Clinical, Imaging, and Serum Biomarker Predictors of Malignant Cerebral Infarction
Abstract
1. Introduction
2. Materials and Methods
- 1.
- Proximal LVO involving the carotid artery or M1 segment of the middle cerebral artery (MCA).
- 2.
- Acute hemispheric syndrome with severe symptoms (National Institute of Health Stroke Scale (NIHSS) > 15 for dominant hemisphere or NIHSS > 13 for non-dominant hemisphere) at admission.
- 3.
- Lack of clinical improvement (more than four points in the NIHSS) after treatment or in the first 24 h after admission.
- 4.
- Admission multimodal CT protocol, including non-contrast CT (NCCT), CT angiography (CTA), and CTP.
- Clinical indicators of intracranial hypertension, such as a decreased level of consciousness (score ≥1 in the corresponding item on NIHSS), anisocoria, death due to cerebral edema, or need for decompressive craniectomy.
- Neuroimaging evidence indicating significant cerebral edema, exemplified by a midline shift ≥6 mm or an infarct encompassing over half of the MCA territory.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCI | Malignant Cerebral Infarction |
| LVO | Large Vessel Occlusion |
| CT | Computed Tomography |
| CTP | Computed Tomography Perfusion |
| rCVB | Relative Cerebral Blood Volume |
| MRI | Magnetic Resonance Imaging |
| NSE | Neuron Specific Enolase |
| VEGF | Vascular Endothelial Growth Factor |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| NIHSS | National Institute of Health Stroke Scale |
| ASPECS | Alberta Stroke Program Early Computed Tomography Score |
| rtPA | Recombinant Tissue Plasminogen Activator |
| mTICI | Modified Treatment in Cerebral Ischemia |
| AUC | Area Under Curve |
| MCA | Middle Cerebral Artery |
| NCCT | Non-Contrast Computed Tomography |
| CTA | Computed Tomography Angiography |
| mRS | Modified Rankin Score |
| rCBF | Cerebral Blood Flow |
| MTT | Mean Transient Time |
| DT | Delay Time |
| Tmax | Time-To-Maximum |
| DWI | Diffusion-Weighted Imaging |
| FLAIR | Fluid Attenuation Inversion Recovery |
| PWI | Perfusion-Weighted Imaging |
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; De Georgia, M.; von Kummer, R. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996, 53, 309–315. [Google Scholar] [CrossRef]
- White, O.B.; Norris, J.W.; Hachinski, V.C.; Lewis, A. Death in early stroke, causes and mechanisms. Stroke 1979, 10, 743. [Google Scholar] [CrossRef]
- Soinne, L.; Sundararajan, S.; Strbian, D. Malignant hemispheric infarction: Diagnosis and management by hemicraniectomy. Stroke 2014, 45, e185–e187. [Google Scholar] [CrossRef]
- Huttner, H.B.; Schwab, S. Malignant middle cerebral artery infarction: Clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. 2009, 8, 949–958. [Google Scholar] [CrossRef]
- Jüttler, E.; Schellinger, P.D.; Aschoff, A.; Zweckberger, K.; Unterberg, A.; Hacke, W. Clinical review: Therapy for refractory intracranial hypertension in ischaemic stroke. Crit. Care 2007, 11, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyer, M.J.; Megyesi, J.; Meythaler, J.; Murie-Fernandez, M.; Aubut, J.A.; Foley, N.; Salter, K.; Bayley, M.; Marshall, S.; Teasell, R. Acute management of acquired brain injury part II: An evidence-based review of pharmacological interventions. Brain Inj. 2010, 24, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Bardutzky, J.; Schwab, S. Antiedema therapy in ischemic stroke. Stroke 2007, 38, 3084–3094. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, K.; Hofmeijer, J.; Juettler, E.; Vicaut, E.; George, B.; Algra, A.; Amelink, G.J.; Schmiedeck, P.; Schwab, S.; Rothwell, P.M.; et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007, 6, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, K.; Vicaut, E.; Mateo, J.; Kurtz, A.; Orabi, M.; Guichard, J.P.; Boutron, C.; Couvreur, G.; Rouanet, F.; Touzé, E.; et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 2007, 38, 2506–2517. [Google Scholar] [CrossRef]
- Hofmeijer, J.; Kappelle, L.J.; Algra, A.; Amelink, G.J.; van Gijn, J.; van der Worp, H.B. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009, 8, 326–333. [Google Scholar] [CrossRef]
- Jüttler, E.; Schwab, S.; Schmiedek, P.; Unterberg, A.; Hennerici, M.; Woitzik, J.; Witte, S.; Jenetzky, E.; Hacke, W. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): A randomized, controlled trial. Stroke 2007, 38, 2518–2525. [Google Scholar] [CrossRef]
- Vibbert, M.; Mayer, S.A. Early decompressive hemicraniectomy following malignant ischemic stroke: The crucial role of timing. Curr. Neurol. Neurosci. Rep. 2010, 10, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Thomalla, G.; Hartmann, F.; Juettler, E.; Singer, O.C.; Lehnhardt, F.G.; Köhrmann, M.; Kersten, J.F.; Krützelmann, A.; Humpich, M.C.; Sobesky, J.; et al. Prediction of malignant middle cerebral artery infarction by magnetic resonance imaging within 6 hours of symptom onset: A prospective multicenter observational study. Ann. Neurol. 2010, 68, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, A.; Laredo, C.; Reyes, L.; Dolz, G.; Doncel-Moriano, A.; Llansó, L.; Rudilosso, S.; Llull, L.; Renú, A.; Amaro, S.; et al. Computed tomography perfusion as an early predictor of malignant cerebral infarction. Eur. Stroke J. 2024, 13, 23969873241260965. [Google Scholar] [CrossRef]
- Ryoo, J.W.; Na, D.G.; Kim, S.S.; Lee, K.H.; Lee, S.J.; Chung, C.S.; Choi, D.S. Malignant middle cerebral artery infarction in hyperacute ischemic stroke: Evaluation with multiphasic perfusion computed tomography maps. J. Comput. Assist. Tomogr. 2004, 28, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Semerano, A.; Strambo, D.; Martino, G.; Comi, G.; Filippi, M.; Roveri, L.; Bacigaluppi, M. Leukocyte Counts and Ratios Are Predictive of Stroke Outcome and Hemorrhagic Complications Independently of Infections. Front. Neurol. 2020, 11, 201. [Google Scholar] [CrossRef]
- Barros, A.G.d.A.; e Silva, L.R.; Pessoa, A.; Eiras Falcão, A.; Viana Magno, L.A.; Valadão Freitas Rosa, D.; Aurelio Romano Silva, M.; Marques de Miranda, D.; Nicolato, R. Use of biomarkers for predicting a malignant course in acute ischemic stroke: An observational case-control study. Sci. Rep. 2023, 13, 16097. [Google Scholar] [CrossRef]
- Foerch, C.; Otto, B.; Singer, O.C.; Neumann-Haefelin, T.; Yan, B.; Berkefeld, J.; Steinmetz, H.; Sitzer, M. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke 2004, 35, 2160–2164. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; He, W.; Zhang, S.; Liu, M.; Wu, S. Association between serum NLRP3 and malignant brain edema in patients with acute ischemic stroke. BMC Neurol. 2021, 21, 341. [Google Scholar] [CrossRef]
- Maheshwari, S.; Um, I.H.; Donachie, S.; Asghar, N.; McDade, K.; Millar, T.; Harrison, D.J.; Tello, J.A. Kisspeptin is elevated in the brain after intracerebral haemorrhagic stroke. Sci. Rep. 2024, 14, 32046. [Google Scholar] [CrossRef]
- Shan, W.; Lan, H.; Wu, Y.; Xu, Q.; You, M.; Ma, R. Kisspeptin-54 Restores Blood-Brain Barrier Integrity via GATA-4 in Ischemic Stroke. Chem. Biol. Drug Des. 2025, 105, e70134. [Google Scholar] [CrossRef]
- Gacoń, J.; Badacz, R.; Stępień, E.; Karch, I.; Enguita, F.J.; Żmudka, K.; Przewłocki, T.; Kabłak-Ziembicka, A. Diagnostic and prognostic micro-RNAs in ischaemic stroke due to carotid artery stenosis and in acute coronary syndrome: A four-year prospective study. Kardiol Pol. 2018, 76, 362–369. [Google Scholar] [CrossRef]
- Neag, M.A.; Mitre, A.O.; Burlacu, C.C.; Inceu, A.I.; Mihu, C.; Melincovici, C.S.; Bichescu, M.; Buzoianu, A.D. miRNA Involvement in Cerebral Ischemia-Reperfusion Injury. Front. Neurosci. 2022, 16, 901360. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Sakai, N.; Yamagami, H.; Uchida, K.; Beppu, M.; Toyoda, K.; Matsumaru, Y.; Matsumoto, Y.; Kimura, K.; Takeuchi, M.; et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. N. Engl. J. Med. 2022, 386, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Ma, G.; Tong, X.; Zhang, X.; Pan, Y.; Nguyen, T.N.; Yuan, G.; Han, H.; Chen, W.; Wei, M.; et al. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N. Engl. J. Med. 2023, 388, 1272–1283. [Google Scholar] [CrossRef]
- Sarraj, A.; Hassan, A.E.; Abraham, M.G.; Ortega-Gutierrez, S.; Kasner, S.E.; Hussain, M.S.; Chen, M.; Blackburn, S.; Sitton, C.W.; Churilov, L.; et al. Trial of Endovascular Thrombectomy for Large Ischemic Strokes. N. Engl. J. Med. 2023, 388, 1259–1271, Erratum in N. Engl. J. Med. 2024, 390, 388. https://doi.org/10.1056/NEJMx230009. [Google Scholar] [CrossRef]
- Bendszus, M.; Fiehler, J.; Subtil, F.; Bonekamp, S.; Aamodt, A.H.; Fuentes, B.; Gizewski, E.R.; Hill, M.D.; Krajina, A.; Pierot, L.; et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: Multicentre, open-label, randomised trial. Lancet 2023, 402, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Costalat, V.; Jovin, T.G.; Albucher, J.F.; Cognard, C.; Henon, H.; Nouri, N.; Gory, B.; Richard, S.; Marnat, G.; Sibon, I.; et al. Trial of Thrombectomy for Stroke with a Large Infarct of Unrestricted Size. N. Engl. J. Med. 2024, 390, 1677–1689. [Google Scholar] [CrossRef]
- Luger, S.; Koerbel, K.; Martinez Oeckel, A.; Schneider, H.; Maurer, C.J.; Hintereder, G.; Wagner, M.; Hattingen, E.; Foerch, C. Role of S100B Serum Concentration as a Surrogate Outcome Parameter After Mechanical Thrombectomy. Neurology 2021, 97, e2185–e2194. [Google Scholar] [CrossRef]
- Anogianakis, G.; Daios, S.; Topouzis, N.; Barmpagiannos, K.; Kaiafa, G.; Myrou, A.; Ztriva, E.; Tsankof, A.; Karlafti, E.; Anogeianaki, A.; et al. Current Trends in Stroke Biomarkers: The Prognostic Role of S100 Calcium-Binding Protein B and Glial Fibrillary Acidic Protein. Life 2024, 14, 1247. [Google Scholar] [CrossRef] [PubMed]
- Mochetti, M.M.; Silva, E.G.P.; Correa, A.A.F.; Cabette, M.R.; Perissinotti, I.N.; E Silva, L.O.J.; Pessoa, A.S.; de Oliveira, R.C.; da Silva, L.F.F.; de Souza, H.P.; et al. Neuron-specific enolase at admission as a predictor for stroke volume, severity and outcome in ischemic stroke patients: A prognostic biomarker review. Sci. Rep. 2024, 14, 2688. [Google Scholar] [CrossRef]
- Freitas, T.E.; Costa, A.I.; Neves, L.; Barros, C.; Martins, M.; Freitas, P.; Noronha, D.; Freitas, P.; Faria, T.; Borges, S.; et al. Neuron-specific enolase as a prognostic biomarker in acute ischemic stroke patients treated with reperfusion therapies. Front. Neurol. 2024, 15, 1408111. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. 2013, 70, 1753–1761. [Google Scholar] [CrossRef]
- Seidkhani-Nahal, A.; Khosravi, A.; Mirzaei, A.; Basati, G.; Abbasi, M.; Noori-Zadeh, A. Serum vascular endothelial growth factor (VEGF) levels in ischemic stroke patients: A systematic review and meta-analysis of case-control studies. Neurol. Sci. 2021, 42, 1811–1820. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Feng, D.; Wang, X. Serum ICAM-1 as a Predictor of Prognosis in Patients with Acute Ischemic Stroke. BioMed Res. Int. 2021, 2021, 5539304. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Treadwell, S.D.; Thanvi, B. Malignant middle cerebral artery (MCA) infarction: Pathophysiology, diagnosis and management. Postgrad. Med. J. 2010, 86, 235–242. [Google Scholar] [CrossRef]
- Bivard, A.; Levi, C.; Spratt, N.; Parsons, M. Perfusion CT in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology 2013, 267, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, C.; Samson, Y.; Manaï, R.; Lalam, T.; Vandamme, X.; Crozier, S.; Srour, A.; Cornu, P.; Dormont, D.; Rancurel, G.; et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke 2000, 31, 2175–2181. [Google Scholar] [CrossRef]
- Laredo, C.; Renú, A.; Tudela, R.; Lopez-Rueda, A.; Urra, X.; Llull, L.; Macías, N.G.; Rudilosso, S.; Obach, V.; Amaro, S.; et al. The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: A comprehensive whole-brain computed tomography perfusion study. J. Cereb. Blood Flow Metab. 2020, 40, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Scalzo, F.; Saver, J.L.; Pautot, F.; Mitulescu, A.; Chaibi, Y.; Girard, N.; Salamon, N.; Liebeskind, D.S. The combination of baseline magnetic resonance perfusion-weighted imaging-derived tissue volume with severely prolonged arterial-tissue delay and diffusion-weighted imaging lesion volume is predictive of MCA-M1 recanalization in patients treated with endovascular thrombectomy. Neuroradiology 2014, 56, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Laredo, C.; Rodríguez, A.; Oleaga, L.; Hernández-Pérez, M.; Renú, A.; Puig, J.; Román, L.S.; Planas, A.M.; Urra, X.; Chamorro, Á. Adjunct Thrombolysis Enhances Brain Reperfusion following Successful Thrombectomy. Ann. Neurol. 2022, 92, 860–870. [Google Scholar] [CrossRef]
- Missler, U.; Wiesmann, M.; Friedrich, C.; Kaps, M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 1997, 28, 1956–1960. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, J.G.; Na, S.J.; Park, J.H.; Choi, Y.C.; Kim, W.J. Prediction of early clinical severity and extent of neuronal damage in anterior-circulation infarction using the initial serum neuron-specific enolase level. Arch. Neurol. 2003, 60, 37–41. [Google Scholar] [CrossRef][Green Version]
- Brea, D.; Sobrino, T.; Blanco, M.; Cristobo, I.; Rodríguez-González, R.; Rodríguez-Yañez, M.; Moldes, O.; Agulla, J.; Leira, R.; Castillo, J. Temporal profile and clinical significance of serum neuron-specific enolase and S100 in ischemic and hemorrhagic stroke. Clin. Chem. Lab Med. 2009, 47, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Serena, J. Applicability of biomarkers in ischemic stroke. Cerebrovasc. Dis. 2007, 24 (Suppl. S1), 7–15. [Google Scholar] [CrossRef]
- Whiteley, W.; Chong, W.L.; Sengupta, A.; Sandercock, P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke 2009, 40, e380–e389. [Google Scholar] [CrossRef]

| Total n = 73 | Non-Malignant n = 55 (76%) | Malignant n = 18 (24%) | p-Value | |
|---|---|---|---|---|
| Female, n (%) | 33 (45) | 30 (54) | 3 (16) | 0.003 |
| Age (years), median (IQR) | 76 (63–83) | 78 (65–84) | 64 (53–76) | 0.005 |
| Premorbid mRS, median (IQR) | 0 (0–1) | 0 (0–1) | 0.961 | |
| Hypertension, n (%) | 50 (68) | 38 (69) | 12 (66) | 0.848 |
| Dyslipidemia, n (%) | 28 (38) | 19 (34) | 9 (50) | 0.242 |
| Diabetes mellitus, n (%) | 17 (23) | 11 (20) | 6 (33) | 0.245 |
| Smoker, n (%) | 16 (21) | 12 (21) | 4 (22) | 0.971 |
| Alcohol intake (any dose), n (%) | 10 (13) | 6 (10) | 4 (22) | 0.226 |
| Ischemic cardiopathy, n (%) | 11 (15) | 6 (10) | 5 (27) | 0.082 |
| Atrial fibrillation, n (%) | 18 (24) | 14 (25) | 4 (22) | 0.728 |
| Previous stroke, n (%) | 11 (15) | 10 (18) | 1 (5) | 0.194 |
| Chronic kidney disease, n (%) | 10 (13) | 6 (10) | 4 (22) | 0.226 |
| Occlusion site | 0.247 | |||
| M1, n (%) | 42 (57) | 34 (61) | 8 (44) | |
| ICA, n (%) | 16 (21) | 9 (16) | 7 (38) | |
| Tandem, n (%) | 16 (21) | 12 (21) | 4 (22) | |
| NIHSS at admission, median (IQR) | 20 (17–24) | 20 (17–24) | 20 (18–22) | 0.515 |
| NIHSS at 24 h, median (IQR) | 17 (13–22) | 16 (11–20) | 21 (17–33) | <0.001 |
| Treatment | 0.007 | |||
| None, n (%) | 14 (19) | 8 (14) | 6 (33) | |
| rtPA, n (%) | 1 (1) | 0 (0) | 1 (5) | |
| MT, n (%) | 39 (53) | 28 (50) | 11 (61) | |
| MT + rtPA, n (%) | 19 (35) | 19 (34) | 0 (0) | |
| mTICI | 0.188 | |||
| 0, n (%) | 17 (23) | 9 (16) | 8 (44) | |
| 1, n (%) | 0 (0) | 0 (0) | 0 (0) | |
| 2a, n (%) | 6 (8) | 1 (1) | 0 (0) | |
| 2b, n (%) | 25 (34) | 23 (41) | 5 (27) | |
| 2c, n (%) | 8 (10) | 4 (7) | 2 (11) | |
| 3, n (%) | 37 (50) | 18 (32) | 4 (22) | |
| Time-to-CTP in hours, mean (SD) | 7 (6) | 7 (6) | 8 (6) | 0.571 |
| Systolic blood pressure (mmHg), mean (SD) | 144 (27) | 145 (25) | 143 (32) | 0.828 |
| Hemorrhagic transformation | ||||
| None, n (%) | 24 (32) | 22 (40) | 2 (11) | |
| HI1, n (%) | 5 (6) | 4 (7) | 1 (5) | |
| HI2, n (%) | 13 (17) | 9 (16) | 4 (22) | |
| PH1, n (%) | 16 (21) | 14 (25) | 2 (11) | |
| PH2, n (%) | 4 (5) | 3 (5) | 1 (5) | |
| SAH, n (%) | 6 (8) | 1 (1) | 5 (27) | |
| Symptomatic hemorrhagic transformation, n% | 6 (8) | 2 (3) | 4 (22) | 0.005 |
| TOAST | 0.320 | |||
| LAA, n (%) | 16 (21) | 12 (21) | 4 (22) | |
| Cardioembolism, n (%) | 34 (46) | 29 (52) | 5 (27) | |
| Undetermined, n (%) | 14 (19) | 9 (16) | 5 (27) | |
| Other, n (%) | 8 (10) | 5 (9) | 3 (15) | |
| mRS at 90 days, median (IQR) | 4 (3–6) | 4 (2–5) | 6 (5–6) | 0.050 |
| Total n = 73 | Non-Malignant n = 55 (76%) | Malignant n = 18 (24%) | p-Value | |
|---|---|---|---|---|
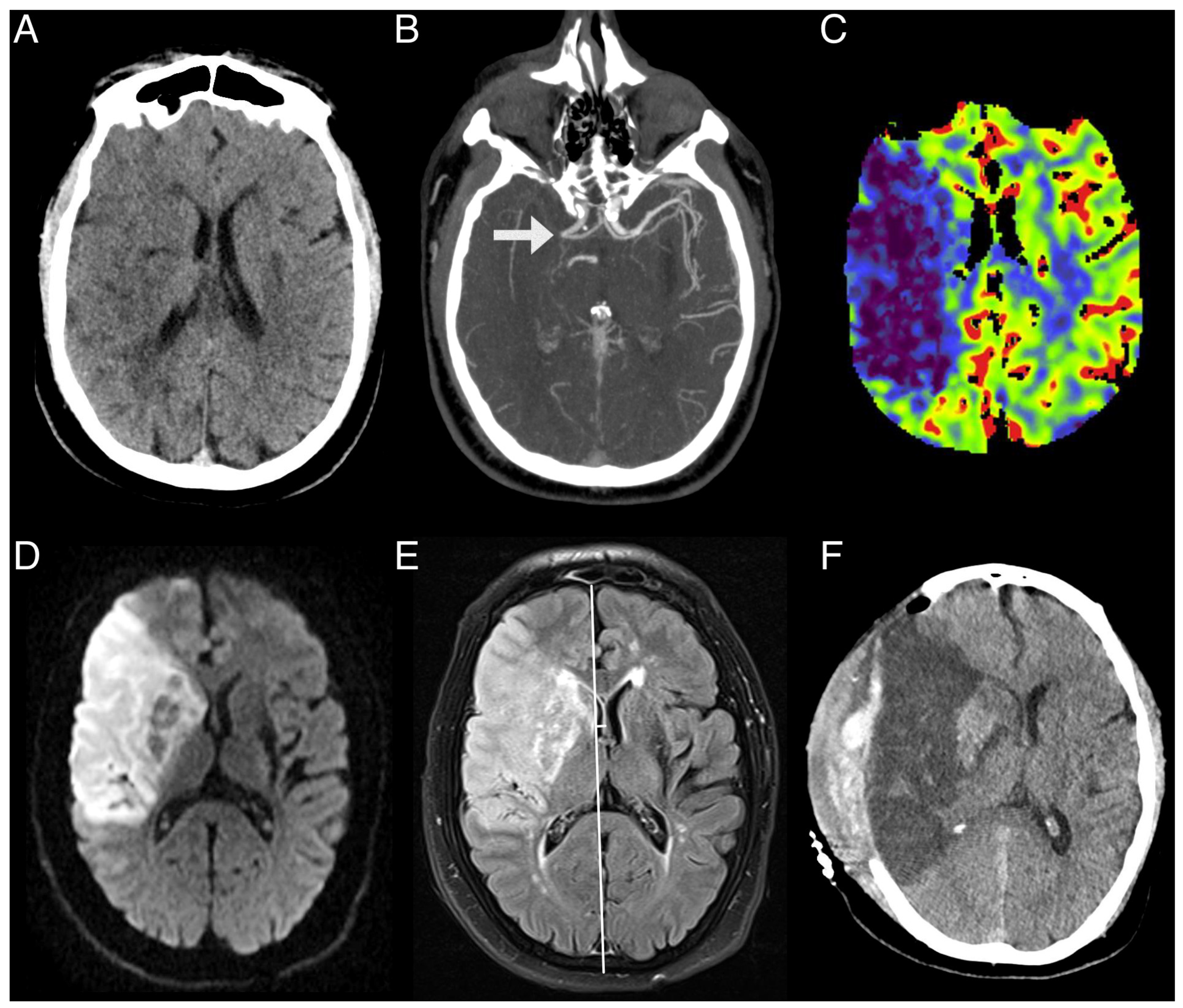
| Infarct volume (DWI-MRI) in mL, mean (SD) | 73 (57) | 66 (56) | 105 (48) | 0.010 |
| Brain edema (FLAIR-MRI) in mL, mean (SD) | 100 (70) | 78 (52) | 182 (73) | <0.001 |
| Persistent hypoperfusion (PWI-MRI) in mL, mean (SD) | 37 (58) | 26 (46) | 82 (82) | 0.025 |
| ASPECTS, median (IQR) | 7 (5–9) | 8 (6–9) | 6 (3–8) | 0.006 |
| rCBF < 30% (CTP) in mL, mean (SD) | 36 (36) | 29 (28) | 57 (49) | 0.005 |
| rCBV < 30% (CTP) in mL, mean (SD) | 16 (21) | 11 (14) | 31 (30) | <0.001 |
| Basal glycaemia (mg/dL), mean (SD) | 141 (40) | 133 (28) | 164 (61) | 0.046 |
| Glycaemia at 24 h (mg/dL), mean (SD) | 125 (41) | 119 (36) | 143 (50) | 0.038 |
| Leucocytes at admission (counts per 109/L), mean (SD) | 10.685 (3.471) | 10.164 (3.411) | 12.250 (3.255) | 0.025 |
| Leucocytes at 24 h (counts per 109/L), mean (SD) | 11.358 (3.819) | 10.282 (2.782) | 14.646 (4.689) | <0.001 |
| Neutrophils at admission(counts per 109/L), mean (SD) | 8.524 (3.623) | 7.952 (3.554) | 10.239 (3.359) | 0.016 |
| Neutrophils at 24 h (counts per 109/L), mean (SD) | 9.438 (2.804) | 8.416 (2.943) | 12.561 (4.480) | <0.001 |
| Lymphocytes at admission (counts per 109/L), mean (SD) | 1.446 (0.766) | 1.508 (0.785) | 1.261 (0.692) | 0.214 |
| Lymphocytes at 24 h (counts per 109/L), mean (SD) | 1.360 (1.580) | 1.415 (1.796) | 1.192 (0.542) | 0.985 |
| Platelets at admission (counts per 109/L), mean (SD) | 219 (73) | 226 (65) | 199 (92) | 0.188 |
| Platelets at 24 h(counts per 109/L), mean (SD) | 216 (79) | 218 (67) | 211 (109) | 0.763 |
| s100b at 24 h (µg/mL), mean (SD) | 1.256 (2.431) | 0.869 (1.130) | 2.375 (4.284) | 0.019 |
| NSE at 24 h (ng/mL), mean (SD) | 22.635 (14.462) | 20.891 (9.668) | 27.352 (22.696) | 0.030 |
| VEGF at 24 h (pg/mL), mean (SD) | 115 (159) | 115 (172) | 114 (118) | 0.986 |
| ICAM-1 at 24 h (ng/mL), mean (SD) | 165,170 (69,072) | 160,883 (64,062) | 177,578 (82,574) | 0.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Vázquez, A.; Rudilosso, S.; Doncel-Moriano, A.; Cabero-Arnold, A.; Laredo, C.; Ramis, D.; Moraleja, D.; Serrano, M.; González-Romero, Y.; Renú, A.; et al. Clinical, Imaging, and Serum Biomarker Predictors of Malignant Cerebral Infarction. J. Cardiovasc. Dev. Dis. 2025, 12, 392. https://doi.org/10.3390/jcdd12100392
Rodríguez-Vázquez A, Rudilosso S, Doncel-Moriano A, Cabero-Arnold A, Laredo C, Ramis D, Moraleja D, Serrano M, González-Romero Y, Renú A, et al. Clinical, Imaging, and Serum Biomarker Predictors of Malignant Cerebral Infarction. Journal of Cardiovascular Development and Disease. 2025; 12(10):392. https://doi.org/10.3390/jcdd12100392
Chicago/Turabian StyleRodríguez-Vázquez, Alejandro, Salvatore Rudilosso, Antonio Doncel-Moriano, Andrea Cabero-Arnold, Carlos Laredo, Darío Ramis, David Moraleja, Mònica Serrano, Yolanda González-Romero, Arturo Renú, and et al. 2025. "Clinical, Imaging, and Serum Biomarker Predictors of Malignant Cerebral Infarction" Journal of Cardiovascular Development and Disease 12, no. 10: 392. https://doi.org/10.3390/jcdd12100392
APA StyleRodríguez-Vázquez, A., Rudilosso, S., Doncel-Moriano, A., Cabero-Arnold, A., Laredo, C., Ramis, D., Moraleja, D., Serrano, M., González-Romero, Y., Renú, A., Bartolomé-Arenas, I., Rosa-Batlle, I., Dolz, G., Torné, R., Vargas, M., Urra, X., & Chamorro, Á. (2025). Clinical, Imaging, and Serum Biomarker Predictors of Malignant Cerebral Infarction. Journal of Cardiovascular Development and Disease, 12(10), 392. https://doi.org/10.3390/jcdd12100392






