Clinical Significance of Serum Omega-3 Fatty Acids on Endothelial Function in Patients with Coronary Artery Disease Under Statin Therapy
Abstract
1. Introduction
2. Materials and Methods
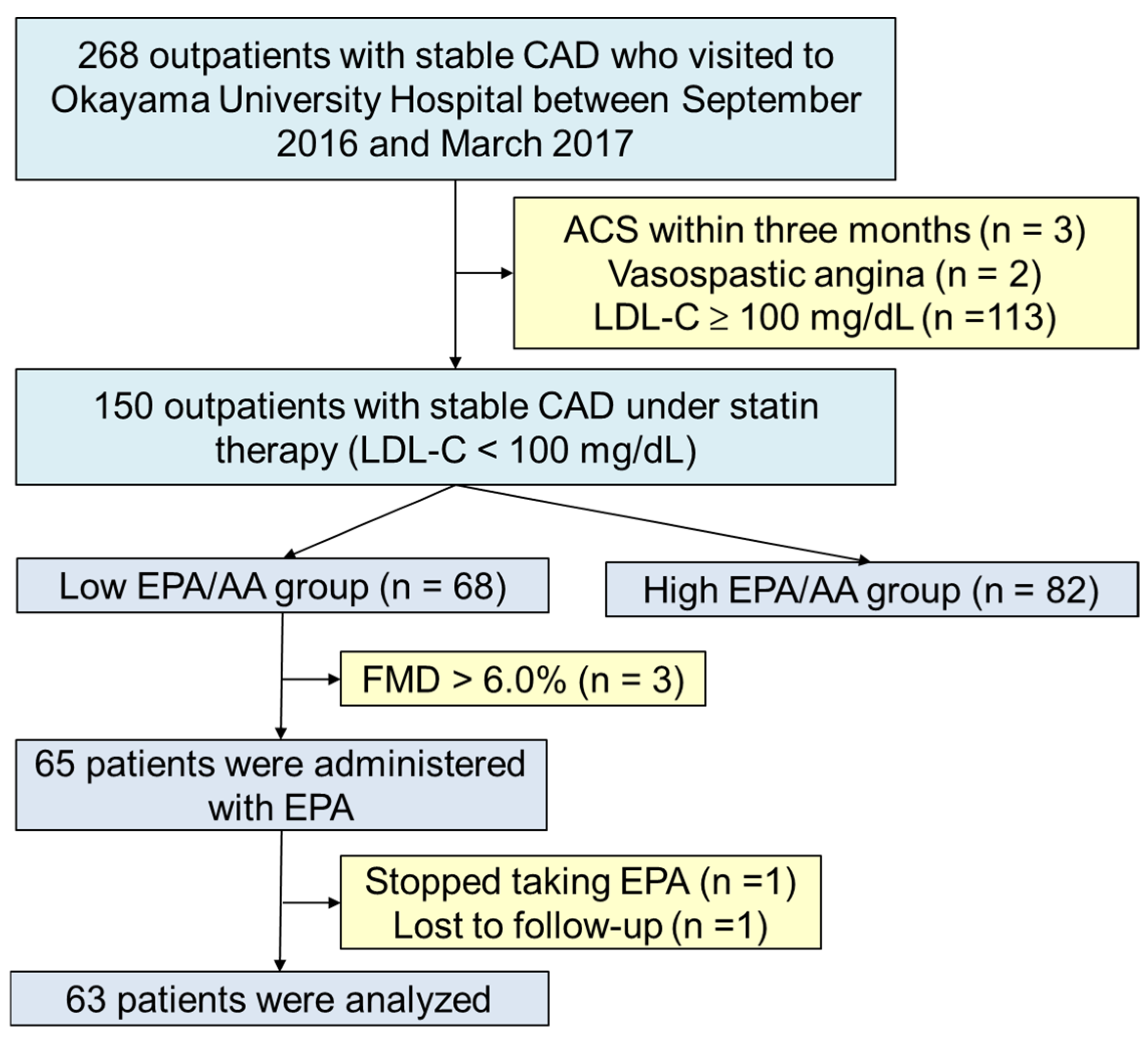
2.1. Study Design and Participants
2.2. Definition of Risk Factors
2.3. Biochemical Analysis
2.4. Assessment of Endothelial Vasomotor Function
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Serum PUFA Levels and Endothelial Function Inpatients with CAD During Optimal Statin Therapy
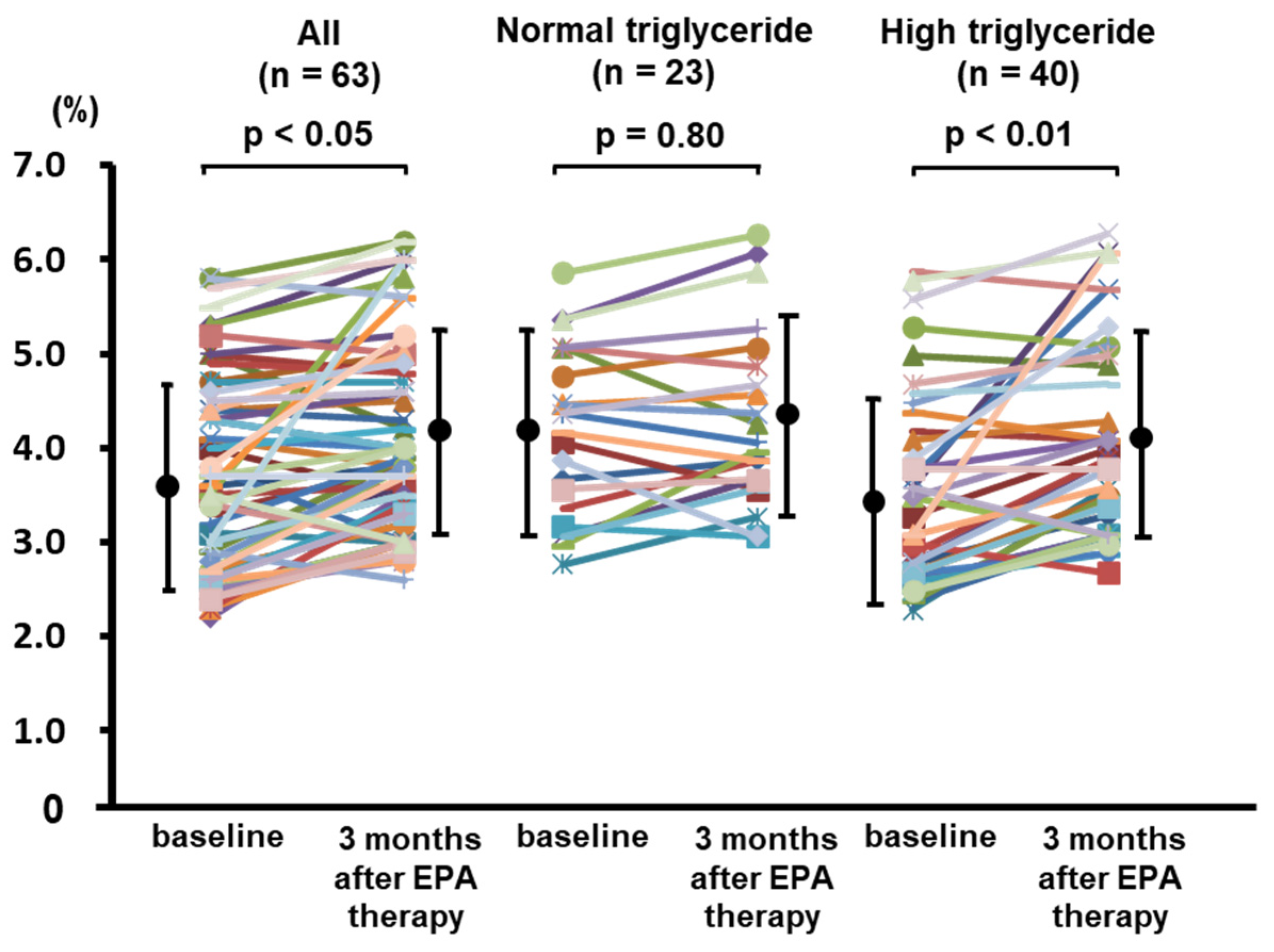
3.3. Effects of EPA Therapy on Lipid Profile and Endothelial Function in CAD Patients with High Triglyceride and Low EPA/AA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Scandinavian Simvastatin Survival Study Group Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of Cardiovascular Events and Death with Pravastatin in Patients with Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels. N. Engl. J. Med. 1998, 339, 1349–1357. [CrossRef]
- LaRosa, J.C.; He, J.; Vupputuri, S. Effect of Statins on Risk of Coronary Disease. JAMA 1999, 282, 2340–2346. [Google Scholar] [CrossRef] [PubMed]
- German, C.A.; Liao, J.K. Understanding the molecular mechanisms of statin pleiotropic effects. Arch. Toxicol. 2023, 97, 1529–1545. [Google Scholar] [CrossRef]
- Ganda, O.P.; Bhatt, D.L.; Mason, R.P.; Miller, M.; Boden, W.E. Unmet Need for Adjunctive Dyslipidemia Therapy in Hypertriglyceridemia Management. J. Am. Coll. Cardiol. 2018, 72, 330–343. [Google Scholar] [CrossRef]
- Liao, J.; Xiong, Q.; Yin, Y.; Ling, Z.; Chen, S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Front. Cardiovasc. Med. 2022, 8, 802306. [Google Scholar] [CrossRef]
- Miyoshi, T.; Noda, Y.; Ohno, Y.; Sugiyama, H.; Oe, H.; Nakamura, K.; Kohno, K.; Ito, H. Omega-3 fatty acids improve postprandial lipemia and associated endothelial dysfunction in healthy individuals—a randomized cross-over trial. Biomed. Pharmacother. 2014, 68, 1071–1077. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Miyauchi, K.; Iwata, H.; Nishizaki, Y.; Inoue, T.; Hirayama, A.; Kimura, K.; Ozaki, Y.; Murohara, T.; Ueshima, K.; Kuwabara, Y.; et al. Randomized Trial for Evaluation in Secondary Prevention Efficacy of Combination Therapy–Statin and Eicosapentaenoic Acid (RESPECT-EPA). Circulation 2024, 150, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kwon, T.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Hear. Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kitta, Y.; Obata, J.-E.; Nakamura, T.; Hirano, M.; Kodama, Y.; Fujioka, D.; Saito, Y.; Kawabata, K.-I.; Sano, K.; Kobayashi, T.; et al. Persistent Impairment of Endothelial Vasomotor Function Has a Negative Impact on Outcome in Patients With Coronary Artery Disease. Circ. 2009, 53, 323–330. [Google Scholar] [CrossRef]
- Kubo, M.; Miyoshi, T.; Oe, H.; Ohno, Y.; Nakamura, K.; Ito, H. Prognostic significance of endothelial dysfunction in patients undergoing percutaneous coronary intervention in the era of drug-eluting stents. BMC Cardiovasc. Disord. 2015, 15, 102. [Google Scholar] [CrossRef]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M.; et al. Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan ^|^mdash;2012 Version. J. Atheroscler. Thromb. 2013, 20, 517–523. [Google Scholar] [CrossRef]
- Yagi, S.; Aihara, K.-I.; Fukuda, D.; Takashima, A.; Hara, T.; Hotchi, J.; Ise, T.; Yamaguchi, K.; Tobiume, T.; Iwase, T.; et al. Effects of Docosahexaenoic Acid on the Endothelial Function in Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2015, 22, 447–454. [Google Scholar] [CrossRef]
- Nozue, T.; Yamamoto, S.; Tohyama, S.; Fukui, K.; Umezawa, S.; Onishi, Y.; Kunishima, T.; Sato, A.; Nozato, T.; Miyake, S.; et al. Low serum docosahexaenoic acid is associated with progression of coronary atherosclerosis in statin-treated patients with diabetes mellitus: Results of the treatment with statin on atheroma regression evaluated by intravascular ultrasound with virtual histology (TRUTH) study. Cardiovasc. Diabetol. 2014, 13, 13. [Google Scholar] [CrossRef]
- Richard, D.; Wolf, C.; Barbe, U.; Kefi, K.; Bausero, P.; Visioli, F. Docosahexaenoic acid down-regulates endothelial Nox 4 through a sPLA2 signalling pathway. Biochem. Biophys. Res. Commun. 2009, 389, 516–522. [Google Scholar] [CrossRef]
- Chen, J.; Shearer, G.C.; Chen, Q.; Healy, C.L.; Beyer, A.J.; Nareddy, V.B.; Gerdes, A.M.; Harris, W.S.; O’Connell, T.D.; Wang, D. Omega-3 Fatty Acids Prevent Pressure Overload–Induced Cardiac Fibrosis Through Activation of Cyclic GMP/Protein Kinase G Signaling in Cardiac Fibroblasts. Circulation 2011, 123, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Watts, G.F.; Burke, V.; Hilme, E.; Puddey, I.B.; Beilin, L.J. Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Vascular Reactivity of the Forearm Microcirculation in Hyperlipidemic, Overweight Men. Circulation 2000, 102, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Fujioka, Y.; Morimoto, S.; Tsuboi, S.; Masai, M.; Tsujino, T.; Ohyanagi, M.; Iwasaki, T. Eicosapentaenoic Acid Improves Endothelial Function in Hypertriglyceridemic Subjects Despite Increased Lipid Oxidizability. Am. J. Med Sci. 2002, 324, 247–253. [Google Scholar] [CrossRef]
- Ohnishi, H.; Saito, Y. Eicosapentaenoic Acid (EPA) Reduces Cardiovascular Events: Relationship with the EPA/Arachidonic Acid Ratio. J. Atheroscler. Thromb. 2013, 20, 861–877. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yunoki, K.; Nakamura, K.; Miyoshi, T.; Enko, K.; Kubo, M.; Murakami, M.; Hata, Y.; Kohno, K.; Morita, H.; Kusano, K.F.; et al. Impact of Hypertriglyceridemia on Endothelial Dysfunction During Statin ± Ezetimibe Therapy in Patients With Coronary Heart Disease. Am. J. Cardiol. 2011, 108, 333–339. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Glickstein, S.B.; Rowe, J.D.; Soni, P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J. Clin. Lipidol. 2012, 6, 5–18. [Google Scholar] [CrossRef]
- Wei, M.Y.; Jacobson, T.A. Effects of Eicosapentaenoic Acid Versus Docosahexaenoic Acid on Serum Lipids: A Systematic Review and Meta-Analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef]
- Itakura, H.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Kita, T.; et al. The Change in Low-Density Lipoprotein Cholesterol Concentration is Positively Related to Plasma Docosahexaenoic Acid but not Eicosapentaenoic Acid. J. Atheroscler. Thromb. 2012, 19, 673–679. [Google Scholar] [CrossRef][Green Version]
- Okamura, T.; Tsukamoto, K.; Arai, H.; Fujioka, Y.; Ishigaki, Y.; Koba, S.; Ohmura, H.; Shoji, T.; Yokote, K.; Yoshida, H.; et al. Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2022. J. Atheroscler. Thromb. 2024, 31, 641–853. [Google Scholar] [CrossRef]
- Ninomiya, T.; Nagata, M.; Hata, J.; Hirakawa, Y.; Ozawa, M.; Yoshida, D.; Ohara, T.; Kishimoto, H.; Mukai, N.; Fukuhara, M.; et al. Association between ratio of serum eicosapentaenoic acid to arachidonic acid and risk of cardiovascular disease: The Hisayama Study. Atherosclerosis 2013, 231, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Domei, T.; Yokoi, H.; Kuramitsu, S.; Soga, Y.; Arita, T.; Ando, K.; Shirai, S.; Kondo, K.; Sakai, K.; Goya, M.; et al. Ratio of Serum n-3 to n-6 Polyunsaturated Fatty Acids and the Incidence of Major Adverse Cardiac Events in Patients Undergoing Percutaneous Coronary Intervention. Circ. J. 2012, 76, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Hishikari, K.; Kimura, S.; Yamakami, Y.; Kojima, K.; Sagawa, Y.; Otani, H.; Sugiyama, T.; Kuwahara, T.; Hikita, H.; Takahashi, A.; et al. The prognostic value of the serum eicosapentaenoic acid to arachidonic acid ratio in relation to clinical outcomes after endovascular therapy in patients with peripheral artery disease caused by femoropopliteal artery lesions. Atherosclerosis 2015, 239, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Itakura, H.; Hishida, H.; Kita, T.; et al. Relationship between Coronary Artery Disease and Non-HDL-C, and Effect of Highly Purified EPA on the Risk of Coronary Artery Disease in Hypercholesterolemic Patients Treated with Statins: Sub-Analysis of the Japan EPA Lipid Intervention Study (JELIS). J. Atheroscler. Thromb. 2012, 19, 194–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saito, Y.; Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: Sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2008, 200, 135–140. [Google Scholar] [CrossRef]

| Variable | All | High EPA/AA (≥ 0.3) | Low EPA/AA (<0.3) | p-Value |
|---|---|---|---|---|
| (n = 82) | (n = 68) | |||
| Age (years) | 70 ± 9 | 70 ± 9 | 68 ± 8 | 0.09 |
| Men | 116 (77%) | 60 (73%) | 56 (82%) | 0.18 |
| BMI (kg/m2) | 24.1 ± 3.5 | 23.6 ± 3.7 | 24.7 ± 3.2 | 0.07 |
| Hypertension | 112 (75%) | 60 (73%) | 52 (76%) | 0.64 |
| Diabetes mellitus | 74 (49%) | 38 (46%) | 36 (53%) | 0.42 |
| Current smoker | 110 (73%) | 55 (67%) | 55 (81%) | 0.06 |
| Angina pectoris/OMI | 136 (90%)/34 (23%) | 68 (45%)/14 (9%) | 48 (32%)/20 (13%) | 0.07 |
| Multivessel disease | 79 (52%) | 38 (46%) | 41 (60%) | 0.09 |
| CABG | 8 (5%) | 3 (4%) | 5 (7%) | 0.32 |
| Cerebral vascular disease | 21 (14) | 12 (15%) | 9 (13%) | 0.81 |
| Peripheral artery disease | 14 (9%) | 6 (7%) | 8 (12%) | 0.35 |
| Antiplatelet | 150 (100%) | 82 (100%) | 68 (100%) | > 0.99 |
| ACEi or ARB | 113 (75%) | 61 (74%) | 52 (76%) | 0.77 |
| β-blocker | 82 (55%) | 45 (55%) | 37 (54%) | 0.95 |
| Statin | 119 (79%) | 82 (100%) | 68 (100%) | > 0.99 |
| CCB | 65 (43%) | 32 (39%) | 33 (49%) | 0.24 |
| Nitrates | 21 (14%) | 12 (15%) | 9 (13%) | 0.88 |
| Ezetimibe | 14 (9%) | 7 (9%) | 7 (10%) | 0.71 |
| Sulfonylurea | 21 (14%) | 11 (13%) | 10 (15%) | 0.82 |
| Insulin | 16 (11%) | 9 (11%) | 7 (10%) | 0.89 |
| Triglyceride (mg/dL) | 127 ± 46 | 96 ± 30 | 164 ± 65 | <0.0001 |
| LDL cholesterol (mg/dL) | 77 ± 12 | 76 ± 13 | 79 ± 10 | 0.11 |
| HDL cholesterol (mg/dL) | 43 ± 11 | 46 ± 12 | 40 ± 9 | <0.001 |
| Uric acid (mg/dL) | 6.1 ± 1.6 | 6.1 ± 1.6 | 6.2 ± 1.7 | 0.56 |
| FBG (mg/dL) | 126 ± 44 | 121 ± 43 | 133 ± 46 | <0.005 |
| HemoglobinA1c (%) | 5.9 ± 0.9 | 5.8 ± 0.8 | 6.1 ± 1.2 | 0.64 |
| hsCRP (mg/dL) | 0.30 ± 0.53 | 0.41 ± 0.79 | 0.18 ± 0.23 | 0.77 |
| eGFR (ml/min/1.73 m2) | 62.0 ± 21.7 | 62.9 ± 21.8 | 61.0 ± 21.7 | 0.61 |
| EPA (μg/mL) | 44.9 (29.4–68.2) | 72.5 (53.8–81.0) | 30.4 (25.8–43.7) | <0.001 |
| AA (μg/mL) | 134.6 (110.9–182.0) | 124.8 (101.2–145.2) | 150.0 (119.7–193.9) | <0.001 |
| DHA (μg/mL) | 112.8 (80.2–144.2) | 140.5 (97.4–159.8) | 96.8 (63.5–132.6) | <0.001 |
| DHLA (μg/mL) | 29.8 (22.8–37.6) | 30.1 (25.6–35.4) | 29.2 (21.2–38.0) | 0.72 |
| Baseline brachial artery diameter (mm) | 4.04 ± 0.48 | 3.99 ± 0.53 | 4.12 ± 0.43 | 0.19 |
| FMD (%) | 4.1 ± 1.3 | 4.3 ± 1.3 | 3.9 ± 1.3 | <0.05 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| r | p-Value | β | p-Value | |
| Model 1 | ||||
| Age, years | −0.277 | <0.001 | −0.277 | <0.001 |
| Men | −0.224 | <0.01 | −0.031 | 0.69 |
| Body mass index | −0.132 | 0.11 | ||
| Systolic blood pressure | 0.036 | 0.66 | ||
| Diastolic blood pressure | 0.167 | <0.05 | 0.181 | <0.05 |
| Baseline brachial artery diameter * | −0.293 | <0.001 | −0.148 | <0.05 |
| Triglyceride * | −0.277 | <0.001 | −0.486 | <0.0001 |
| Low-density lipoprotein cholesterol | 0.088 | 0.28 | ||
| High-density lipoprotein cholesterol * | 0.151 | 0.06 | −0.003 | 0.97 |
| Uric acid * | −0.248 | <0.01 | −0.121 | 0.10 |
| Fasting blood glucose * | −0.064 | 0.43 | ||
| Hemoglobin A1c * | −0.074 | 0.38 | ||
| C-reactive protein * | 0.023 | 0.78 | ||
| EPA * | 0.111 | 0.18 | −0.024 | 0.79 |
| DHA * | 0.142 | 0.08 | 0.214 | <0.05 |
| AA * | 0.100 | 0.22 | −0.011 | 0.90 |
| DHLA* | 0.031 | 0.71 | 0.112 | 0.25 |
| Model 2 | ||||
| Age, years | −0.277 | <0.001 | −0.260 | <0.01 |
| Men | −0.224 | <0.01 | −0.735 | 0.46 |
| Diastolic blood pressure | 0.167 | <0.05 | 0.181 | <0.05 |
| Baseline brachial artery diameter * | −0.293 | <0.001 | −0.160 | <0.05 |
| Triglyceride * | −0.277 | <0.001 | −0.401 | <0.0001 |
| High-density lipoprotein cholesterol * | 0.151 | 0.06 | 0.010 | 0.90 |
| Uric acid * | −0.248 | <0.01 | −0.130 | 0.08 |
| EPA+DHA * | 0.115 | 0.16 | 0.163 | <0.05 |
| AA+DHLA * | 0.129 | 0.12 | 0.054 | 0.51 |
| Model 3 | ||||
| Age, years | −0.277 | <0.001 | −0.276 | <0.001 |
| Men | −0.224 | <0.01 | −0.082 | 0.28 |
| Diastolic blood pressure | 0.167 | <0.05 | 0.190 | <0.05 |
| Baseline brachial artery diameter * | −0.293 | <0.001 | −0.166 | <0.05 |
| Triglyceride * | −0.277 | <0.001 | −0.366 | <0.0001 |
| High-density lipoprotein cholesterol * | 0.151 | 0.06 | 0.058 | 0.48 |
| Uric acid * | −0.248 | <0.01 | −0.139 | 0.06 |
| EPA/AA | −0.005 | 0.95 | −0.069 | 0.49 |
| DHA/AA | −0.038 | 0.65 | 0.154 | 0.13 |
| Variables | Baseline | 3 Months | p-Value |
|---|---|---|---|
| Triglyceride (mg/dL) | 167 ± 66 | 141 ± 44 | <0.05 |
| LDL cholesterol (mg/dL) | 79 ± 11 | 76 ± 8 | 0.06 |
| HDL cholesterol (mg/dL) | 40 ± 9 | 41 ± 7 | 0.20 |
| Uric acid (mg/dL) | 6.3 ± 1.5 | 6.3 ± 1.7 | 0.93 |
| Fasting blood glucose (mg/dL) | 134 ± 47 | 126 ± 29 | 0.54 |
| HemoblobinA1c (%) | 6.1 ± 1.2 | 6.1 ± 1.0 | 0.76 |
| hsCRP (mg/dL) | 0.19 ± 0.23 | 0.10 ± 0.13 | <0.01 |
| EPA (μg/mL) | 49 ± 24 | 147 ± 36 | <0.0001 |
| AA (μg/mL) | 146 ± 47 | 130 ± 38 | <0.05 |
| DHA (μg/mL) | 115 ± 45 | 109 ± 42 | 0.49 |
| EPA/AA | 0.36 ± 0.21 | 1.20 ± 0.38 | <0.0001 |
| DHA/AA | 0.84 ± 0.36 | 0.88 ± 0.37 | 0.48 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| r | p-Value | β | p-Value | |
| ΔTG | −0.321 | <0.05 | −0.317 | <0.05 |
| ΔLDL cholesterol | −0.015 | 0.91 | ||
| ΔHDL cholesterol | 0.022 | 0.86 | ||
| ΔUric acid | 0.021 | 0.87 | ||
| ΔFasting blood glucose | −0.098 | 0.45 | ||
| ΔHemoglobinbA1c | −0.162 | 0.21 | ||
| ΔCRP | −0.032 | 0.81 | ||
| ΔEPA | −0.218 | 0.09 | −0.193 | 0.16 |
| ΔAA | 0.050 | 0.69 | ||
| ΔDHA | 0.019 | 0.88 | ||
| ΔEPA/AA | −0.170 | 0.18 | −0.048 | 0.73 |
| ΔDHA/AA | 0.023 | 0.86 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunoki, K.; Matsumi, H.; Miyoshi, T.; Kubo, M.; Hata, Y.; Yuasa, S. Clinical Significance of Serum Omega-3 Fatty Acids on Endothelial Function in Patients with Coronary Artery Disease Under Statin Therapy. J. Cardiovasc. Dev. Dis. 2025, 12, 60. https://doi.org/10.3390/jcdd12020060
Yunoki K, Matsumi H, Miyoshi T, Kubo M, Hata Y, Yuasa S. Clinical Significance of Serum Omega-3 Fatty Acids on Endothelial Function in Patients with Coronary Artery Disease Under Statin Therapy. Journal of Cardiovascular Development and Disease. 2025; 12(2):60. https://doi.org/10.3390/jcdd12020060
Chicago/Turabian StyleYunoki, Kei, Hiroaki Matsumi, Toru Miyoshi, Motoki Kubo, Yoshiki Hata, and Shinsuke Yuasa. 2025. "Clinical Significance of Serum Omega-3 Fatty Acids on Endothelial Function in Patients with Coronary Artery Disease Under Statin Therapy" Journal of Cardiovascular Development and Disease 12, no. 2: 60. https://doi.org/10.3390/jcdd12020060
APA StyleYunoki, K., Matsumi, H., Miyoshi, T., Kubo, M., Hata, Y., & Yuasa, S. (2025). Clinical Significance of Serum Omega-3 Fatty Acids on Endothelial Function in Patients with Coronary Artery Disease Under Statin Therapy. Journal of Cardiovascular Development and Disease, 12(2), 60. https://doi.org/10.3390/jcdd12020060







