Prognostic Role of Pan-Immune-Inflammatory Value in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Endpoint
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.S.; Jeong, M.H. Differences in the Korea Acute Myocaridal Infarction Registry compared with Western registries. Korean Circ. J. 2017, 67, 811–822. [Google Scholar] [CrossRef]
- Han, X.; Bai, L.; Jeong, M.H.; Ahn, J.H.; Hyun, D.Y.; Cho, K.H.; Kim, M.C.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; et al. Higher long-term mortlaity in patients with non-ST elevaation myocardial infarction than ST-elevation myocardial infraction after discharge. Yonsei Med. J. 2021, 62, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef]
- Hofbauer, T.M.; Mangold, A.; Scherz, T.; Seidl, V.; Panzenböck, A.; Ondracek, A.S.; Müller, J.; Schneider, M.; Binder, T.; Hell, L.; et al. Neutrophil extracellular traps and fibrocytes in ST-segment elevation myocardial infarction. Basic. Res. Cardiol. 2019, 114, 33. [Google Scholar] [CrossRef] [PubMed]
- Gombozhapova, A.; Rogovskaya, Y.; Shurupov, V.; Rebenkova, M.; Kzhyshkowska, J.; Popov, S.V.; Karpov, R.S.; Ryabov, V. Macrophage activation and polarization in post-infarction cardiac remodeling. J. Biomed. Sci. 2017, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Moore, X.L.; Dart, A.M.; Wang, L.M. Systemic inflammatory response following acute myocardial infarction. J. Geriatr. Cardiol. 2015, 12, 305–312. [Google Scholar] [CrossRef]
- Núñez, J.; Miñana, G.; Bodí, V.; Núñez, E.; Sanchis, J.; Husser, O.; Llàcer, A. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 2011, 18, 3226–3233. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Schönbeck, U.; Yan, Z.Q. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ. Res. 2002, 91, 281–291. [Google Scholar] [CrossRef]
- Waehre, T.; Damås, J.K.; Yndestad, A.; Taskén, K.; Pedersen, T.M.; Smith, C.; Halvorsen, B.; Frøland, S.S.; Solum, N.O.; Aukrust, P. Effect of activated platelets on expression of cytokines in peripheral blood mononuclear cells—Potential role of prostaglandin E2. Thromb. Haemost. 2004, 92, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Jang, H.J.; Oh, I.Y.; Yoon, C.H.; Suh, J.W.; Cho, Y.S.; Youn, T.J.; Cho, G.Y.; Chae, I.H.; Choi, D.J. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am. J. Cardiol. 2013, 111, 636–642. [Google Scholar] [CrossRef]
- Azab, B.; Shah, N.; Akerman, M.; McGinn, J.T., Jr. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J. Thromb. Thrombolysis 2012, 34, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, A.; Yarlioglues, M.; Murat, S.N.; Ergun, G.; Duran, M.; Kasapkara, H.A.; Demircelik, M.B.; Cetin, M.; Ocek, A.H. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am. J. Cardiol. 2014, 114, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.I.; Ann, S.H.; Singh, G.B.; Her, A.Y.; Shin, E.S. Combined Usefulness of the Platelet-to-Lymphocyte Ratio and the Neutrophil-to-Lymphocyte Ratio in Predicting the Long-Term Adverse Events in Patients Who Have Undergone Percutaneous Coronary Intervention with a Drug-Eluting Stent. PLoS ONE 2015, 10, e0133934. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Q.; Wang, R.; Ji, H.; Chen, Y.; Quan, X.; Zhang, C. Systemic Immune-Inflammatory Index Predicts Clinical Outcomes for Elderly Patients with Acute Myocardial Infarction Receiving Percutaneous Coronary Intervention. Med. Sci. Monit. 2019, 25, 9690–9701. [Google Scholar] [CrossRef] [PubMed]
- Marchi, F.; Pylypiv, N.; Parlanti, A.; Storti, S.; Gaggini, M.; Paradossi, U.; Berti, S.; Vassalle, C. Systemic Immune-Inflammation Index and Systemic Inflammatory Response Index as Predictors of Mortality in ST-Elevation Myocardial Infarction. J. Clin. Med. 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Murat, B.; Murat, S.; Ozgeyik, M.; Bilgin, M. Comparison of pan-immune-inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur. J. Clin. Investig. 2023, 53, e13872. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Diao, J.; Qi, C.; Jin, J.; Li, L.; Gao, X.; Gong, L.; Wu, W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 75. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y.; Geng, X.B.; Tan, Z.; Wang, J.H.; Cui, C.; Wang, H.L.; Shang, X.M. Platelet-to-lymphocyte ratio relates to poor prognosis in elderly patients with acute myocardial infarction. Aging Clin. Exp. Res. 2021, 33, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; Erdöl, M.A.; Öztürk, S.; Durmaz, T. Systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Hu, X.; Zhang, R.; Li, X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: A systematic review and meta-analysis. J. Transl. Med. 2023, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Shi, D.; Yang, L.; Wang, Z.; Li, Y.; Gao, F.; Liu, Y.; Ma, X.; Zhou, Y. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann. Med. 2022, 54, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; Guarini, V.; Antoniotti, C.; Morano, F.; Moretto, R.; Corallo, S.; Marmorino, F.; Lonardi, S.; Rimassa, L.; Sartore-Bianchi, A.; et al. The Pan-Immune-Inflammation Value is a new prognostic biomarker in metastatic colorectal cancer: Results from a pooled-analysis of the Valentino and TRIBE first-line trials. Br. J. Cancer 2020, 123, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, J.; Chen, M.; Wang, Y.; Li, J.; Zhang, J. Pan-Immune-Inflammatory Value is Superior to Other Inflammatory Indicators in Predicting Inpatient Major Adverse Cardiovascular Events and Severe Coronary Artery Stenosis after Percutaneous Coronary Intervention in STEMI Patients. Rev. Cardiovasc. Med. 2024, 25, 294. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Z.; Kelesoglu, S.; Tuncay, A.; Yilmaz, Y.; Karaca, Y.; Karasu, M.; Secen, O.; Cinar, A.; Harman, M.; Sahin, S.; et al. The Role of Pan-Immune-Inflammation Value in Determining the Severity of Coronary Artery Disease in NSTEMI Patients. J. Clin. Med. 2024, 13, 1295. [Google Scholar] [CrossRef] [PubMed]
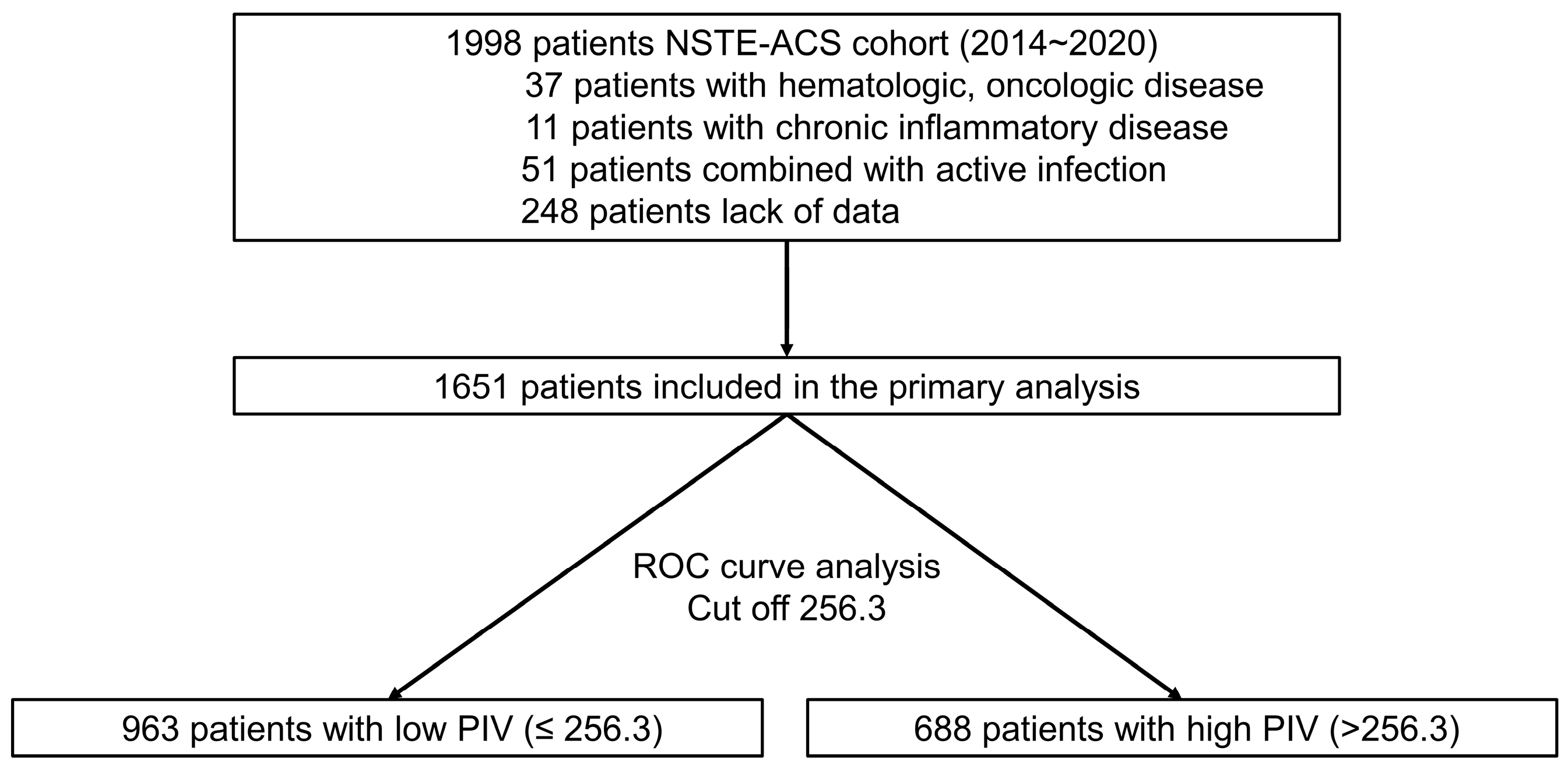
| PIV ≤ 256.3 (n = 963) | PIV > 256.3 (n = 688) | p-Value | |
|---|---|---|---|
| Age (years) | 65.9 ± 10.3 | 66.9 ± 11.5 | 0.082 |
| Male (%) | 656 (68.1) | 456 (66.3) | 0.431 |
| Body mass index (kg/m2) | 24.9 ± 3.0 | 24.7 ± 3.7 | 0.450 |
| Hypertension (%) | 605 (62.8) | 486 (70.6) | 0.001 |
| Diabetes mellitus (%) | 370 (38.4) | 342 (49.7) | <0.001 |
| Current smoker (%) | 234 (24.3) | 215 (31.3) | 0.002 |
| Previous coronary intervention (%) | 95 (9.9) | 75 (10.9) | 0.495 |
| Diagnosis (%) | <0.001 | ||
| Unstable angina | 841 (87.3) | 495 (71.9) | |
| Non-ST-segment elevation MI | 122 (12.7) | 193 (28.1) | |
| Ejection fraction (%) | 60.2 ± 11.1 | 55.4 ± 13.0 | <0.001 |
| Hemoglobin (g/dL) | 13.6 ± 1.8 | 13.2 ± 2.1 | <0.001 |
| Neutrophil count (103/μL) | 3.6 ± 1.1 | 6.6 ± 2.6 | <0.001 |
| Lymphocyte count (103/μL) | 2.4 ± 0.9 | 2.1 ± 0.8 | <0.001 |
| Monocyte count (103/μL) | 0.5 ± 0.1 | 0.7 ± 0.2 | <0.001 |
| Platelet count (103/μL) | 211.0 ± 49.2 | 250.4 ± 68.7 | <0.001 |
| eGFR (mL/min/1.73 m2) | 77.5 ± 23.0 | 73.0 ± 31.3 | 0.001 |
| Troponin T (ng/mL) | 0.23 ± 0.76 | 0.44 ± 1.02 | <0.001 |
| LDL cholesterol (mg/dL) | 106.4 ± 40.3 | 106.4 ± 41.7 | 0.992 |
| C-reactive protein (mg/L) | 2.8 ± 5.0 | 9.9 ± 22.3 | <0.001 |
| Culprit lesion (%) | 0.189 | ||
| Left main | 52 (5.4) | 29 (4.2) | |
| Left anterior descending | 479 (49.7) | 318 (46.2) | |
| Left circumflex | 189 (19.6) | 139 (20.2) | |
| Right coronary artery | 243 (25.2) | 202 (29.4) | |
| Multivessel disease (%) | 447 (46.4) | 341 (49.6) | 0.207 |
| Stent number per patient | 1.6 ± 0.9 | 1.6 ± 1.0 | 0.714 |
| Minimal stent diameter (mm) | 2.9 ± 0.4 | 2.9 ± 0.4 | 0.534 |
| Total stent length (mm) | 43.3 ± 27.7 | 44.7 ± 29.6 | 0.320 |
| Discharge medication (%) | |||
| Aspirin | 961 (99.8) | 688 (100) | 0.514 |
| P2Y12 inhibitors | 951 (98.8) | 674 (98.0) | 0.204 |
| Beta blocker | 606 (62.9) | 454 (66.0) | 0.201 |
| ACEI/ARB | 826 (85.8) | 603 (87.6) | 0.272 |
| Statin | 941 (97.7) | 673 (97.8) | 0.888 |
| PIV ≤ 256.3 (n = 963) | PIV > 256.3 (n = 688) | Adjusted HR (95% CI) | p-Value | |
|---|---|---|---|---|
| All-cause death | 9 (0.9) | 27 (3.9) | 4.03 (1.89, 8.57) | <0.001 |
| Cardiac death | 7 (0.7) | 17 (2.5) | 3.24 (1.34, 7.81) | 0.009 |
| Myocardial infarction | 16 (1.7) | 13 (1.9) | 1.14 (0.55, 2.37) | 0.725 |
| Stroke | 5 (0.5) | 12 (1.7) | 3.30 (1.16, 9.36) | 0.025 |
| Any revascularization | 26 (2.7) | 17 (2.5) | 0.92 (0.50, 1.70) | 0.799 |
| Rehospitalization for heart failure | 9 (0.9) | 12 (1.7) | 1.85 (0.78, 4.40) | 0.162 |
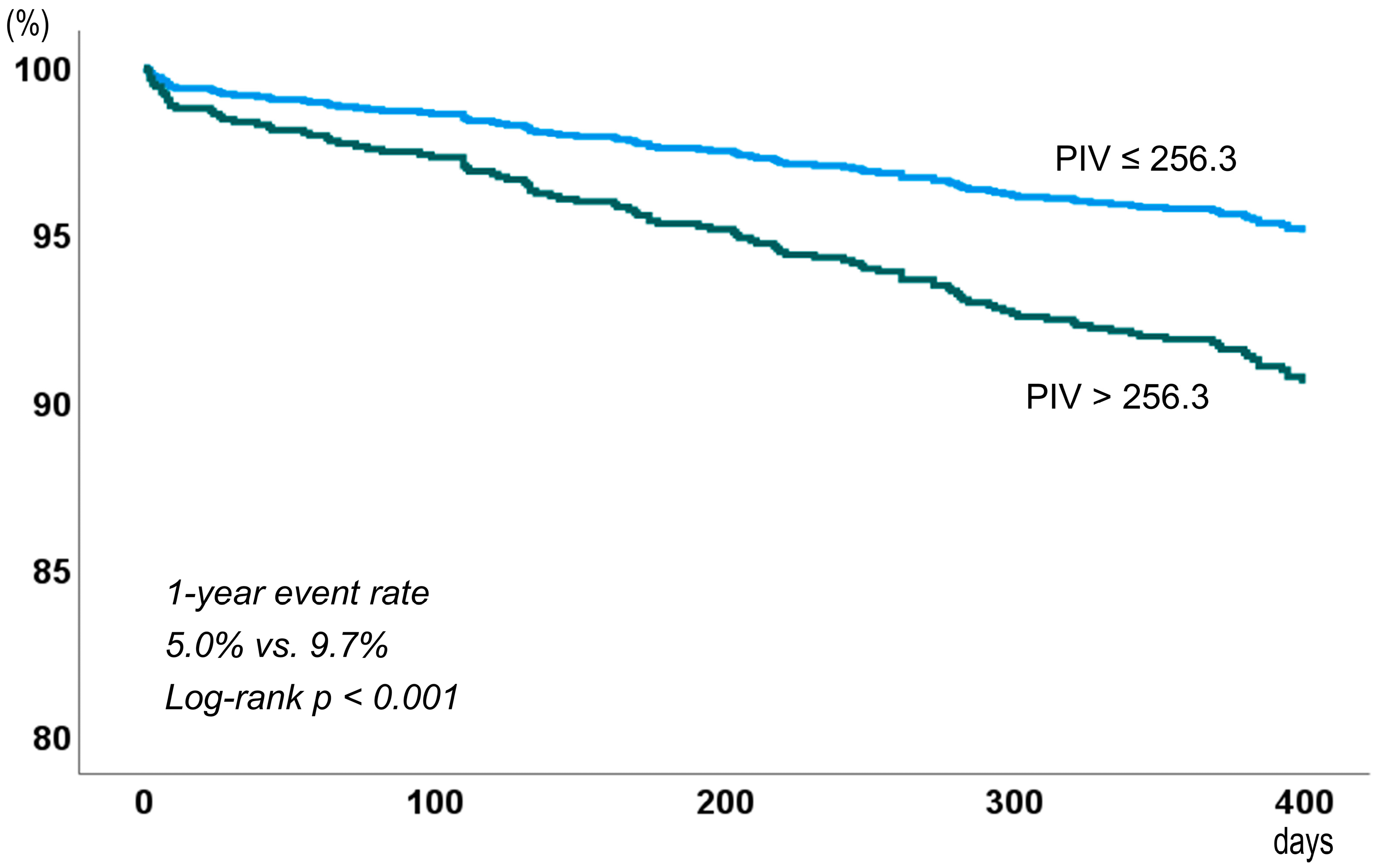
| Major adverse cardiovascular events | 48 (5.0) | 67 (9.7) | 1.96 (1.36, 2.85) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |
| Age > 65 years | 2.29 (1.50, 3.48) | <0.001 | 1.75 (1.12, 2.72) | 0.014 |
| Male gender | 1.11 (0.76, 1.63) | 0.591 | ||
| Hypertension | 1.50 (1.19, 1.90) | 0.001 | 1.41 (0.86, 2.31) | 0.171 |
| Diabetes mellitus | 1.38 (1.15, 1.66) | 0.001 | 1.47 (0.99, 2.18) | 0.057 |
| Myocardial infarction | 1.62 (1.08, 2.45) | 0.021 | 1.37 (0.90, 2.10) | 0.146 |
| Hemoglobin < 12 g/dL | 2.43 (1.67, 3.55) | <0.001 | 1.34 (0.88, 2.05) | 0.178 |
| eGFR < 60 mL/min/1.73 m2 | 2.69 (1.86, 3.89) | <0.001 | 1.73 (1.23, 2.64) | 0.012 |
| C-reactive protein > 2 mg/L | 1.75 (1.20, 2.54) | 0.003 | 1.32 (0.89, 1.95) | 0.171 |
| Ejection fraction < 50% | 1.25 (1.03, 1.53) | 0.024 | 1.05 (0.69, 1.61) | 0.817 |
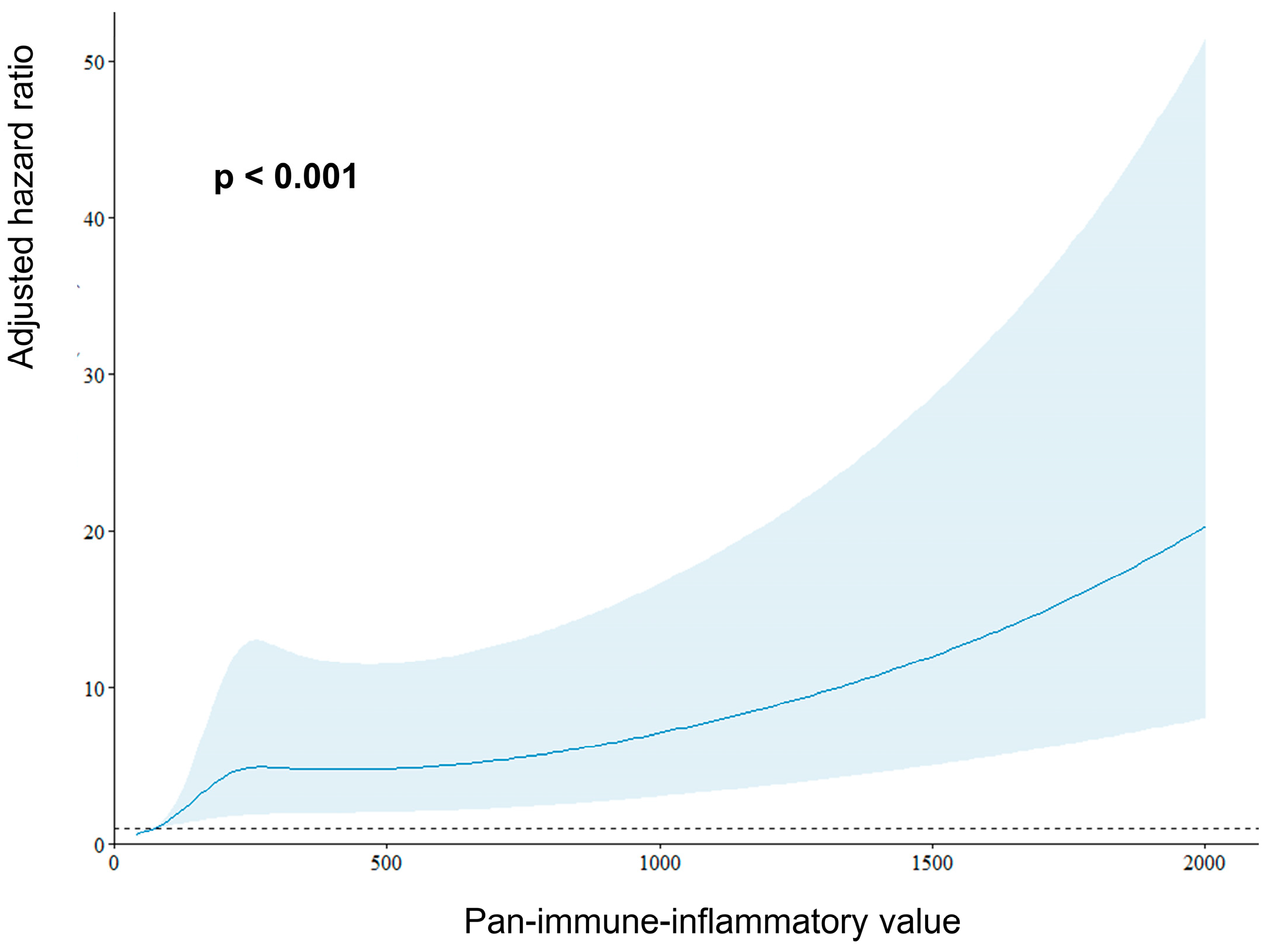
| PIV > 256.3 | 2.01 (1.39, 2.91) | <0.001 | 1.49 (1.01, 2.22) | 0.048 |
| Subgroup | PIV ≤ 256.3 | PIV > 256.3 | HR (95% CI) | p-Value | p-Value for Interaction |
|---|---|---|---|---|---|
| Age, years | <0.001 | ||||
| <65 | 18/428 (4.2) | 11/273 (4.0) | 0.95 (0.45, 2.01) | 0.895 | |
| ≥65 | 30/535 (5.6) | 58/415 (13.5) | 2.54 (1.63, 3.95) | <0.001 | |
| Gender | 0.885 | ||||
| Male | 34/656 (5.2) | 41/456 (9.0) | 1.76 (1.12, 2.78) | 0.015 | |
| Female | 14/307 (4.6) | 26/232 (11.2) | 2.60 (1.36, 4.97) | 0.004 | |
| Hypertension | <0.001 | ||||
| Yes | 35/605 (5.8) | 58/486 (11.9) | 2.12 (1.40, 3.23) | <0.001 | |
| No | 13/358 (3.6) | 9/202 (4.5) | 1.25 (0.54, 2.93) | 0.605 | |
| Diabetes | 0.077 | ||||
| Yes | 28/370 (7.6) | 39/342 (11.4) | 1.55 (0.95, 2.52) | 0.077 | |
| No | 20/593 (3.4) | 28/346 (8.1) | 2.45 (1.38, 4.36) | 0.002 | |
| Clinical presentation | 0.317 | ||||
| Unstable angina | 43/841 (5.1) | 41/495 (8.3) | 1.65 (1.08, 2.53) | 0.022 | |
| Non-ST-segment elevation MI | 5/122 (4.1) | 26/196 (13.5) | 3.44 (1.32, 8.96) | 0.011 | |
| Multivessel disease | 0.050 | ||||
| Yes | 28/447 (6.8) | 41/341 (12.0) | 1.99 (1.23, 3.22) | 0.005 | |
| No | 20/516 (3.9) | 26/347 (7.5) | 1.97 (1.10, 3.54) | 0.022 | |
| Hemoglobin, g/dL | 0.073 | ||||
| <12 | 15/168 (8.9) | 28/175 (16.0) | 1.95 (1.04, 3.65) | 0.037 | |
| ≥12 | 33/795 (4.2) | 39/513 (7.6) | 1.85 (1.16, 2.94) | 0.009 | |
| eGFR, mL/min/1.73 m2 | 0.003 | ||||
| <60 | 15/178 (8.4) | 35/213 (16.4) | 2.03 (1.11, 3.71) | 0.022 | |
| ≥60 | 33/785 (4.2) | 32/475 (6.7) | 1.62 (0.99, 2.64) | 0.051 | |
| C-reactive protein, mg/L | 0.052 | ||||
| ≤2.0 | 27/645 (4.2) | 25/313 (8.0) | 1.94 (1.12, 3.33) | 0.017 | |
| >2.0 | 19/286 (6.6) | 40/362 (11.0) | 1.72 (1.00, 2.97) | 0.052 |
| Variables | Area Under Curve | p-Value | Cut Off | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Pan-immune-inflammatory value | 0.658 | <0.001 | 256.3 | 60.7% | 59.3% |
| Neutrophil-to-lymphocyte ratio | 0.623 | <0.001 | 3.06 | 42.7% | 79.6% |
| Platelet-to-lymphocyte ratio | 0.633 | <0.001 | 83.2 | 89.6% | 30.7% |
| Systemic immune-inflammation index | 0.635 | <0.001 | 752.7 | 39.1% | 81.4% |
| Systemic inflammation response index | 0.652 | <0.001 | 1.43 | 52.2% | 70.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byoun, J.T.; Yun, K.H.; Jo, S.; Joo, D.; Cho, J.Y. Prognostic Role of Pan-Immune-Inflammatory Value in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. J. Cardiovasc. Dev. Dis. 2025, 12, 79. https://doi.org/10.3390/jcdd12020079
Byoun JT, Yun KH, Jo S, Joo D, Cho JY. Prognostic Role of Pan-Immune-Inflammatory Value in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Journal of Cardiovascular Development and Disease. 2025; 12(2):79. https://doi.org/10.3390/jcdd12020079
Chicago/Turabian StyleByoun, Jeong Tae, Kyeong Ho Yun, Sungho Jo, Donghyeon Joo, and Jae Young Cho. 2025. "Prognostic Role of Pan-Immune-Inflammatory Value in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome" Journal of Cardiovascular Development and Disease 12, no. 2: 79. https://doi.org/10.3390/jcdd12020079
APA StyleByoun, J. T., Yun, K. H., Jo, S., Joo, D., & Cho, J. Y. (2025). Prognostic Role of Pan-Immune-Inflammatory Value in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Journal of Cardiovascular Development and Disease, 12(2), 79. https://doi.org/10.3390/jcdd12020079





