Sex-Specific Expression Patterns of MYH6 and MYH7 Gene Transcripts in Large Cohorts of Non-Failing and Failing Human Left Ventricular Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Data Extraction
2.2. Statistical Analysis
3. Results
3.1. Healthy Hearts
3.2. Failing Hearts
3.3. Comparison of Healthy and Failing Hearts
3.4. Threshold Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadal-Ginard, B.; Mahdavi, V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J. Clin. Investig. 1989, 84, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Minobe, W.; Bristow, M.R.; Leinwand, L.A. Myosin Heavy Chain Isoform Expression in the Failing and Nonfailing Human Heart. Circ. Res. 2000, 86, 386–390. [Google Scholar] [CrossRef]
- Reiser, P.J.; Portman, M.A.; Ning, X.-H.; Moravec, C.S. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am. J. Physiol. Circ. Physiol. 2001, 280, H1814–H1820. [Google Scholar] [CrossRef]
- Nakao, K.; Minobe, W.; Roden, R.; Bristow, M.R.; Leinwand, L.A. Myosin heavy chain gene expression in human heart failure. J. Clin. Invest. 1997, 100, 2362–2370. [Google Scholar] [CrossRef]
- Alpert, N.R.; Mulieri, L.A. Increased myothermal economy of isometric force generation in compensated cardiac hypertrophy induced by pulmonary artery constriction in the rabbit. A characterization of heat liberation in normal and hypertrophied right ventricular papillary muscles. Circ. Res. 1982, 50, 491–500. [Google Scholar] [CrossRef]
- Holubarsch, C.; Goulette, R.P.; Litten, R.Z.; Martin, B.J.; Mulieri, L.A.; Alpert, N.R. The economy of isometric force development, myosin isoenzyme pattern and myofibrillar ATPase activity in normal and hypothyroid rat myocardium. Circ. Res. 1985, 56, 78–86. [Google Scholar] [CrossRef]
- Herron, T.J.; McDonald, K.S. Small Amounts of α-Myosin Heavy Chain Isoform Expression Significantly Increase Power Output of Rat Cardiac Myocyte Fragments. Circ. Res. 2002, 90, 1150–1152. [Google Scholar] [CrossRef]
- Harris, D.E.; Work, S.S.; Wright, R.K.; Alpert, N.R.; Warshaw, D.M. Smooth, cardiac and skeletal muscle myosin force and motion generation assessed by cross-bridge mechanical interactions in vitro. J. Muscle Res. Cell Motil. 1994, 15, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Kobayakawa, N.; Fujita, H.; Yamashita, H.; Momomura, S.I.; Chaen, S.; Omata, M.; Sugi, H. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: Molecular basis for cardiac adaptation. Circ. Res. 1998, 82, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and beta myosin isoforms and human atrial and ventricular contraction. Cell. Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef]
- Abraham, W.T.; Gilbert, E.M.; Lowes, B.D.; Minobe, W.A.; Larrabee, P.; Roden, R.L.; Dutcher, D.; Sederberg, J.; Lindenfeld, J.A.; Wolfel, E.E.; et al. Coordinate Changes in Myosin Heavy Chain Isoform Gene Expression Are Selectively Associated With Alterations in Dilated Cardiomyopathy Phenotype. Mol. Med. 2002, 8, 750–760. [Google Scholar] [CrossRef]
- Lowes, B.D.; Gilbert, E.M.; Abraham, W.T.; Minobe, W.A.; Larrabee, P.; Ferguson, D.; Wolfel, E.E.; Lindenfeld, J.; Tsvetkova, T.; Robertson, A.D.; et al. Myocardial Gene Expression in Dilated Cardiomyopathy Treated with Beta-Blocking Agents. N. Engl. J. Med. 2002, 346, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Flam, E.; Jang, C.; Murashige, D.; Yang, Y.; Morley, M.P.; Jung, S.; Kantner, D.S.; Pepper, H.; Bedi, K.C.; Brandimarto, J.; et al. Integrated landscape of cardiac metabolism in end-stage human nonischemic dilated cardiomyopathy. Nat. Cardiovasc. Res. 2022, 1, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for functional genomics data sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Wahr, P.A.; Michele, D.E.; Metzger, J.M. Effects of aging on single cardiac myocyte function in Fischer 344 x Brown Norway rats. Am. J. Physiol. Circ. Physiol. 2000, 279, H559–H565. [Google Scholar] [CrossRef]
- Grey, E.M.; Chan, C.K.; Chen, Y.; Hoffman, P.A. Age-related functional effects linked to phosphatase activity in ventricular myocytes. Am. J. Physiol. Circ. Physiol. 2003, 285, H90–H96. [Google Scholar] [CrossRef]
- Buttrick, P.; Malhotra, A.; Factor, S.; Greenen, D.; Leinwand, L.; Scheuer, J. Effect of aging and hypertension on myosin biochemistry and gene expression in the rat heart. Circ. Res. 1991, 68, 645–652. [Google Scholar] [CrossRef]
- Patrizio, M.; Musumeci, M.; Piccone, A.; Raggi, C.; Mattei, E.; Marano, G. Hormonal regulation of β-myosin heavy chain expression in the mouse left ventricle. J. Endocrinol. 2012, 216, 287–296. [Google Scholar] [CrossRef]
- Calovini, T.; Haase, H.; Morano, I. Steroid-hormone regulation of myosin subunit expression in smooth and cardiac muscle. J. Cell. Biochem. 1995, 59, 69–78. [Google Scholar] [CrossRef]
- Villar, A.V.; Llano, M.; Cobo, M.; Expósito, V.; Merino, R.; Martín-Durán, R.; Hurlé, M.A.; Nistal, J.F. Gender differences of echocardiographic and gene expression patterns in human pressure overload left ventricular hypertrophy. J. Mol. Cell. Cardiol. 2009, 46, 526–535. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Borlaug, B.A.; Rodeheffer, R.J.; Kass, D.A. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation 2005, 112, 2254–2262. [Google Scholar] [CrossRef]
- Adekunle, A.O.; Adzika, G.K.; Mprah, R.; Ndzie Noah, M.L.; Adu-Amankwaah, J.; Rizvi, R.; Akhter, N.; Sun, H. Predominance of Heart Failure with Preserved Ejection Fraction in Postmenopausal Women: Intra and Extra-Cardiomyocyte Maladaptive Alterations Scaffolded by Estrogen Deficiency. Front. Cell Dev. Biol. 2021, 9, 685996. [Google Scholar] [CrossRef]
- Panguluri, S.K.; Tur, J.; Chapalamadugu, K.C.; Katnik, C.; Cuevas, J.; Tipparaju, S.M. MicroRNA-301a Mediated Regulation of Kv4.2 in Diabetes: Identification of Key Modulators. PLoS ONE 2013, 8, e60545. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Ishii, D.N.; Backx, P.H.; Pulford, B.E.; Birks, B.R.; Tamkun, M.M. Altered K+ channel gene expression in diabetic rat ventricle: Isoform switching between Kv4.2 and Kv1.4. Am. J. Physiol. Circ. Physiol. 2001, 281, H1800–H1807. [Google Scholar] [CrossRef] [PubMed]
- Goodale, T.; Sadhu, A.; Petak, S.; Robbins, R. Testosterone and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]




| Gene | Comparison | Group 1 (Mean ± SD) | Group 2 (Mean ± SD) | p-Value |
|---|---|---|---|---|
| MYH6 | F ≤ 50 vs. F > 50 | 895.33 ± 729.97 | 486.82 ± 456.97 | 0.009 * |
| M ≤ 50 vs. M > 50 | 705.73 ± 498.91 | 682.69 ± 450.94 | 0.936 | |
| F ≤ 50 vs. M ≤ 50 | 895.33 ± 729.97 | 705.73 ± 498.91 | 0.471 | |
| F > 50 vs. M > 50 | 486.82 ± 456.97 | 682.69 ± 450.94 | 0.001 * | |
| MYH7 | F ≤ 50 vs. F > 50 | 2358.33 ± 885.88 | 2295.90 ± 947.47 | 0.892 |
| M ≤ 50 vs. M > 50 | 1834.85 ± 727.39 | 2263.71 ± 1172.16 | 0.187 | |
| F ≤ 50 vs. M ≤ 50 | 2358.33 ± 885.88 | 1834.85 ± 727.39 | 0.158 | |
| F > 50 vs. M > 50 | 2295.90 ± 947.47 | 2263.71 ± 1172.16 | 0.418 |
| MYH6 | |||
|---|---|---|---|
| Pairwise Comparisons | Mean ± SD | p-Value | |
| F ≤ 50 vs. F > 50 | 881.30 ± 786.81 | 569.83 ± 416.04 | 0.309 |
| F ≤ 50 vs. F > 50 HpT | 881.30 ± 786.81 | 456.07 ± 467.51 | 0.016 |
| F > 50 vs. F > 50 HpT | 569.83 ± 416.04 | 456.07 ± 467.51 | 0.175 |
| M ≤ 50 vs. M ≤ 50 HpT | 699.24 ± 452.72 | 712.73 ± 581.31 | 0.822 |
| M ≤ 50 vs. M > 50 | 699.24 ± 452.72 | 561.33 ± 354.90 | 0.395 |
| M ≤ 50 vs. M > 50 HpT | 699.24 ± 452.72 | 734.47 ± 497.21 | 0.870 |
| M ≤ 50 HpT vs. M >50 | 712.73 ± 581.31 | 561.33 ± 354.90 | 0.790 |
| M ≤ 50 HpT vs. M > 50 HpT | 712.73 ± 581.31 | 734.47 ± 497.21 | 0.632 |
| M > 50 vs. M > 50 HpT | 561.33 ± 354.90 | 734.47 ± 497.21 | 0.340 |
| F ≤ 50 vs. M ≤ 50 | 881.30 ± 786.81 | 699.24 ± 452.72 | 0.841 |
| F ≤ 50 vs. M ≤ 50 HpT | 881.30 ± 786.81 | 712.73 ± 581.31 | 0.738 |
| F ≤ 50 vs. M > 50 | 881.30 ± 786.81 | 561.33 ± 354.90 | 0.397 |
| F ≤ 50 vs. M > 50 HpT | 881.30 ± 786.81 | 734.47 ± 497.21 | 0.965 |
| F > 50 vs. M > 50 | 569.83 ± 416.04 | 561.33 ± 354.90 | 0.705 |
| F > 50 vs. M > 50 HpT | 569.83 ± 416.04 | 734.47 ± 497.21 | 0.187 |
| F > 50 vs. M ≤ 50 | 569.83 ± 416.04 | 699.24 ± 452.72 | 0.306 |
| F > 50 vs. M ≤ 50 HpT | 569.83 ± 416.04 | 712.73 ± 581.31 | 0.478 |
| F > 50 HpT vs. M ≤ 50 | 456.07 ± 467.51 | 699.24 ± 452.72 | 0.008 * |
| F > 50 HpT vs. M ≤ 50 HpT | 456.07 ± 467.51 | 712.73 ± 581.31 | 0.02 |
| F > 50 HpT vs. M > 50 | 456.07 ± 467.51 | 561.33 ± 354.90 | 0.027 |
| F > 50 HpT vs. M > 50 HpT | 456.07 ± 467.51 | 734.47 ± 497.21 | 0.0005 * |
| MYH7 | |||
| Pairwise comparisons | Mean ± SD | p-value | |
| F ≤ 50 vs. F > 50 | 2253.20 ± 931.04 | 2281.46 ± 1141.35 | 0.914 |
| F ≤ 50 vs. F > 50 HpT | 2253.20 ± 931.04 | 2301.24 ± 864.64 | 0.669 |
| F > 50 vs. F > 50 HpT | 2281.46 ± 1141.35 | 2301.24 ± 864.64 | 0.780 |
| M ≤ 50 vs. M ≤ 50 HpT | 1535.29 ± 686.24 | 2157.47 ± 625.31 | 0.022 |
| M ≤ 50 vs. M > 50 | 1535.29 ± 686.24 | 2590.00 ± 1408.74 | 0.020 |
| M ≤ 50 vs. M > 50 HpT | 1535.29 ± 686.24 | 2142.65 ± 1016.65 | 0.057 |
| M ≤ 50 HpT vs. M >50 | 2157.47 ± 625.31 | 2590.00 ± 1408.74 | 0.811 |
| M ≤ 50 HpT vs. M > 50 HpT | 2157.47 ± 625.31 | 2142.65 ± 1016.65 | 0.795 |
| M > 50 vs. M > 50 HpT | 2590.00 ± 1408.74 | 2142.65 ± 1016.65 | 0.505 |
| F ≤ 50 vs. M ≤ 50 | 2253.20 ± 931.04 | 1535.29 ± 686.24 | 0.056 |
| F ≤ 50 vs. M ≤ 50 HpT | 2253.20 ± 931.04 | 2157.47 ± 625.31 | 0.693 |
| F ≤ 50 vs. M > 50 | 2253.20 ± 931.04 | 2590.00 ± 1408.74 | 0.849 |
| F ≤ 50 vs. M > 50 HpT | 2253.20 ± 931.04 | 2142.65 ± 1016.65 | 0.827 |
| F > 50 vs. M > 50 | 2281.46 ± 1141.35 | 2590.00 ± 1408.74 | 0.780 |
| F > 50 vs. M > 50 HpT | 2281.46 ± 1141.35 | 2142.65 ± 1016.65 | 0.634 |
| F > 50 vs. M ≤ 50 | 2281.46 ± 1141.35 | 1535.29 ± 686.24 | 0.039 |
| F > 50 vs. M ≤ 50 HpT | 2281.46 ± 1141.35 | 2157.47 ± 625.31 | 0.758 |
| F > 50 HpT vs. M ≤ 50 | 2301.24 ± 864.64 | 1535.29 ± 686.24 | 0.003 * |
| F > 50 HpT vs. M ≤ 50 HpT | 2301.24 ± 864.64 | 2157.47 ± 625.31 | 0.557 |
| F > 50 HpT vs. M > 50 | 2301.24 ± 864.64 | 2590.00 ± 1408.74 | 0.914 |
| F > 50 HpT vs. M > 50 HpT | 2301.24 ± 864.64 | 2142.65 ± 1016.65 | 0.296 |
| Gene | Comparison | Group 1 (Mean ± SD) | Group 2 (Mean ± SD) | p-Value |
|---|---|---|---|---|
| MYH6 | F ≤ 50 vs. F > 50 | 202.15 ± 127.33 | 160.99 ± 91.52 | 0.183 |
| M ≤ 50 vs. M > 50 | 156.77 ± 89.89 | 190.19 ± 127.15 | 0.269 | |
| F ≤ 50 vs. M ≤ 50 | 202.15 ± 127.33 | 156.77 ± 89.89 | 0.175 | |
| F > 50 vs. M > 50 | 160.99 ± 91.52 | 190.19 ± 127.15 | 0.334 | |
| MYH7 | F ≤ 50 vs. F > 50 | 3076.46 ± 120.87 | 2613.48 ± 784.86 | 0.152 |
| M ≤ 50 vs. M > 50 | 2736.27 ± 972.66 | 2743.73 ± 883.05 | 0.782 | |
| F ≤ 50 vs. M ≤ 50 | 3076.46 ± 120.87 | 2736.27 ± 972.66 | 0.434 | |
| F > 50 vs. M > 50 | 2613.48 ± 784.86 | 2743.73 ± 883.05 | 0.599 |
| MYH6 | |||
|---|---|---|---|
| Pairwise Comparisons | Mean ± SD | p-Value | |
| F ≤ 50 vs. F ≤ 50 DCM | 895.33 ± 62.42 | 202.15 ± 30.32 | <0.0001 * |
| F > 50 vs. F > 50 DCM | 486.82 ± 60.09 | 160.99 ± 92.63 | <0.0001 * |
| F ≤ 50 vs. F > 50 DCM | 895.33 ± 62.42 | 160.99 ± 92.63 | <0.0001 * |
| F > 50 vs. F ≤ 50 DCM | 486.82 ± 60.09 | 202.15 ± 30.32 | <0.0001 * |
| M ≤ 50 vs. M ≤ 50 DCM | 705.73 ± 508.42 | 156.77 ± 91.17 | <0.0001 * |
| M > 50 vs. M > 50 DCM | 682.69 ± 455.71 | 190.19 ± 28.23 | <0.0001 * |
| M ≤ 50 vs. M > 50 DCM | 705.73 ± 508.42 | 190.19 ± 28.23 | <0.0001 * |
| M > 50 vs. M ≤ 50 DCM | 682.69 ± 455.71 | 156.77 ± 91.17 | <0.0001 * |
| MYH7 | |||
| Pairwise comparisons | Mean ± SD | p-value | |
| F ≤ 50 vs. F ≤ 50 DCM | 2358.33 ± 25.27 | 3076.46 ± 147.24 | 0.049 |
| F > 50 vs. F > 50 DCM | 2295.90 ± 53.94 | 2613.48 ± 794.37 | 0.048 |
| F ≤ 50 vs. F > 50 DCM | 2358.33 ± 25.27 | 2613.48 ± 794.37 | 0.221 |
| F > 50 vs. F ≤ 50 DCM | 2295.90 ± 53.94 | 3076.46 ± 147.24 | 0.003 * |
| M ≤ 50 vs. M ≤ 50 DCM | 1834.85 ± 41.24 | 2736.27 ± 986.46 | 0.0003 * |
| M > 50 vs. M > 50 DCM | 2263.71 ± 184.57 | 2743.74 ± 890.50 | 0.0012 * |
| M ≤ 50 vs. M > 50 DCM | 1834.85 ± 741.24 | 2743.74 ± 890.50 | <0.0001 * |
| M > 50 vs. M ≤ 50 DCM | 2263.71 ± 184.57 | 2736.27 ± 986.46 | 0.012 |
| MYH6 | |||
|---|---|---|---|
| Pairwise Comparisons | Mean ± SD | p-Value | |
| F ≤ 50 vs. F ≤ 50 DCM | 881.3 ± 29.37 | 202.15 ± 30.32 | <0.0001 * |
| F ≤ 50 vs. F > 50 DCM | 881.3 ± 29.37 | 160.99 ± 92.63 | <0.0001 * |
| F > 50 vs. F ≤ 50 DCM | 569.83 ± 26.84 | 202.15 ± 130.32 | <0.0001 * |
| F > 50 vs. F > 50 DCM | 569.83 ± 426.84 | 160.99 ± 92.63 | <0.0001 * |
| F > 50 HpT vs. F ≤ 50 DCM | 456.1 ± 471.90 | 202.15 ± 130.32 | <0.0001 * |
| F > 50 HpT vs. F > 50 DCM | 456.1 ± 471.90 | 160.99 ± 92.63 | <0.0001 * |
| M ≤ 50 vs. M ≤ 50 DCM | 699.24 ± 452.72 | 156.77 ± 91.17 | <0.0001 * |
| M ≤ 50 vs. M > 50 DCM | 699.24 ± 452.72 | 190.19 ± 128.23 | <0.0001 * |
| M ≤ 50 HpT vs. M ≤ 50 DCM | 712.73 ± 581.31 | 156.77 ± 91.17 | <0.0001 * |
| M ≤ 50 HpT vs. M > 50 DCM | 712.73 ± 581.31 | 190.19 ± 128.23 | <0.0001 * |
| M > 50 vs. M ≤ 50 DCM | 561.33 ± 354.90 | 156.77 ± 91.17 | <0.0001 * |
| M > 50 vs. M > 50 DCM | 561.33 ± 354.90 | 190.19 ± 128.23 | <0.0001 * |
| M > 50 HpT vs. M ≤ 50 DCM | 734.47 ± 497.22 | 156.77 ± 91.17 | <0.0001 * |
| M > 50 HpT vs. M > 50 DCM | 734.47 ± 497.22 | 190.19 ± 128.23 | <0.0001 * |
| MYH7 | |||
| Pairwise comparisons | Mean ± SD | p-value | |
| F ≤ 50 vs. F ≤ 50 DCM | 2253.2 ± 981.41 | 3076.46 ± 1147.24 | 0.018 |
| F ≤ 50 vs. F > 50 DCM | 2253.2 ± 981.41 | 2613.48 ± 794.37 | 0.097 |
| F > 50 vs. F ≤ 50 DCM | 2281.46 ± 1171.00 | 3076.46 ± 1147.24 | 0.023 |
| F > 50 vs. F > 50 DCM | 2281.46 ± 1171.00 | 2613.48 ± 794.37 | 0.185 |
| F > 50 HpT vs. F ≤ 50 DCM | 2301.24 ± 872.76 | 3076.46 ± 1147.24 | 0.0004 * |
| F > 50 HpT vs. F > 50 DCM | 2301.24 ± 872.76 | 2613.48 ± 794.37 | 0.058 |
| M ≤ 50 vs. M ≤ 50 DCM | 1535.29 ± 712.14 | 2736.27 ± 986.46 | 0.0001 * |
| M ≤ 50 vs. M > 50 DCM | 1535.29 ± 712.14 | 2743.73 ± 890.50 | <0.0001 * |
| M ≤ 50 HpT vs. M ≤ 50 DCM | 2157.46 ± 650.84 | 2736.27 ± 986.46 | 0.037 |
| M ≤ 50 HpT vs. M > 50 DCM | 2157.46 ± 650.84 | 2743.73 ± 890.50 | 0.012 |
| M > 50 vs. M ≤ 50 DCM | 2590.00 ± 1458.18 | 2736.27 ± 986.46 | 0.365 |
| M > 50 vs. M > 50 DCM | 2590.00 ± 1458.18 | 2743.73 ± 890.50 | 0.290 |
| M > 50 HpT vs. M ≤ 50 DCM | 2142.65 ± 1032.92 | 2736.27 ± 986.46 | 0.0006 * |
| M > 50 HpT vs. M > 50 DCM | 2142.65 ± 1032.92 | 2743.73 ± 890.50 | 0.001 * |
| MYH6 | |||
|---|---|---|---|
| Pairwise Comparisons | Mean ± SD | p-Value | |
| F ≤ 50 vs. M ≤ 50 DCM | 881.3 ± 829.37 | 156.77 ± 91.17 | <0.0001 * |
| M ≤ 50 vs. F ≤ 50 DCM | 202.15 ± 130.32 | 699.24 ± 452.72 | <0.0001 * |
| F ≤ 50 vs. M > 50 DCM | 881.3 ± 829.37 | 190.19 ± 128.23 | <0.0001 * |
| M ≤ 50 vs. F > 50 DCM | 160.99 ± 92.63 | 699.24 ± 452.72 | <0.0001 * |
| F > 50 vs. M ≤ 50 DCM | 569.83 ± 426.84 | 156.77 ± 91.17 | <0.0001 * |
| M > 50 vs. F ≤ 50 DCM | 202.15 ± 130.32 | 561.33 ± 354.90 | <0.0001 * |
| F > 50 HpT vs. M ≤ 50 DCM | 456.1 ± 471.90 | 156.77 ± 91.17 | <0.0001 * |
| M > 50 HpT vs. F ≤ 50 DCM | 202.15 ± 130.32 | 734.47 ± 497.22 | <0.0001 * |
| F > 50 HpT vs. M > 50 DCM | 456.1 ± 471.90 | 190.19 ± 128.23 | <0.0001 * |
| M > 50 HpT vs. F > 50 DCM | 160.99 ± 92.63 | 734.47 ± 497.22 | <0.0001 * |
| F > 50 vs. M > 50 DCM | 569.83 ± 426.84 | 190.19 ± 128.23 | <0.0001 * |
| M > 50 vs. F > 50 DCM | 160.99 ± 92.63 | 561.33 ± 354.90 | <0.0001 * |
| F ≤ 50 DCM vs. M ≤ 50 HpT | 202.15 ± 130.32 | 712.73 ± 581.31 | <0.0001 * |
| F > 50 DCM vs. M ≤ 50 HpT | 160.99 ± 92.63 | 712.73 ± 581.31 | <0.0001 * |
| MYH7 | |||
| Pairwise comparisons | Mean ± SD | p-value | |
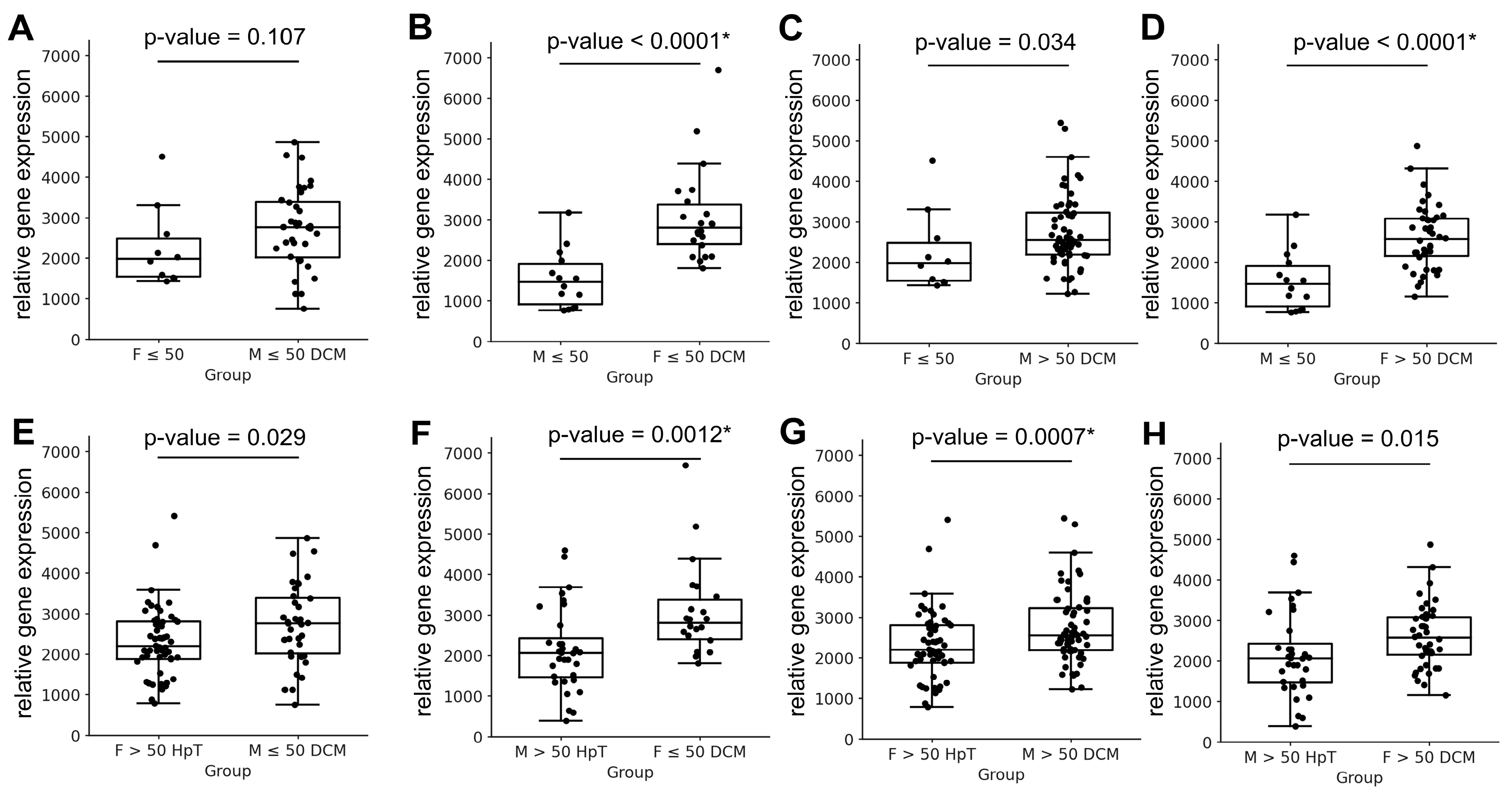
| F ≤ 50 vs. M ≤ 50 DCM | 2253.2 ± 981.41 | 2736.27 ± 986.46 | 0.107 |
| M ≤ 50 vs. F ≤ 50 DCM | 1535.29 ± 712.14 | 3076.46 ± 1147.24 | <0.0001 * |
| F ≤ 50 vs. M > 50 DCM | 2253.2 ± 981.41 | 2743.73 ± 890.50 | 0.034 |
| M ≤ 50 vs. F > 50 DCM | 1535.29 ± 712.14 | 2613.48 ± 794.37 | <0.0001 * |
| F > 50 vs. M ≤ 50 DCM | 2281.46 ± 1171.00 | 2736.27 ± 986.46 | 0.073 |
| M > 50 vs. F ≤ 50 DCM | 2590.00 ± 1458.18 | 3076.46 ± 1147.24 | 0.134 |
| F > 50 HpT vs. M ≤ 50 DCM | 2301.24 ± 872.76 | 2736.27 ± 986.46 | 0.029 |
| M > 50 HpT vs. F ≤ 50 DCM | 2142.65 ± 1032.92 | 3076.46 ± 1147.24 | 0.0012 * |
| F > 50 HpT vs. M > 50 DCM | 2301.24 ± 872.76 | 2743.73 ± 890.50 | 0.007 * |
| M > 50 HpT vs. F > 50 DCM | 2142.65 ± 1032.92 | 2613.48 ± 794.37 | 0.015 |
| F > 50 vs. M > 50 DCM | 2281.46 ± 1171.00 | 2743.73 ± 890.50 | 0.065 |
| M > 50 vs. F > 50 DCM | 2613.48 ± 794.37 | 2 590.00 ± 1458.18 | 0.418 |
| F ≤ 50 DCM vs. M ≤ 50 HpT | 3076.46 ± 1147.24 | 2157.46 ± 650.84 | 0.003 * |
| F > 50 DCM vs. M ≤ 50 HpT | 2613.48 ± 794.37 | 2157.46 ± 650.84 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Červenák, Z.; Somorčík, J.; Jalali, Y.; Zajacová, Ž.; Baldovič, M.; Gažová, A.; Kyselovič, J. Sex-Specific Expression Patterns of MYH6 and MYH7 Gene Transcripts in Large Cohorts of Non-Failing and Failing Human Left Ventricular Tissues. J. Cardiovasc. Dev. Dis. 2025, 12, 447. https://doi.org/10.3390/jcdd12110447
Červenák Z, Somorčík J, Jalali Y, Zajacová Ž, Baldovič M, Gažová A, Kyselovič J. Sex-Specific Expression Patterns of MYH6 and MYH7 Gene Transcripts in Large Cohorts of Non-Failing and Failing Human Left Ventricular Tissues. Journal of Cardiovascular Development and Disease. 2025; 12(11):447. https://doi.org/10.3390/jcdd12110447
Chicago/Turabian StyleČervenák, Zdenko, Ján Somorčík, Yashar Jalali, Žaneta Zajacová, Marian Baldovič, Andrea Gažová, and Ján Kyselovič. 2025. "Sex-Specific Expression Patterns of MYH6 and MYH7 Gene Transcripts in Large Cohorts of Non-Failing and Failing Human Left Ventricular Tissues" Journal of Cardiovascular Development and Disease 12, no. 11: 447. https://doi.org/10.3390/jcdd12110447
APA StyleČervenák, Z., Somorčík, J., Jalali, Y., Zajacová, Ž., Baldovič, M., Gažová, A., & Kyselovič, J. (2025). Sex-Specific Expression Patterns of MYH6 and MYH7 Gene Transcripts in Large Cohorts of Non-Failing and Failing Human Left Ventricular Tissues. Journal of Cardiovascular Development and Disease, 12(11), 447. https://doi.org/10.3390/jcdd12110447








