRef]
- Stangl, D.; Buia, V.; Walascheck, J.; Rittger, H.; Bastian, D.; Vitali-Serdoz, L. The pursuit of gender equality: A substudy of the PACE-CONDUCT trial on gender differences in His bundle pacing implantation. EP Europace 2024, 26, i899–i900. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Sun, W.; Nayak, H.M.; Aziz, Z.; Beaser, A.D.; Ozcan, C.; Tung, R. B-PO01-040 Lead Stability in His Bundle Pacing—Incidence, Predictors, and Timing of Increased Pacing Thresholds. Hear. Rhythm. O2 2021, 18, S67. [Google Scholar] [CrossRef]

| Baseline Characteristics | All | Male | Female | p |
|---|---|---|---|---|
| Number of patients | 73 | 43 | 30 | |
| Age (years, mean ± SD) | 72.8 ± 6.3 | 71.8 ± 6.4 | 74.3 ± 5.9 | 0.10 |
| e GFR (mL/min, mean ± SD) | 62.4 ± 19.5 | 60.4 ± 18.7 | 65.2 ± 20.5 | 0.32 |
| BMI (kg/m2, mean ± SD) | 28.8 ± 4.9 | 28.1 ± 4.5 | 29.2 ± 5.1 | 0.27 |
| Baseline QRS | ||||
| QRS duration (ms, mean ± SD) | 108.6 ± 29.2 | 111.1 ± 29.8 | 105 ± 28.5 | 0.38 |
| Normal QRS (n, %) | 47 (64.4) | 27 (62.8) | 20 (66.7) | 0.80 |
| LBBB (n, %) | 12 (16.4) | 5 (11.6) | 7 (23.3) | 0.21 |
| RBBB (n, %) | 14 (19.2) | 11 (25.6) | 3 (10) | 0.13 |
| Echocardiography | ||||
| LVEF (%, mean ± SD) | 52.5 ± 11.8 | 51.6 ± 11.3 | 53.8 ± 12.6 | 0.43 |
| LA diameter (mm, mean ± SD) | 41.1 ± 6.2 | 43.5 ± 5.9 * | 36.8 ± 4.7 * | 0.01 |
| RA diameter (mm, mean ± SD) | 36.8 ± 6.2 | 37.9.1 ± 6.4 | 34.9 ± 5.2 | 0.10 |
| Comorbidities | ||||
| Hypertension (n, %) | 65 (89) | 37 (86) | 28 (93.3) | 0.46 |
| Diabetes mellitus (n, %) | 26 (35.6) | 16 (37.2) | 10 (33.3) | 0.80 |
| Ischemic disease (n, %) | 19 (26) | 11 (25.6) | 8 (26.7) | 1 |
| Renal failure (n, %) | 29 (39.7) | 14 (32.6) | 15 (50) | 0.15 |
| Persistent AF (n, %) | 15 (20.5) | 9 (20.9) | 6 (20) | 1 |
| Treatment | ||||
| RAAS antagonists (n, %) | 65 (89) | 37 (86) | 28 (93.3) | 0.46 |
| Beta-blockers (n, %) | 54 (74) | 33 (76.7) | 21 (70) | 0.59 |
| MRAs (n, %) | 13 (17.8) | 9 (20.9) | 4 (13.3) | 0.54 |
| Anticoagulants (n, %) | 31 (42.5) | 20 (46.5) | 11 (36.7) | 0.47 |
| Procedural Parameters | Overall | Male (n = 43) | Female (n = 30) | p-Value |
|---|---|---|---|---|
| Baseline QRS duration (ms) | 108.6 ± 29.2 | 111 ± 29.8 | 105 ± 28.5 | 0.38 |
| Paced QRS duration (ms) | 94.5 ± 19.4 | 98.9 ± 20.1 * | 88.1 ± 16.6 * | 0.02 |
| Detection (ms, mean ± SD) | 4.1 ± 2.5 | 4.1 ± 2.3 | 4.1 ± 2.9 | 0.94 |
| Pacing threshold (V, mean ± SD) | 1.1 ± 0.8 | 1.1 ± 0.7 | 1.2 ± 0.9 | 0.50 |
| Selective capture (n, %) | 35 (47.9) | 20 (46.5) | 15 (50) | 0.81 |
| Impedance (Ohm, mean ± SD) | 465.4 ± 107.5 | 468.3 ± 104.8 | 461.3 ± 112.8 | 0.79 |
| Fluoroscopy time (min, mean ± SD) | 8.9 ± 7.5 | 8.8 ± 7.7 | 8.9 ± 7.2 | 0.95 |
| Parameters | HR (95% CI) | p Value | HR (95% CI) | p Value |
|---|---|---|---|---|
| Sex | 0.69 (0.27–1.77) | 0.45 | ||
| Age (years) | 0.96 (0.89–1.03) | 0.26 | ||
| Baseline QRS duration (ms) | 1.01 (0.99–1.02) | 0.49 | ||
| Paced QRS duration (ms) | 1.02 (1.00–1.04) | 0.02 | 1.02 (1.00–1.05) | 0.02 |
| Type of capture | 2.3 (0.86–6.00) | 0.09 | 1.89 (0.69–5.20) | 0.21 |
| Procedural pacing threshold > 1.25 V | 0.67 (0.24–1.86) | 0.44 | ||
| LV ejection fraction (%) | 1.08 (1.01–1.17) | 0.03 | 1.08 (1.02–1.16) | 0.01 |
| LA volume (mL) | 1.07 (0.96–1.19) | 0.21 | ||
| RA volume (mL) | 1.05 (0.94–1.18) | 0.38 | ||
| Ischemic disease | 0.38 (0.15–0.95) | 0.04 | 0.26 (0.10–0.68) | 0.01 |
| Hypertension | 0.71 (0.16–3.09) | 0.65 | ||
| Diabetes Mellitus | 1.99 (0.66–6.02) | 0.22 | ||
| Atrial fibrillation | 1.10 (0.72–1.68) | 0.66 |
| Initial | Follow-Up | Overall | Male (n = 43) | Female (n = 30) | p-Value |
|---|---|---|---|---|---|
| NS-Myo | NS-Myo | 32 | 20 | 12 | 0.34 |
| NS-S | NS-S | 29 | 19 | 10 | 0.46 |
| NS-Myo | NS-S | 5 | 2 | 3 | 0.39 |
| NS-S | NS-Myo | 6 | 1 | 5 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pestrea, C.; Cicala, E.; Lovin, D.; Gheorghe, A.; Ortan, F.; Manea, R. Gender Differences for His Bundle Pacing Long-Term Performance in the Elderly Population. J. Cardiovasc. Dev. Dis. 2025, 12, 88. https://doi.org/10.3390/jcdd12030088
Pestrea C, Cicala E, Lovin D, Gheorghe A, Ortan F, Manea R. Gender Differences for His Bundle Pacing Long-Term Performance in the Elderly Population. Journal of Cardiovascular Development and Disease. 2025; 12(3):88. https://doi.org/10.3390/jcdd12030088
Chicago/Turabian StylePestrea, Catalin, Ecaterina Cicala, Dragos Lovin, Adrian Gheorghe, Florin Ortan, and Rosana Manea. 2025. "Gender Differences for His Bundle Pacing Long-Term Performance in the Elderly Population" Journal of Cardiovascular Development and Disease 12, no. 3: 88. https://doi.org/10.3390/jcdd12030088
APA StylePestrea, C., Cicala, E., Lovin, D., Gheorghe, A., Ortan, F., & Manea, R. (2025). Gender Differences for His Bundle Pacing Long-Term Performance in the Elderly Population. Journal of Cardiovascular Development and Disease, 12(3), 88. https://doi.org/10.3390/jcdd12030088






