The Conducting Tissues of the Mouse Heart
Abstract
1. Introduction
2. Materials and Methods
3. Results
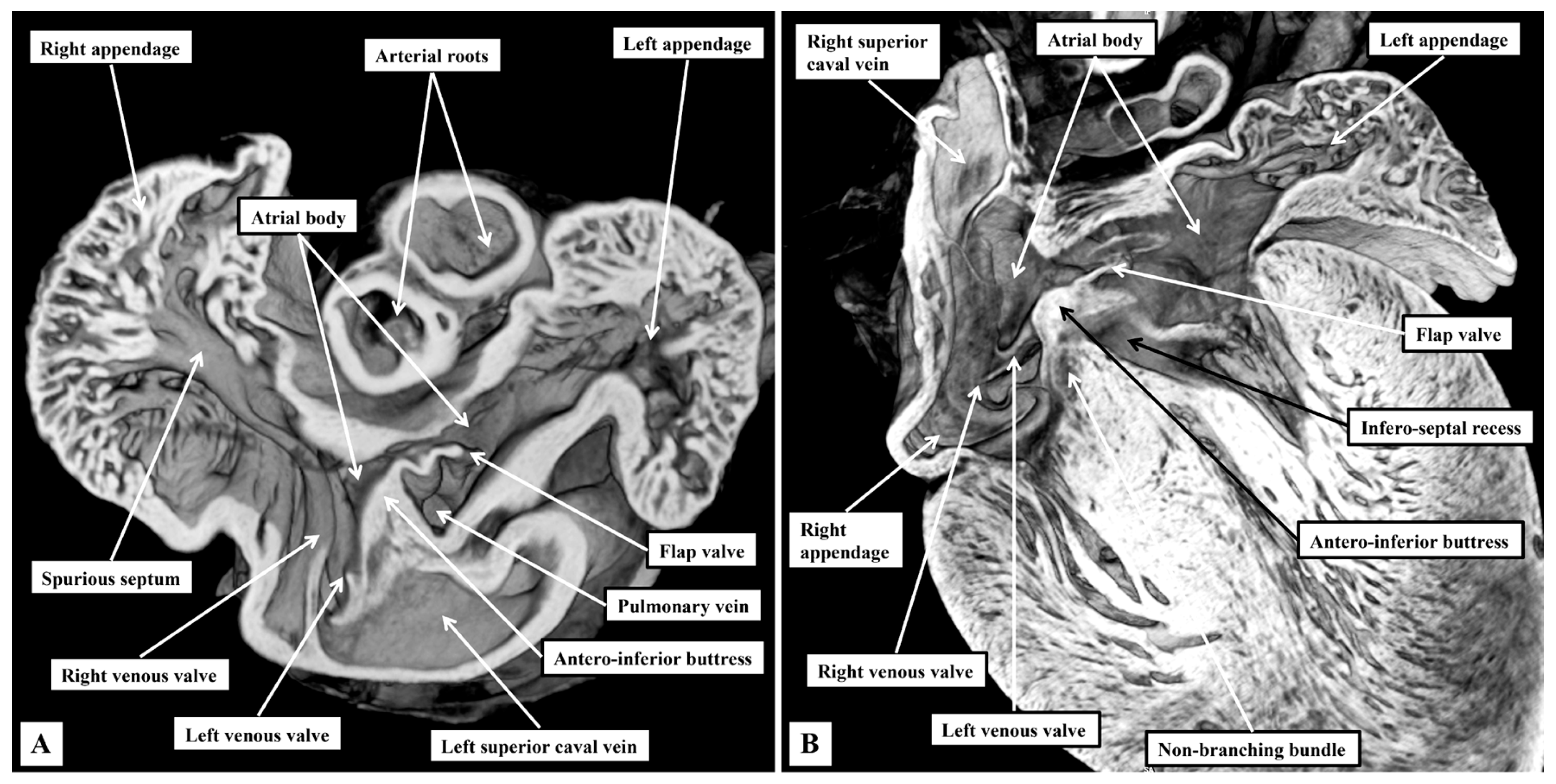
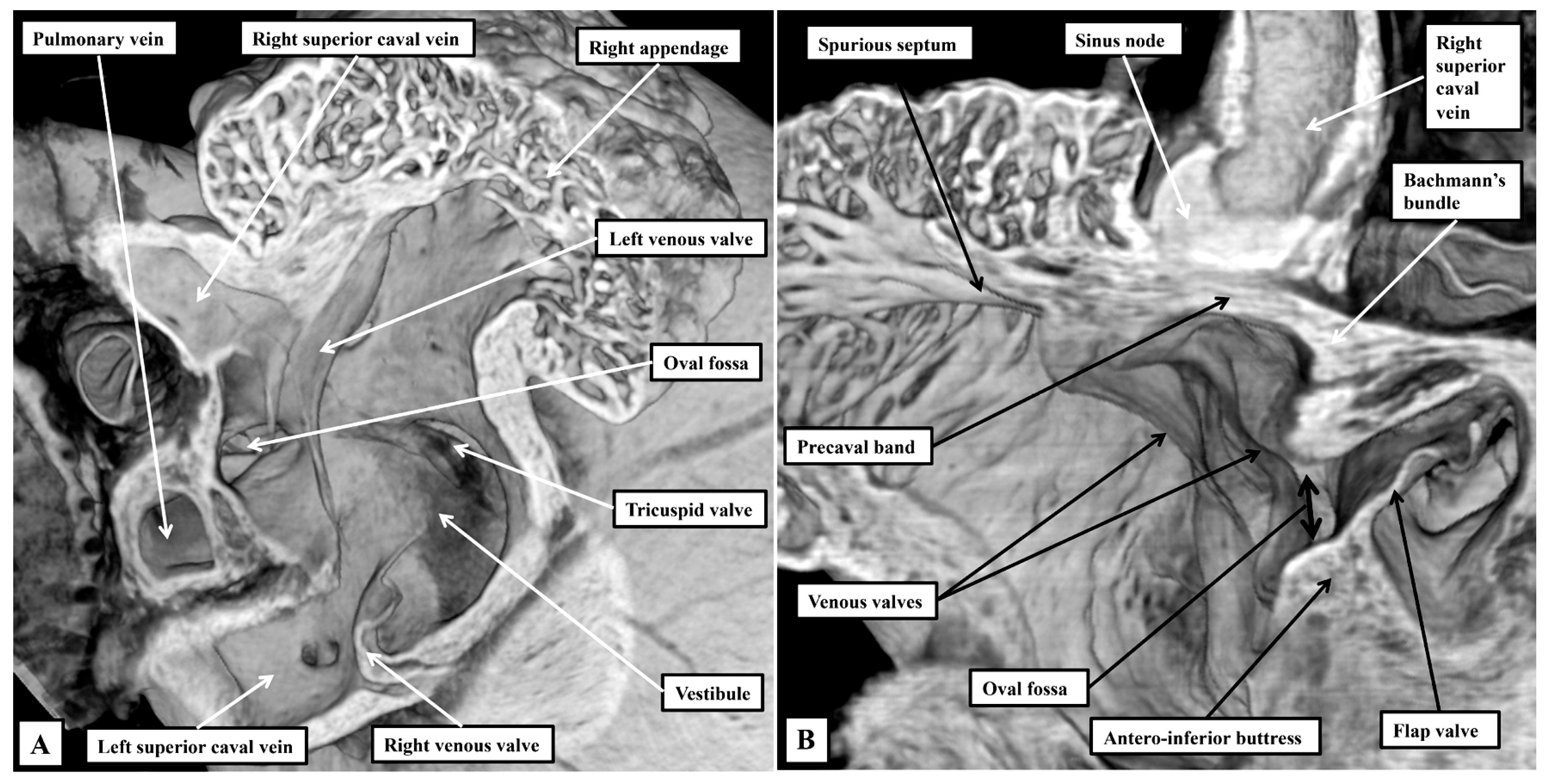
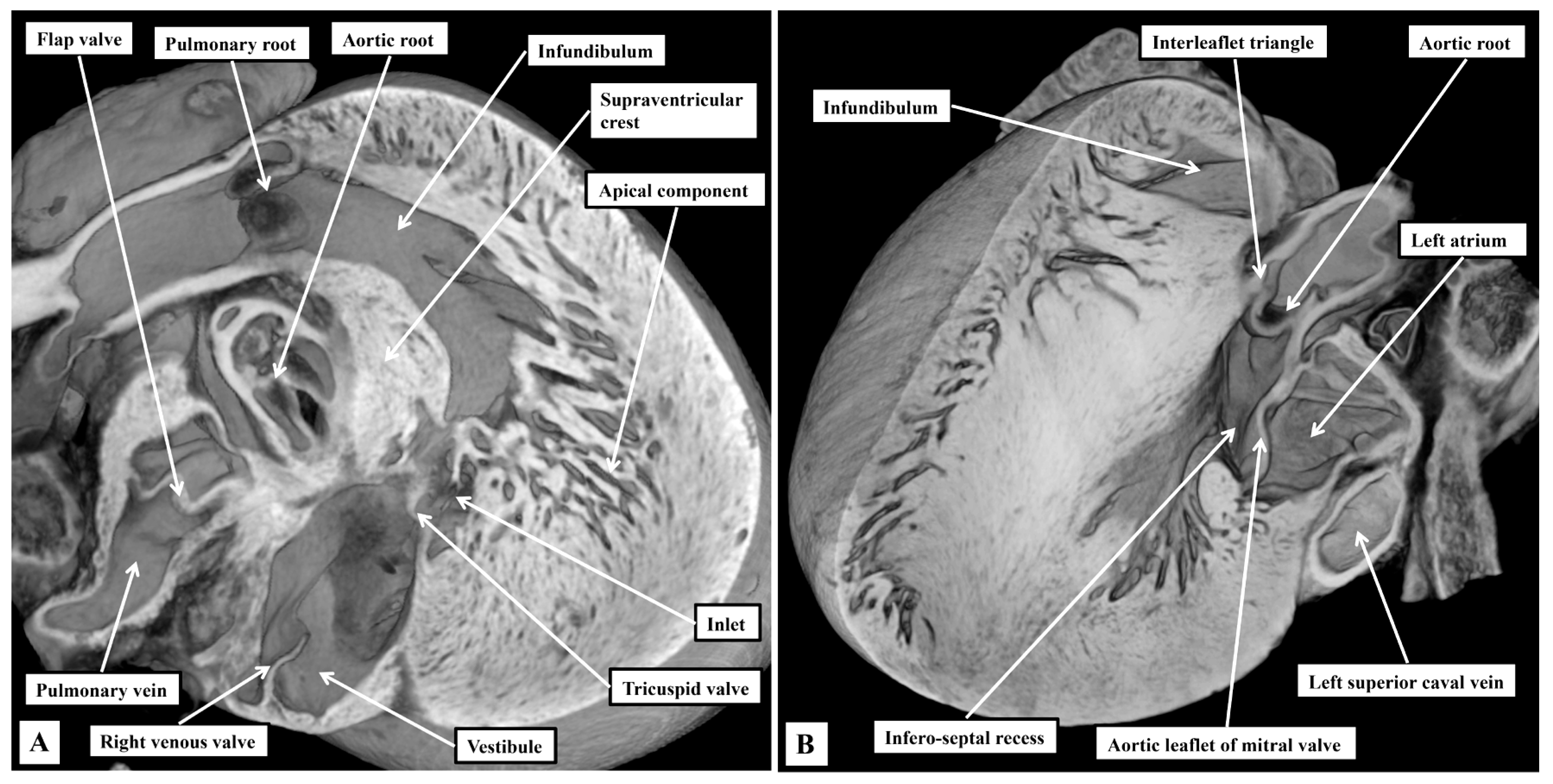
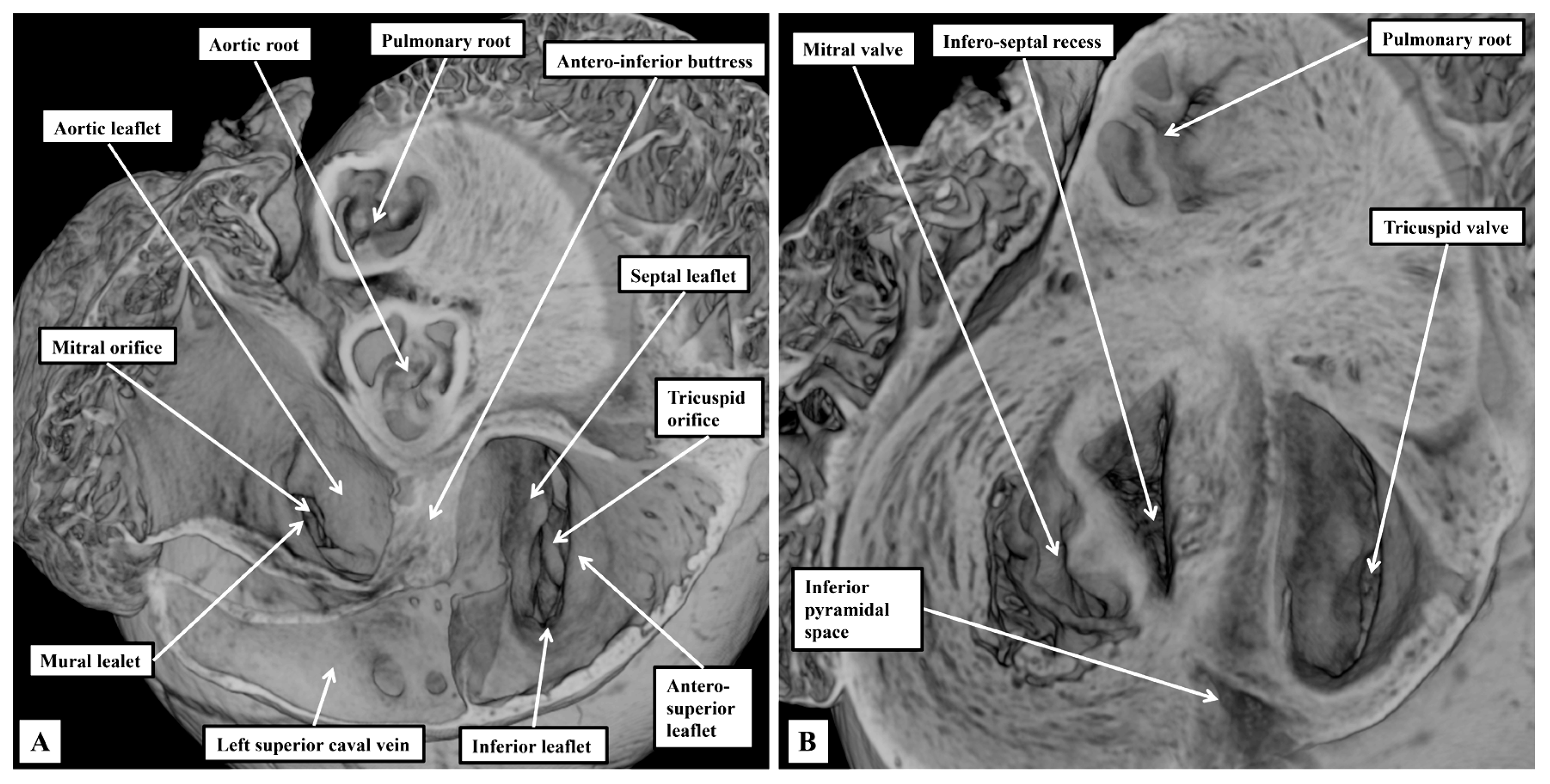
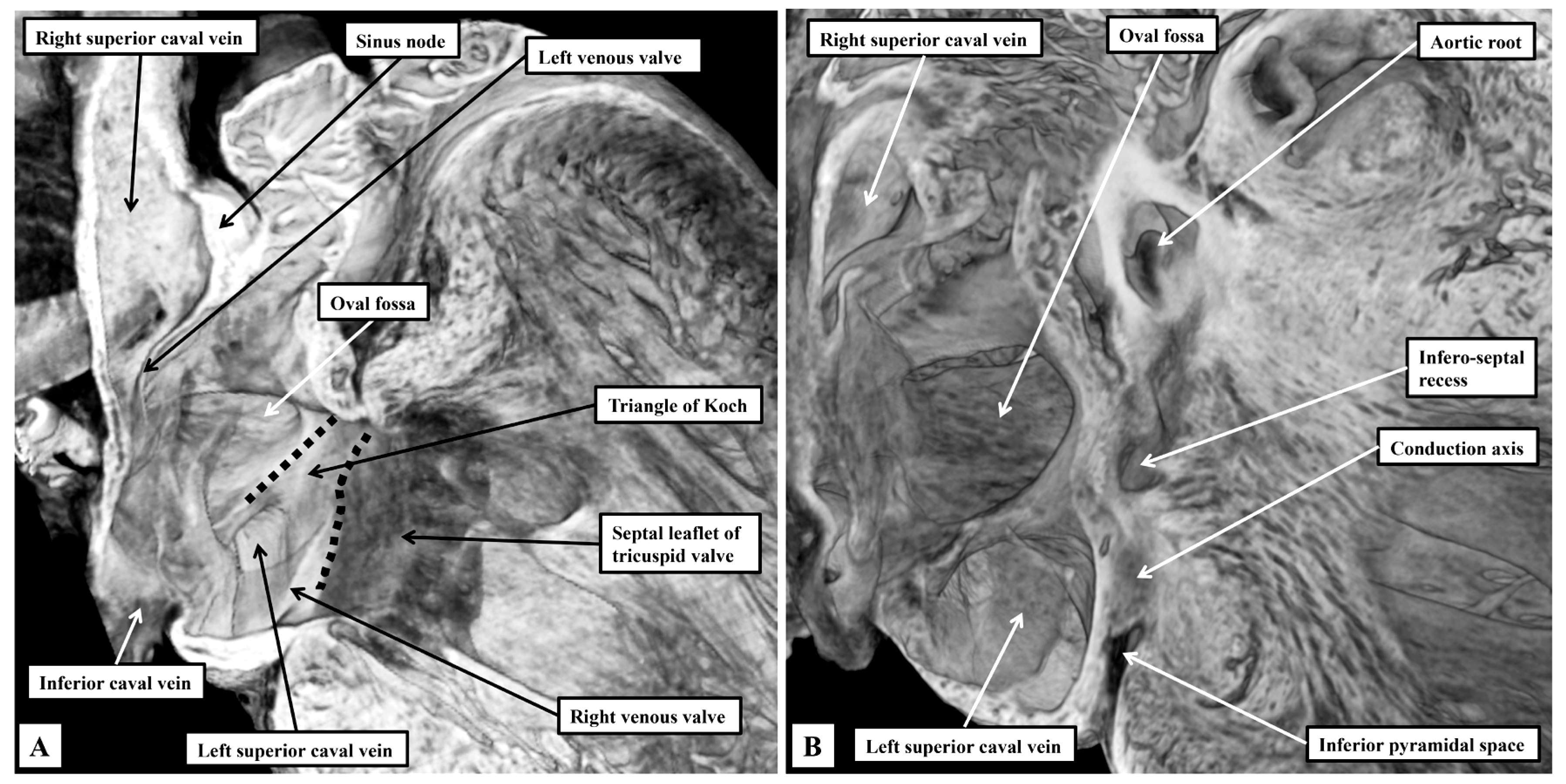
3.1. Gross Anatomy
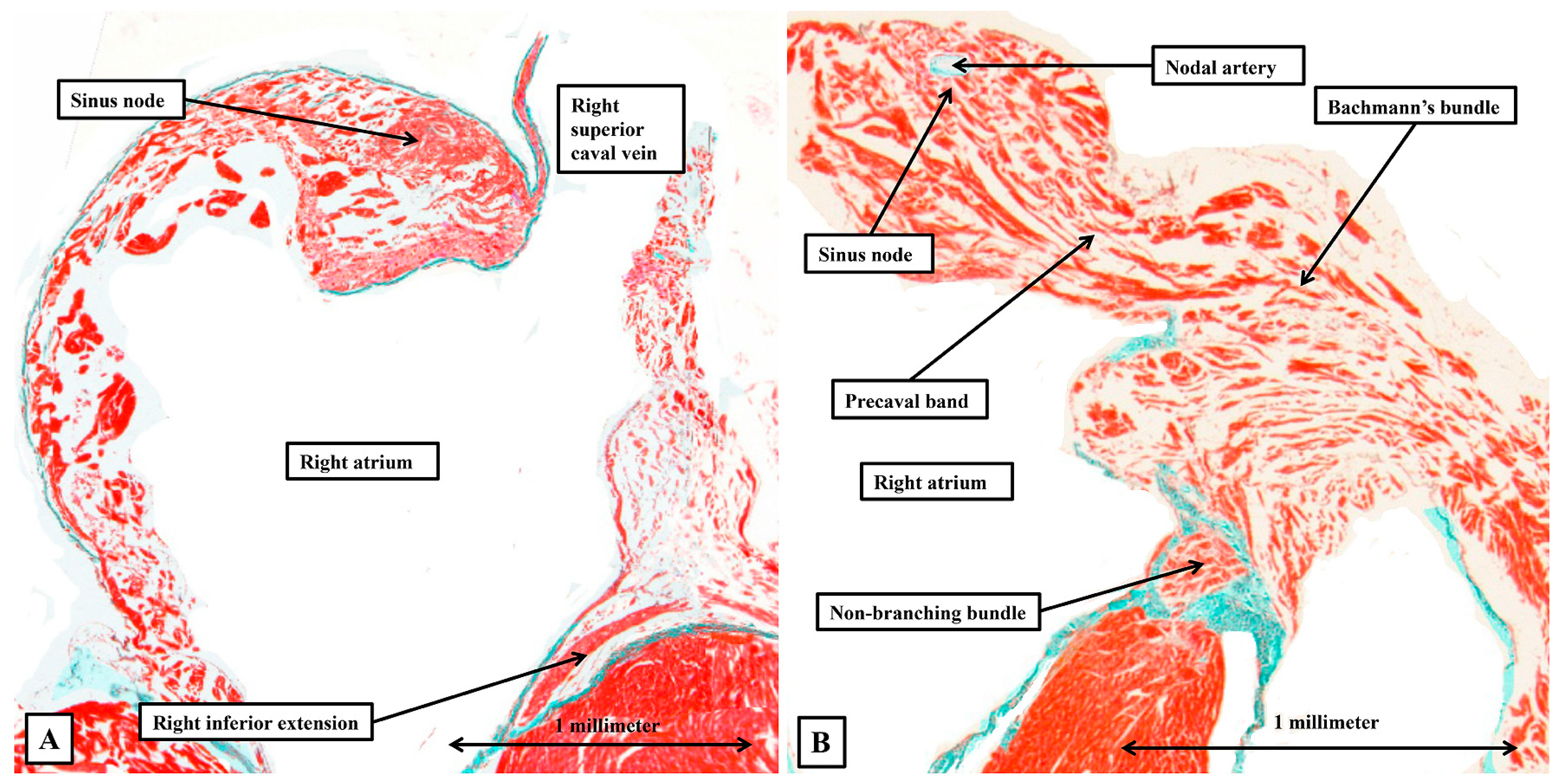
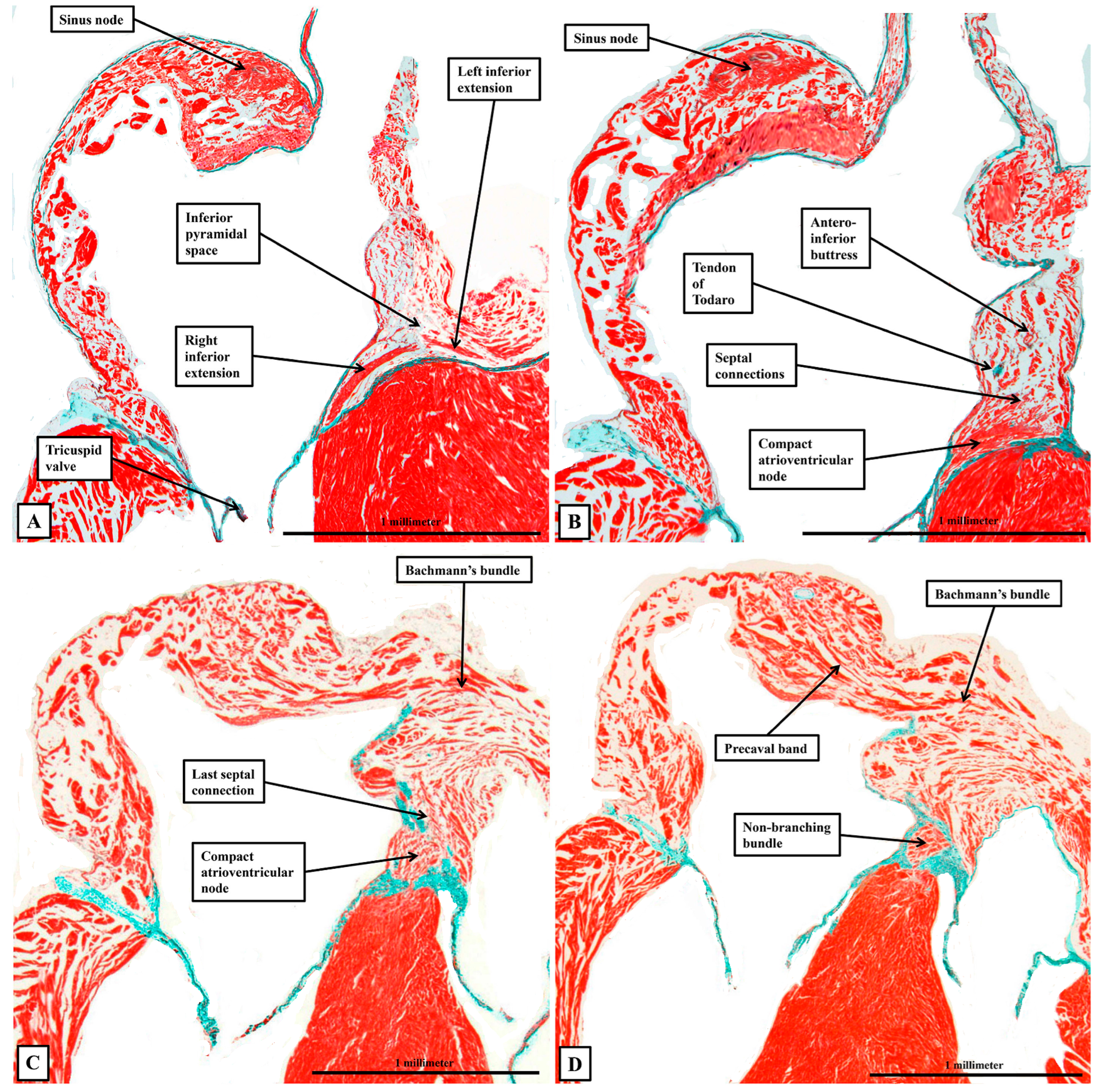
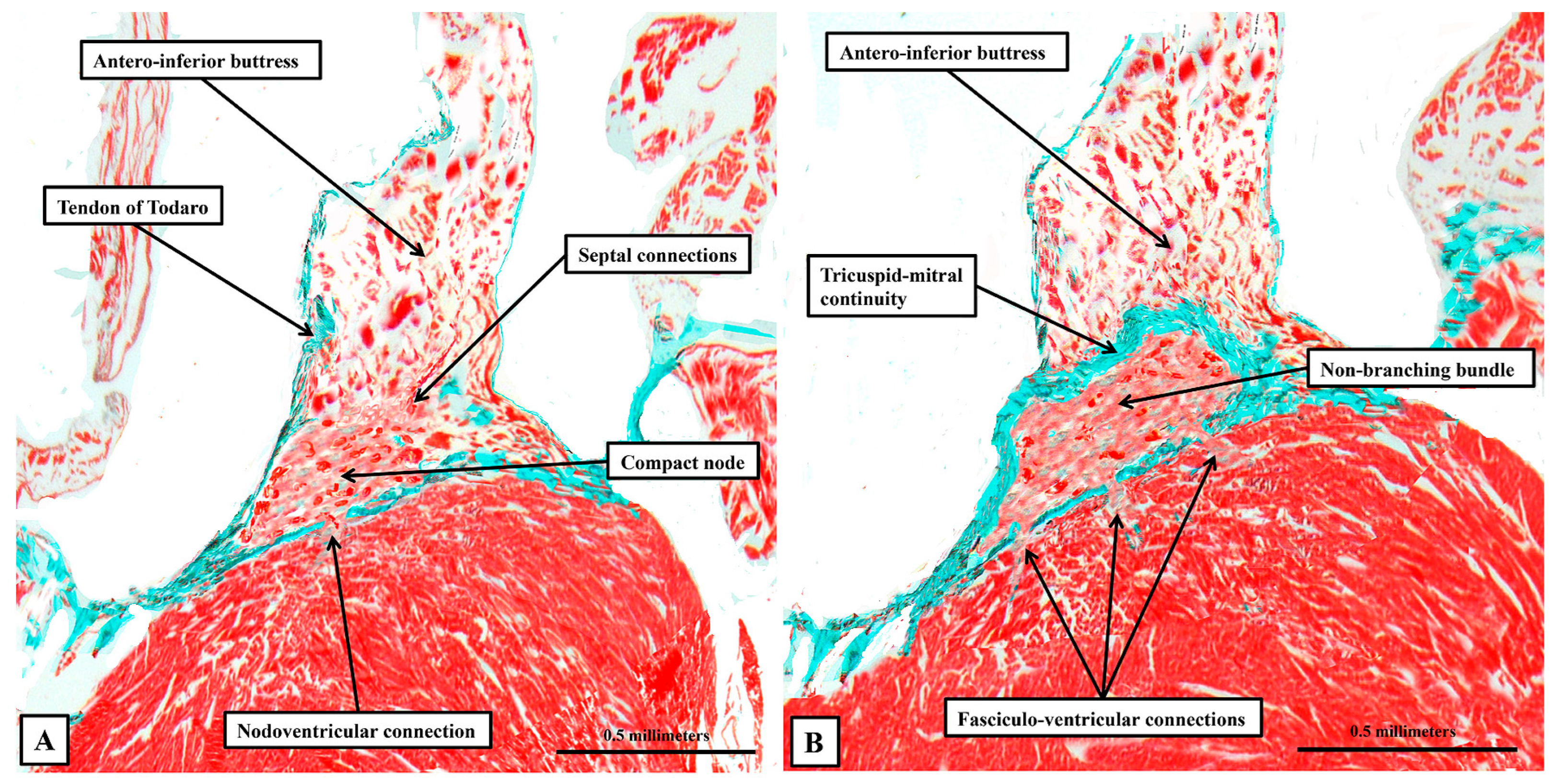
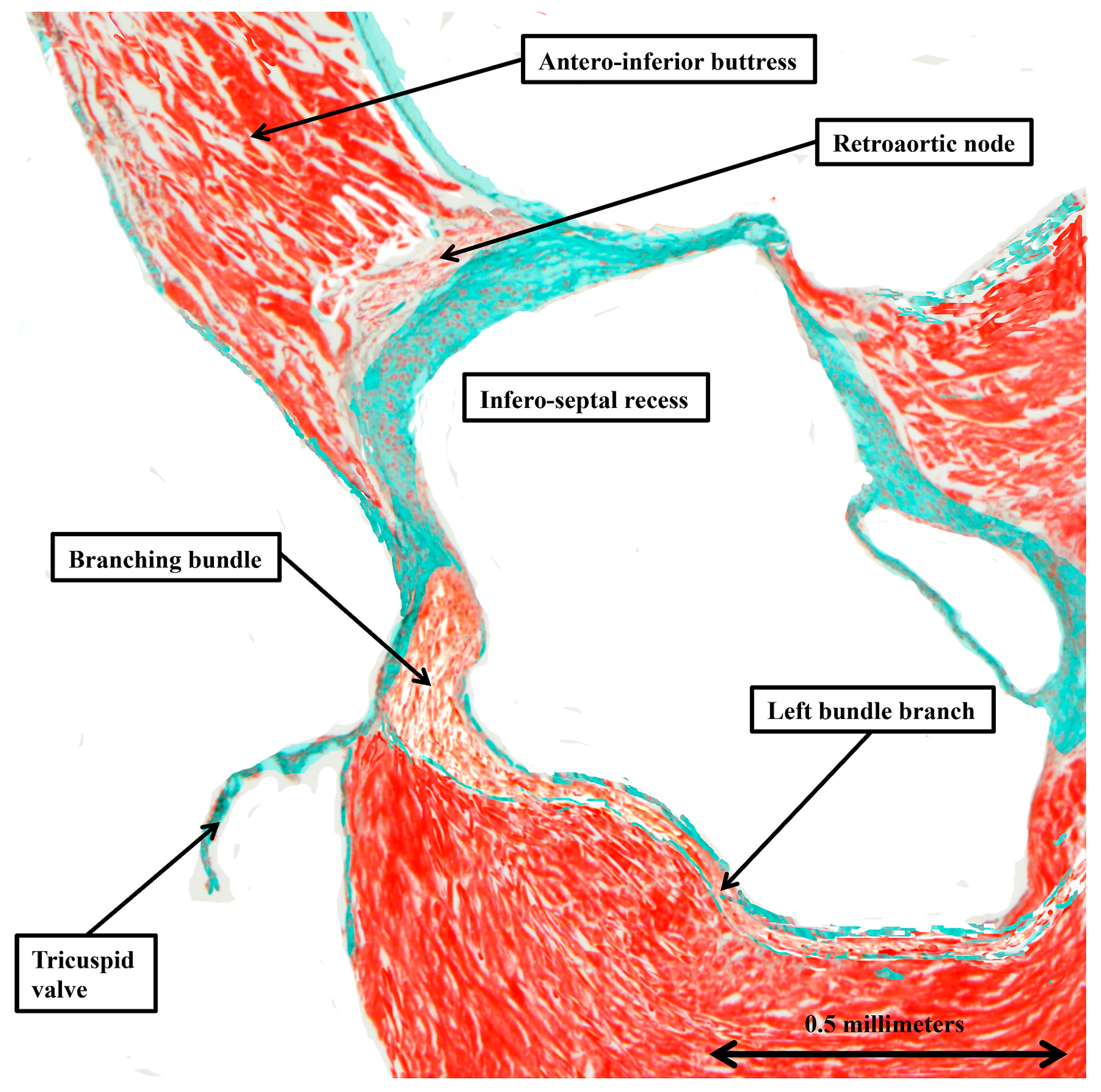
3.2. The Arrangement of the Conduction Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lev, M.; Thaemert, J.C. The conduction system of the mouse heart. Acta Anat. 1973, 85, 342–352. [Google Scholar] [CrossRef]
- Sánchez -Quintana, D.; Macías, Y.; Nevado-Medina, J.; Spicer, D.E.; Anderson, R.H. The Atrioventricular Conduction Axis in Man and Mouse. J. Cardiovasc. Dev. Dis. 2024, 11, 340. [Google Scholar] [CrossRef]
- Anderson, R.H.; Sánchez-Quintana, D.; Nevado-Medina, J.; Spicer, D.E.; Tretter, J.T.; Lamers, W.H.; Hu, Z.; Cook, A.C.; Sternick, E.B.; Katritsis, D.G. The anatomy of the atrioventricular node. J. Cardiovasc. Dev. Dis. 2025, 12, 245. [Google Scholar] [CrossRef]
- Mahaim, I. Kent’s fibers and the AV paraspecific conduction through the upper connection of the bundle of His-Tawara. Am. Heart J. 1947, 33, 651–653. [Google Scholar] [CrossRef]
- Back Sternick, E.; Anderson, R.H. The Continuing Surprises Regarding So-Called Mahaim Conduction. JACC Clin. Electrophysiol. 2021, 7, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- van Rijen, H.V.; van Veen, T.A.; van Kempen, M.J.; Wilms-Schopman, F.J.; Potse, M.; Krueger, O.; Willecke, K.; Opthof, T.; Jongsma, H.J.; de Bakker, J.M. Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circulation 2001, 103, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Durrer, D.; Van Dam, R.T.; Freud, G.E.; Janse, M.J.; Meijler, F.L.; Arzbaecher, R.C. Total excitation of the isolated human heart. Circulation 1970, 41, 899–912. [Google Scholar] [CrossRef]
- Yanni, J.; Boyett, M.R.; Anderson, R.H.; Dobrzynski, H. The extent of the specialized atrioventricular ring tissues. Heart Rhythm 2009, 6, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S. On the structure, distribution and innervation of the special heart muscle systems of the mouse. Cytol. Neurol. Stud. 1952, 10, 211–256. [Google Scholar]
- Duong, N.C.; Yang, Z.; Iaizzo, P.A. Anatomic, electrical, and hemodynamic characterization of Bachmann bundle in the swine heart. Heart Rhythm 2025, 22, 2737–2743. [Google Scholar] [CrossRef]
- Mohun, T.J.; Weninger, W.J. Imaging heart development using high-resolution episcopic microscopy. Curr. Opin. Gen. Devel. 2011, 21, 573–578. [Google Scholar] [CrossRef]
- Cook, A.C.; Anderson, R.H. Attitudinally correct nomenclature. Heart 2002, 87, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, G. The inter-auricular time interval. Am. J. Physiol. 1916, 41, 309–320. [Google Scholar] [CrossRef]
- Bohora, S.; Lokhandwala, Y.; Sternick, E.B.; Anderson, R.H.; Wellens, H.J. Reappraisal and new observations on atrial tachycardia ablated from the non-coronary aortic sinus of Valsalva. EP Eur. 2018, 20, 124–133. [Google Scholar]
- Anderson, R.H.; Sánchez-Quintana, D.; Spicer, D.E.; Farré, J.; Sternick, E.B. How does the cardiac impulse pass from the sinus to the atrioventricular node? Heart Rhythm 2022, 19, 1738–1746. [Google Scholar] [CrossRef]
- Anderson, R.H.; Sutton, R.; Sánchez-Quintana, D. Bachmann’s bundle: How anatomists and electrophysiologists see it. Heart Rhythm 2025, 22, e1077–e1081. [Google Scholar] [CrossRef]
- Macías, Y.; de Almeida, M.C.; Tretter, J.T.; Anderson, R.H.; Spicer, D.E.; Mohun, T.J.; Sánchez-Quintana, D.; Farré, J.; Sternick, E.B. Miniseries 1—Part II: The comparative anatomy of the atrioventricular conduction axis. EP Eur. 2022, 24, 443–454. [Google Scholar] [CrossRef]
- Hucker, W.J.; Mccain, M.L.; Laughner, J.I.; Iaizzo, P.A.; Efimov, I.R. Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat. Rec. 2008, 291, 204–215. [Google Scholar] [CrossRef]
- Hecht, H.H.; Kossmann, C.E.; Childers, R.W.; Langendorf, R.; Lev, M.; Rosen, K.M.; Pruitt, R.D.; Truex, R.C.; Uhley, H.N.; Watt, T.B., Jr. Atrioventricular and intraventricular conduction: Revised nomenclature and concepts. Am. J. Cardiol. 1973, 31, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, R.; Sanchez-Quintana, D.; Macias, Y.; de Almeida, M.C.; Anderson, R.H.; Sternick, E.B. Correction of bundle branch block by so-called nonselective His bundle pacing: The potential role of accessory connections in the ventricular septal crest. Heart Rhythm 2024, 21, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Hillestad, M.L.; Amontree, M.; Mahlberg, R.C.; Bagwell, M.S.; Rizzo, S.A.; Arrell, D.K.; Kargaran, P.K.; Crespo-Diaz, R.J.; Syed, F.F.; Lockhart, E.N.; et al. MYL4 Identifies Intramural Anatomy of Purkinje Fibers in Human Hearts. JACC Clin. Electrophysiol. 2025, 11, 1718–1735. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Kim, E.; Tseng, Z.H.; Gerstenfeld, E.P.; Anderson, R.H.; Sanchez-Quintana, D.; Sternick, E.B.; Hsia, H.H. Atrial tachycardia ablation at the pulmonic valve in a patient with congenitally corrected transposition of great arteries. JACC Clin. Electrophysiol. 2021, 7, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Thaemert, J.C. Fine structure of the atrioventricular node as viewed in serial sections. Am. J. Anat. 1973, 136, 43–65. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías, Y.; Sánchez-Quintana, D.; Sternick, E.B.; Anderson, R.H. The Conducting Tissues of the Mouse Heart. J. Cardiovasc. Dev. Dis. 2025, 12, 452. https://doi.org/10.3390/jcdd12110452
Macías Y, Sánchez-Quintana D, Sternick EB, Anderson RH. The Conducting Tissues of the Mouse Heart. Journal of Cardiovascular Development and Disease. 2025; 12(11):452. https://doi.org/10.3390/jcdd12110452
Chicago/Turabian StyleMacías, Yolanda, Damián Sánchez-Quintana, Eduardo Back Sternick, and Robert H. Anderson. 2025. "The Conducting Tissues of the Mouse Heart" Journal of Cardiovascular Development and Disease 12, no. 11: 452. https://doi.org/10.3390/jcdd12110452
APA StyleMacías, Y., Sánchez-Quintana, D., Sternick, E. B., & Anderson, R. H. (2025). The Conducting Tissues of the Mouse Heart. Journal of Cardiovascular Development and Disease, 12(11), 452. https://doi.org/10.3390/jcdd12110452






