Early Currents: Developmental Electrophysiology and Arrhythmia in Pediatric Congenital Heart Disease
Abstract
1. Introduction
2. Maturation of the Healthy Heart
2.1. Embryology of the CCS
2.2. Electrophysiological Maturation of the Healthy Heart
3. Development of the Pediatric Heart with CHD
3.1. CHD-Associated Conduction System Abnormalities
3.2. Epicardial Mapping in Pediatric Patients with CHD
4. Pediatric Arrhythmias: From Healthy Hearts to Congenital Defects
4.1. Arrhythmias in Children Without CHDs
4.2. Preoperative Arrhythmias in Children with CHDs
4.3. Postoperative Arrhythmias in Children with CHDs
4.3.1. Tachyarrhythmia
4.3.2. Bradyarrhythmia
5. Conclusions
6. Future Directions and Clinical Implications
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shekhar, S.; Agrawal, A.; Pampori, A.; Lak, H.; Windsor, J.; Ramakrishna, H. Mortality in Adult Congenital Heart Disease: Analysis of Outcomes and Risk Stratification. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3379–3388. [Google Scholar] [CrossRef]
- van Zyl, M.; Kapa, S.; Padmanabhan, D.; Chen, F.C.; Mulpuru, S.K.; Packer, D.L.; Munger, T.M.; Asirvatham, S.J.; McLeod, C.J. Mechanism and Outcomes of Catheter Ablation for Ventricular Tachycardia in Adults with Repaired Congenital Heart Disease. Heart Rhythm 2016, 13, 1449–1454. [Google Scholar] [CrossRef]
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Liu, A.; Diller, G.-P.; Moons, P.; Daniels, C.J.; Jenkins, K.J.; Marelli, A. Changing Epidemiology of Congenital Heart Disease: Effect on Outcomes and Quality of Care in Adults. Nat. Rev. Cardiol. 2023, 20, 126–137. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L.; et al. Global, Regional, and National Burden of Congenital Heart Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef]
- van der Bom, T.; Zomer, A.C.; Zwinderman, A.H.; Meijboom, F.J.; Bouma, B.J.; Mulder, B.J.M. The Changing Epidemiology of Congenital Heart Disease. Nat. Rev. Cardiol. 2011, 8, 50–60. [Google Scholar] [CrossRef]
- Kline, J.; Costantini, O. Arrhythmias in Congenital Heart Disease. Med. Clin. N. Am. 2019, 103, 945–956. [Google Scholar] [CrossRef]
- Salameh, S.; Ogueri, V.; Posnack, N.G. Adapting to a New Environment: Postnatal Maturation of the Human Cardiomyocyte. J. Physiol. 2023, 601, 2593–2619. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H.; Chiu, S.-N.; Tseng, W.-C.; Lu, C.-W.; Kao, F.-Y.; Huang, S.-K. Atrial Fibrillation in Adult Congenital Heart Disease and the General Population. Heart Rhythm 2023, 20, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; van Schie, M.S.; Ramdat Misier, N.L.; van Leeuwen, W.J.; Taverne, Y.J.H.J.; van de Woestijne, P.C.; Kammeraad, J.A.E.; Bartelds, B.; Bogers, A.J.J.C.; de Groot, N.M.S. First Evidence of Atrial Conduction Disorders in Pediatric Patients With Congenital Heart Disease. JACC Clin. Electrophysiol. 2020, 6, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; van Schie, M.S.; van Leeuwen, W.J.; Taverne, Y.J.H.J.; Houck, C.A.; Kammeraad, J.A.E.; Bogers, A.J.J.C.; de Groot, N.M.S. First-in-Children Epicardial Mapping of the Heart: Unravelling Arrhythmogenesis in Congenital Heart Disease. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 137–140. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, C.; Freriks, A.I.; Zheng, J.; Linderhof, M.H.C.; Nguyen, H.H.; van Schie, M.S.; Yildirim, V.; Knops, P.; Misier, N.R.; et al. Small Patients, Significant Findings: Electrophysiological Properties of Bachmann’s Bundle in Pediatric Patients. Heart Rhythm 2025, S1547-5271(25)02629-3. [Google Scholar] [CrossRef]
- Mandalenakis, Z.; Rosengren, A.; Lappas, G.; Eriksson, P.; Gilljam, T.; Hansson, P.-O.; Skoglund, K.; Fedchenko, M.; Dellborg, M. Atrial Fibrillation Burden in Young Patients With Congenital Heart Disease. Circulation 2018, 137, 928–937. [Google Scholar] [CrossRef]
- Martín de Miguel, I.; Ávila, P. Atrial Fibrillation in Congenital Heart Disease. Eur. Cardiol. Rev. 2021, 16, e06. [Google Scholar] [CrossRef]
- Kharbanda, R.K.; van Schie, M.S.; Ramdat Misier, N.L.; Wesselius, F.J.; Zwijnenburg, R.D.; van Leeuwen, W.J.; van de Woestijne, P.C.; de Jong, P.L.; Bogers, A.J.J.C.; Taverne, Y.J.H.J.; et al. In-Vivo Sino-Atrial Node Mapping in Children and Adults with Congenital Heart Disease. Front. Pediatr. 2022, 10, 896825. [Google Scholar] [CrossRef]
- Walsh, E.P.; Cecchin, F. Arrhythmias in Adult Patients With Congenital Heart Disease. Circulation 2007, 115, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Miquerol, L.; Beyer, S.; Kelly, R.G. Establishment of the Mouse Ventricular Conduction System. Cardiovasc. Res. 2011, 91, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Munshi, N.V. Development of the Cardiac Conduction System. Cold Spring Harb. Perspect. Biol. 2020, 12, a037408. [Google Scholar] [CrossRef] [PubMed]
- James, T.N. Structure and Function of the Sinus Node, AV Node and His Bundle of the Human Heart: Part I—Structure. Prog. Cardiovasc. Dis. 2002, 45, 235–267. [Google Scholar] [CrossRef]
- James, T.N. Structure and Function of the Sinus Node, AV Node and His Bundle of the Human Heart: Part II—Function. Prog. Cardiovasc. Dis. 2003, 45, 327–360. [Google Scholar] [CrossRef]
- Postma, A.V.; Christoffels, V.M.; Bezzina, C.R. Developmental Aspects of Cardiac Arrhythmogenesis. Cardiovasc. Res. 2011, 91, 243–251. [Google Scholar] [CrossRef][Green Version]
- van Weerd, J.H.; Christoffels, V.M. The Formation and Function of the Cardiac Conduction System. Development 2016, 143, 197–210. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Smits, G.J.; Kispert, A.; Moorman, A.F.M. Development of the Pacemaker Tissues of the Heart. Circ. Res. 2010, 106, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Stroud, D.M.; Gaussin, V.; Burch, J.B.E.; Yu, C.; Mishina, Y.; Schneider, M.D.; Fishman, G.I.; Morley, G.E. Abnormal Conduction and Morphology in the Atrioventricular Node of Mice with Atrioventricular Canal–Targeted Deletion of Alk3/Bmpr1a Receptor. Circulation 2007, 116, 2535–2543. [Google Scholar] [CrossRef]
- Feulner, L.; van Vliet, P.P.; Puceat, M.; Andelfinger, G. Endocardial Regulation of Cardiac Development. J. Cardiovasc. Dev. Dis. 2022, 9, 122. [Google Scholar] [CrossRef]
- Keith, A.; Flack Martin, W. The Auriculo-Ventricular Bundle of the Human Heart. Lancet 1906, 168, 359–364. [Google Scholar] [CrossRef]
- James, T.N. Sudden Death in Babies: New Observations in the Heart. Am. J. Cardiol. 1968, 22, 479–506. [Google Scholar] [CrossRef]
- Nosetti, L.; Zaffanello, M.; Lombardi, C.; Gerosa, A.; Piacentini, G.; Abramo, M.; Agosti, M. Early Screening for Long QT Syndrome and Cardiac Anomalies in Infants: A Comprehensive Study. Clin. Pract. 2024, 14, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, F.; Coll, M.; Grassi, S.; Fernàndez-Falgueras, A.; Nogué-Navarro, L.; Iglesias, A.; Castellà, J.; Oliva, A.; Brugada, R. Investigating Cardiac Genetic Background in Sudden Infant Death Syndrome (SIDS). Int. J. Legal Med. 2024, 138, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Millat, G.; Kugener, B.; Chevalier, P.; Chahine, M.; Huang, H.; Malicier, D.; Rodriguez-Lafrasse, C.; Rousson, R. Contribution of Long-QT Syndrome Genetic Variants in Sudden Infant Death Syndrome. Pediatr. Cardiol. 2009, 30, 502–509. [Google Scholar] [CrossRef]
- Arnestad, M.; Crotti, L.; Rognum, T.O.; Insolia, R.; Pedrazzini, M.; Ferrandi, C.; Vege, A.; Wang, D.W.; Rhodes, T.E.; George, A.L.; et al. Prevalence of Long-QT Syndrome Gene Variants in Sudden Infant Death Syndrome. Circulation 2007, 115, 361–367. [Google Scholar] [CrossRef]
- Liu, J.; Laksman, Z.; Backx, P.H. The Electrophysiological Development of Cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte Maturation: Advances in Knowledge and Implications for Regenerative Medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Duff, H.J. Developmental Changes in Transient Outward Current in Mouse Ventricle. Circ. Res. 1997, 81, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Trépanier-Boulay, V.; Lupien, M.-A.; St-Michel, C.; Fiset, C. Postnatal Development of Atrial Repolarization in the Mouse. Cardiovasc. Res. 2004, 64, 84–93. [Google Scholar] [CrossRef]
- Kilborn, M.J.; Fedida, D. A Study of the Developmental Changes in Outward Currents of Rat Ventricular Myocytes. J. Physiol. 1990, 430, 37–60. [Google Scholar] [CrossRef]
- Xu, H.; Dixon, J.E.; Barry, D.M.; Trimmer, J.S.; Merlie, J.P.; McKinnon, D.; Nerbonne, J.M. Developmental Analysis Reveals Mismatches in the Expression of K+ Channel Alpha Subunits and Voltage-Gated K+ Channel Currents in Rat Ventricular Myocytes. J. Gen. Physiol. 1996, 108, 405–419. [Google Scholar] [CrossRef]
- Guo, W.; Kamiya, K.; Toyama, J. Modulated Expression of Transient Outward Current in Cultured Neonatal Rat Ventricular Myocytes: Comparison with Development in Situ. Cardiovasc. Res. 1996, 32, 524–533. [Google Scholar] [CrossRef]
- Wahler, G.M.; Dollinger, S.J.; Smith, J.M.; Flemal, K.L. Time Course of Postnatal Changes in Rat Heart Action Potential and in Transient Outward Current Is Different. Am. J. Physiol. 1994, 267, H1157–H1166. [Google Scholar] [CrossRef]
- Shimoni, Y.; Fiset, C.; Clark, R.B.; Dixon, J.E.; McKinnon, D.; Giles, W.R. Thyroid Hormone Regulates Postnatal Expression of Transient K+ Channel Isoforms in Rat Ventricle. J. Physiol. 1997, 500, 65–73. [Google Scholar] [CrossRef]
- Leuranguer, V.; Monteil, A.; Bourinet, E.; Dayanithi, G.; Nargeot, J. T-Type Calcium Currents in Rat Cardiomyocytes during Postnatal Development: Contribution to Hormone Secretion. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2540–H2548. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Kumar, R.; Tipparaju, S.M.; Wagner, M.B.; Joyner, R.W. Differences in Transient Outward Current Properties between Neonatal and Adult Human Atrial Myocytes. J. Mol. Cell. Cardiol. 2003, 35, 1083–1092. [Google Scholar] [CrossRef]
- Roca, T.P.; Pigott, J.D.; Clarkson, C.W.; Crumb, W.J. L-Type Calcium Current in Pediatric and Adult Human Atrial Myocytes: Evidence for Developmental Changes in Channel Inactivation. Pediatr. Res. 1996, 40, 462–468. [Google Scholar] [CrossRef][Green Version]
- Escande, D.; Loisance, D.; Planche, C.; Coraboeuf, E. Age-Related Changes of Action Potential Plateau Shape in Isolated Human Atrial Fibers. Am. J. Physiol.-Heart Circ. Physiol. 1985, 249, H843–H850. [Google Scholar] [CrossRef] [PubMed]
- Semizel, E.; Öztürk, B.; Bostan, O.M.; Cil, E.; Ediz, B. The Effect of Age and Gender on the Electrocardiogram in Children. Cardiol. Young 2008, 18, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.-C.; Wu, M.-H.; Wang, J.-K.; Lin, M.-T.; Lu, C.-W.; Chiu, S.-N.; Chen, C.-A.; Wu, E.-T.; Wang, C.-C.; Fu, C.-M.; et al. Normal ECG Standards and Percentile Charts for Infants, Children and Adolescents. Pediatr. Neonatol. 2023, 64, 256–273. [Google Scholar] [CrossRef]
- Bratincsák, A.; Kimata, C.; Limm-Chan, B.N.; Vincent, K.P.; Williams, M.R.; Perry, J.C. Electrocardiogram Standards for Children and Young Adults Using Z-Scores. Circ. Arrhythm. Electrophysiol. 2020, 13, e008253. [Google Scholar] [CrossRef]
- Zubrzycki, M.; Schramm, R.; Costard-Jäckle, A.; Grohmann, J.; Gummert, J.F.; Zubrzycka, M. Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I. Int. J. Mol. Sci. 2024, 25, 7117. [Google Scholar] [CrossRef]
- Jongbloed, M.R.M.; Vicente Steijn, R.; Hahurij, N.D.; Kelder, T.P.; Schalij, M.J.; Gittenberger-de Groot, A.C.; Blom, N.A. Normal and Abnormal Development of the Cardiac Conduction System; Implications for Conduction and Rhythm Disorders in the Child and Adult. Differentiation 2012, 84, 131–148. [Google Scholar] [CrossRef]
- Jongbloed, M.R.M.; Mahtab, E.a.F.; Blom, N.A.; Schalij, M.J.; Gittenberger-de Groot, A.C. Development of the Cardiac Conduction System and the Possible Relation to Predilection Sites of Arrhythmogenesis. Sci. World J. 2008, 8, 160851. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.R.; Perry, J.C. Arrhythmias and Conduction Disorders Associated with Atrial Septal Defects. J. Thorac. Dis. 2018, 10, S2940–S2944. [Google Scholar] [CrossRef]
- Lin, L.; Liu, J.; Guo, X.; Chen, H.; Huang, Y.; Zheng, H.; Chen, W.; Chen, L.; Chen, L.; Chen, Z. Risk Factors for Atrioventricular Block after Occlusion for Perimembranous Ventricular Septal Defect. Heart Rhythm 2022, 19, 389–396. [Google Scholar] [CrossRef]
- Feins, E.N.; Nido, P.J. del Conduction in Congenital Heart Surgery. J. Thorac. Cardiovasc. Surg. 2023, 166, 1182–1188. [Google Scholar] [CrossRef]
- Di Mambro, C.; Calvieri, C.; Silvetti, M.S.; Tamburri, I.; Giannico, S.; Baban, A.; Albanese, S.; Brancaccio, G.; Carotti, A.; Iorio, F.S.; et al. Bradyarrhythmias in Repaired Atrioventricular Septal Defects: Single-Center Experience Based on 34 Years of Follow-up of 522 Patients. Pediatr. Cardiol. 2018, 39, 1590–1597. [Google Scholar] [CrossRef]
- Waldmann, V.; Hebe, J.; Walsh, E.P.; Khairy, P.; Ernst, S. Catheter Ablation of Atrioventricular Nodal Reentrant Tachycardia in Patients with Congenital Heart Disease. Circ. Arrhythm. Electrophysiol. 2022, 15, e010631. [Google Scholar] [CrossRef]
- Wallis, G.A.; Debich-Spicer, D.; Anderson, R.H. Congenitally Corrected Transposition. Orphanet J. Rare Dis. 2011, 6, 22. [Google Scholar] [CrossRef]
- Cardell, B.S. Corrected Transposition of the Great Vessels. Br. Heart J. 1956, 18, 186–192. [Google Scholar] [CrossRef][Green Version]
- Ozawa, Y.; Asakai, H.; Shiraga, K.; Shindo, T.; Hirata, Y.; Hirata, Y.; Inuzuka, R. Cardiac Rhythm Disturbances in Heterotaxy Syndrome. Pediatr. Cardiol. 2019, 40, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.S.; Willes, R.J.; Kovach, J.R.; Anderson, R.H. Chronic Arrhythmias in the Setting of Heterotaxy: Differences between Right and Left Isomerism. Congenit. Heart Dis. 2016, 11, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Attenhofer Jost, C.H.; Connolly, H.M.; Dearani, J.A.; Edwards, W.D.; Danielson, G.K. Ebstein’s Anomaly. Circulation 2007, 115, 277–285. [Google Scholar] [CrossRef]
- Baruteau, A.; Abrams, D.J.; Ho, S.Y.; Thambo, J.; McLeod, C.J.; Shah, M.J. Cardiac Conduction System in Congenitally Corrected Transposition of the Great Arteries and Its Clinical Relevance. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2017, 6, e007759. [Google Scholar] [CrossRef]
- Silvetti, M.S.; Favoccia, C.; Saputo, F.A.; Tamburri, I.; Mizzon, C.; Campisi, M.; Gimigliano, F.; Rinelli, G.; Rava, L.; Drago, F. Three-Dimensional-Mapping-Guided Permanent Conduction System Pacing in Paediatric Patients with Congenitally Corrected Transposition of the Great Arteries. Europace 2023, 25, 1482–1490. [Google Scholar] [CrossRef]
- Huhta, J.; Maloney, J.; Ritter, D.; Ilstrup, D.; Feldt, R. Complete Atrioventricular Block in Patients with Atrioventricular Discordance. Circulation 1983, 67, 1374–1377. [Google Scholar] [CrossRef]
- Shah, M.J.; Silka, M.J.; Avari Silva, J.N.; Balaji, S.; Beach, C.M.; Benjamin, M.N.; Berul, C.I.; Cannon, B.; Cecchin, F.; Cohen, M.I.; et al. 2021 PACES Expert Consensus Statement on the Indications and Management of Cardiovascular Implantable Electronic Devices in Pediatric Patients. Indian Pacing Electrophysiol. J. 2021, 21, 367–393. [Google Scholar] [CrossRef]
- van Geldorp, I.E.; Vanagt, W.Y.; Bauersfeld, U.; Tomaske, M.; Prinzen, F.W.; Delhaas, T. Chronic Left Ventricular Pacing Preserves Left Ventricular Function in Children. Pediatr. Cardiol. 2009, 30, 125–132. [Google Scholar] [CrossRef][Green Version]
- Gebauer, R.A.; Tomek, V.; Kubus, P.; Rázek, V.; Matejka, T.; Salameh, A.; Kostelka, M.; Janousek, J. Differential Effects of the Site of Permanent Epicardial Pacing on Left Ventricular Synchrony and Function in the Young: Implications for Lead Placement. EP Eur. 2009, 11, 1654–1659. [Google Scholar] [CrossRef]
- van Geldorp, I.E.; Delhaas, T.; Gebauer, R.A.; Frias, P.; Tomaske, M.; Friedberg, M.K.; Tisma-Dupanovic, S.; Elders, J.; Früh, A.; Gabbarini, F.; et al. Impact of the Permanent Ventricular Pacing Site on Left Ventricular Function in Children: A Retrospective Multicentre Survey. Heart Br. Card. Soc. 2011, 97, 2051–2055. [Google Scholar] [CrossRef]
- Janoušek, J.; van Geldorp, I.E.; Krupičková, S.; Rosenthal, E.; Nugent, K.; Tomaske, M.; Früh, A.; Elders, J.; Hiippala, A.; Kerst, G.; et al. Permanent Cardiac Pacing in Children: Choosing the Optimal Pacing Site. Circulation 2013, 127, 613–623. [Google Scholar] [CrossRef]
- Tomaske, M.; Breithardt, O.A.; Bauersfeld, U. Preserved Cardiac Synchrony and Function with Single-Site Left Ventricular Epicardial Pacing during Mid-Term Follow-up in Paediatric Patients. EP Eur. 2009, 11, 1168–1176. [Google Scholar] [CrossRef]
- Salameh, A.; Dhein, S.; Blanke, K.; Rastan, A.; Hiyasat, B.; Dietze, A.; Sobiraij, A.; Dähnert, I.; Janousek, J. Right or Left Ventricular Pacing in Young Minipigs with Chronic Atrioventricular Block: Long-Term in Vivo Cardiac Performance, Morphology, Electrophysiology, and Cellular Biology. Circulation 2012, 125, 2578–2587. [Google Scholar] [CrossRef]
- Khairy, P. Ventricular Arrhythmias and Sudden Cardiac Death in Adults with Congenital Heart Disease. Heart 2016, 102, 1703–1709. [Google Scholar] [CrossRef]
- Ban, J.-E. Neonatal Arrhythmias: Diagnosis, Treatment, and Clinical Outcome. Korean J. Pediatr. 2017, 60, 344–352. [Google Scholar] [CrossRef]
- Nagashima, M.; Matsushima, M.; Ogawa, A.; Ohsuga, A.; Kaneko, T.; Yazaki, T.; Okajima, M. Cardiac Arrhythmias in Healthy Children Revealed by 24-Hour Ambulatory ECG Monitoring. Pediatr. Cardiol. 1987, 8, 103–108. [Google Scholar] [CrossRef]
- Lubocka, P.; Sabiniewicz, R. Respiratory Sinus Arrhythmia in Children—Predictable or Random? Front. Cardiovasc. Med. 2021, 8, 643846. [Google Scholar] [CrossRef]
- Azak, E.; Cetin, I.I. Premature Cardiac Beats in Children with Structurally Normal Heart: Autonomic Dysregulation. Pediatr. Int. 2021, 63, 1433–1440. [Google Scholar] [CrossRef]
- Southall, D.P.; Richards, J.; Mitchell, P.; Brown, D.J.; Johnston, P.G.; Shinebourne, E.A. Study of Cardiac Rhythm in Healthy Newborn Infants. Br. Heart J. 1980, 43, 14–20. [Google Scholar] [CrossRef]
- Sharieff, G.Q.; Rao, S.O. The Pediatric ECG. Emerg. Med. Clin. N. Am. 2006, 24, 195–208. [Google Scholar] [CrossRef]
- Turner, C.J.; Wren, C. The Epidemiology of Arrhythmia in Infants: A Population-Based Study. J. Paediatr. Child Health 2013, 49, 278–281. [Google Scholar] [CrossRef]
- Srinivasan, C.; Balaji, S. Neonatal Supraventricular Tachycardia. Indian Pacing Electrophysiol. J. 2019, 19, 222–231. [Google Scholar] [CrossRef]
- Blaufox, A.D.; Rhodes, J.F.; Fishberger, S.B. Age Related Changes in Dual AV Nodal Physiology. Pacing Clin. Electrophysiol. 2000, 23, 477–480. [Google Scholar] [CrossRef]
- Brugada, J.; Blom, N.; Sarquella-Brugada, G.; Blomstrom-Lundqvist, C.; Deanfield, J.; Janousek, J.; Abrams, D.; Bauersfeld, U.; Brugada, R.; Drago, F.; et al. Pharmacological and Non-Pharmacological Therapy for Arrhythmias in the Pediatric Population: EHRA and AEPC-Arrhythmia Working Group Joint Consensus Statement. EP Eur. 2013, 15, 1337–1382. [Google Scholar] [CrossRef]
- Tasci, O.; Karadeniz, C. Ivabradine in a 15-Day-Old Male Neonate with Refractory Focal Atrial Tachycardia. Pacing Clin. Electrophysiol. 2023, 46, 924–927. [Google Scholar] [CrossRef]
- Van Hare, G.F.; Javitz, H.; Carmelli, D.; Saul, J.P.; Tanel, R.E.; Fischbach, P.S.; Kanter, R.J.; Schaffer, M.; Dunnigan, A.; Colan, S.; et al. Prospective Assessment after Pediatric Cardiac Ablation: Demographics, Medical Profiles, and Initial Outcomes. J. Cardiovasc. Electrophysiol. 2004, 15, 759–770. [Google Scholar] [CrossRef]
- Bharati, S.; Krongrad, E.; Lev, M. Study of the Conduction System in a Population of Patients with Sudden Infant Death Syndrome. Pediatr. Cardiol. 1985, 6, 29–40. [Google Scholar] [CrossRef]
- Ottaviani, G.; Buja, L.M. Anatomopathological Changes of the Cardiac Conduction System in Sudden Cardiac Death, Particularly in Infants: Advances over the Last 25 Years. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2016, 25, 489–499. [Google Scholar] [CrossRef]
- Matturri, L.; Ottaviani, G.; Ramos, S.G.; Rossi, L. Sudden Infant Death Syndrome (SIDS): A Study of Cardiac Conduction System. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2000, 9, 137–145. [Google Scholar] [CrossRef]
- Brida, M.; Chessa, M.; Celermajer, D.; Li, W.; Geva, T.; Khairy, P.; Griselli, M.; Baumgartner, H.; Gatzoulis, M.A. Atrial Septal Defect in Adulthood: A New Paradigm for Congenital Heart Disease. Eur. Heart J. 2022, 43, 2660–2671. [Google Scholar] [CrossRef]
- Deanfield, J.E.; McKenna, W.J.; Presbitero, P.; England, D.; Graham, G.R.; Hallidie-Smith, K. Ventricular Arrhythmia in Unrepaired and Repaired Tetralogy of Fallot. Relation to Age, Timing of Repair, and Haemodynamic Status. Br. Heart J. 1984, 52, 77–81. [Google Scholar] [CrossRef]
- Houck, C.A.; Ramdjan, T.T.T.K.; Yaksh, A.; Teuwen, C.P.; Lanters, E.A.H.; Bogers, A.J.J.C.; de Groot, N.M.S. Intraoperative Arrhythmias in Children with Congenital Heart Disease: Transient, Innocent Events? EP Eur. 2018, 20, e115–e123. [Google Scholar] [CrossRef]
- Khairy, P.; Van Hare, G.F.; Balaji, S.; Berul, C.I.; Cecchin, F.; Cohen, M.I.; Daniels, C.J.; Deal, B.J.; Dearani, J.A.; Groot, N.D.; et al. PACES/HRS Expert Consensus Statement on the Recognition and Management of Arrhythmias in Adult Congenital Heart Disease. Can. J. Cardiol. 2014, 30, e1–e63. [Google Scholar] [CrossRef]
- Delaney, J.W.; Moltedo, J.M.; Dziura, J.D.; Kopf, G.S.; Snyder, C.S. Early Postoperative Arrhythmias after Pediatric Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2006, 131, 1296–1300. [Google Scholar] [CrossRef]
- Lapmahapaisan, S.; Sateantantikul, N.; Maisat, W. Revisiting Risk Factors and Incidence of Postoperative Tachyarrhythmias in Pediatric Cardiac Surgery. Sci. Rep. 2025, 15, 7297. [Google Scholar] [CrossRef]
- Rękawek, J.; Kansy, A.; Miszczak-Knecht, M.; Manowska, M.; Bieganowska, K.; Brzezinska-Paszke, M.; Szymaniak, E.; Turska-Kmieć, A.; Maruszewski, P.; Burczyński, P.; et al. Risk Factors for Cardiac Arrhythmias in Children with Congenital Heart Disease after Surgical Intervention in the Early Postoperative Period. J. Thorac. Cardiovasc. Surg. 2007, 133, 900–904. [Google Scholar] [CrossRef]
- Öztürk, E.; Kafalı, H.C.; Tanıdır, İ.C.; Tunca Şahin, G.; Onan, İ.S.; Haydin, S.; Güzeltaş, A.; Ergül, Y. Early Postoperative Arrhythmias in Patients Undergoing Congenital Heart Surgery. Turk. J. Thorac. Cardiovasc. Surg. 2021, 29, 27–35. [Google Scholar] [CrossRef]
- Kerr, S.; O’Leary, E.; DeWitt, E.S.; Mah, D.Y.; Alexander, M.E.; Kheir, J.N.; Feins, E.N.; Walsh, E.P.; Triedman, J.K.; Dionne, A. Efficacy and Safety of Early Postoperative Ablation in Patients with Congenital Heart Disease. Heart Rhythm 2024, 22, 1330–1336. [Google Scholar] [CrossRef]
- Joye, R.; Beghetti, M.; Wacker, J.; Malaspinas, I.; Bouhabib, M.; Polito, A.; Bordessoule, A.; Shah, D.C. Early and Late Postoperative Tachyarrhythmias in Children and Young Adults Undergoing Congenital Heart Disease Surgery. Pediatr. Cardiol. 2023, 44, 312–324. [Google Scholar] [CrossRef]
- Mildh, L.; Hiippala, A.; Rautiainen, P.; Pettilä, V.; Sairanen, H.; Happonen, J.-M. Junctional Ectopic Tachycardia after Surgery for Congenital Heart Disease: Incidence, Risk Factors and Outcome. Eur. J. Cardiothorac. Surg. 2011, 39, 75–80. [Google Scholar] [CrossRef]
- Andreasen, J.B.; Johnsen, S.P.; Ravn, H.B. Junctional Ectopic Tachycardia after Surgery for Congenital Heart Disease in Children. Intensive Care Med. 2008, 34, 895–902. [Google Scholar] [CrossRef]
- Hoffman, T.M.; Bush, D.M.; Wernovsky, G.; Cohen, M.I.; Wieand, T.S.; Gaynor, J.W.; Spray, T.L.; Rhodes, L.A. Postoperative Junctional Ectopic Tachycardia in Children: Incidence, Risk Factors, and Treatment. Ann. Thorac. Surg. 2002, 74, 1607–1611. [Google Scholar] [CrossRef]
- Dodge-Khatami, A.; Miller, O.I.; Anderson, R.H.; Goldman, A.P.; Gil-Jaurena, J.M.; Elliott, M.J.; Tsang, V.T.; De Leval, M.R. Surgical Substrates of Postoperative Junctional Ectopic Tachycardia in Congenital Heart Defects. J. Thorac. Cardiovasc. Surg. 2002, 123, 624–630. [Google Scholar] [CrossRef]
- Makhoul, M.; Oster, M.; Fischbach, P.; Das, S.; Deshpande, S. Junctional Ectopic Tachycardia after Congenital Heart Surgery in the Current Surgical Era. Pediatr. Cardiol. 2013, 34, 370–374. [Google Scholar] [CrossRef]
- Dodge-Khatami, A.; Miller, O.I.; Anderson, R.H.; Gil-Jaurena, J.M.; Goldman, A.P.; de Leval, M.R. Impact of Junctional Ectopic Tachycardia on Postoperative Morbidity Following Repair of Congenital Heart Defects. Eur. J. Cardiothorac. Surg. 2002, 21, 255–259. [Google Scholar] [CrossRef]
- Erickson, S.J. Guidelines for the Management of Junctional Ectopic Tachycardia Following Cardiac Surgery in Children. Curr. Paediatr. 2006, 16, 275–278. [Google Scholar] [CrossRef]
- Rosales, A.M.; Walsh, E.P.; Wessel, D.L.; Triedman, J.K. Postoperative Ectopic Atrial Tachycardia in Children with Congenital Heart Disease. Am. J. Cardiol. 2001, 88, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Berger, J.T.; Berul, C.I.; Jonas, R.A.; Kaltman, J.R.; Lapsa, J.; Nath, D.S.; Sherwin, E.D.; Sinha, P.; Zurakowski, D.; et al. Risk Factors for Development of Ectopic Atrial Tachycardia in Post-Operative Congenital Heart Disease. Pediatr. Cardiol. 2018, 39, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Uniat, J.; Hill, A.C.; Shwayder, M.; Silka, M.J.; Bar-Cohen, Y. Ectopic Atrial Tachycardia in Infants Following Congenital Heart Disease Surgery. Pediatr. Cardiol. 2023, 44, 479–486. [Google Scholar] [CrossRef]
- Stephenson, E.A.; Lu, M.; Berul, C.I.; Etheridge, S.P.; Idriss, S.F.; Margossian, R.; Reed, J.H.; Prakash, A.; Sleeper, L.A.; Vetter, V.L.; et al. Arrhythmias in a Contemporary Fontan Cohort: Prevalence and Clinical Associations in a Multicenter Cross-Sectional Study. J. Am. Coll. Cardiol. 2010, 56, 890–896. [Google Scholar] [CrossRef]
- Durongpisitkul, K.; Porter, C.J.; Cetta, F.; Offord, K.P.; Slezak, J.M.; Puga, F.J.; Schaff, H.V.; Danielson, G.K.; Driscoll, D.J. Predictors of Early- and Late-Onset Supraventricular Tachyarrhythmias after Fontan Operation. Circulation 1998, 98, 1099–1107. [Google Scholar] [CrossRef]
- Fishberger, S.B.; Wernovsky, G.; Gentles, T.L.; Gauvreau, K.; Burnett, J.; Mayer, J.E.; Walsh, E.P. Factors That Influence the Development of Atrial Flutter after the Fontan Operation. J. Thorac. Cardiovasc. Surg. 1997, 113, 80–86. [Google Scholar] [CrossRef]
- Gelatt, M.; Hamilton, R.M.; McCrindle, B.W.; Gow, R.M.; Williams, W.G.; Trusler, G.A.; Freedom, R.M. Risk Factors for Atrial Tachyarrhythmias after the Fontan Operation. J. Am. Coll. Cardiol. 1994, 24, 1735–1741. [Google Scholar] [CrossRef]
- Ganea, G.; Cinteză, E.E.; Filip, C.; Iancu, M.A.; Balta, M.D.; Vătășescu, R.; Vasile, C.M.; Cîrstoveanu, C.; Bălgrădean, M. Postoperative Cardiac Arrhythmias in Pediatric and Neonatal Patients with Congenital Heart Disease—A Narrative Review. Life 2023, 13, 2278. [Google Scholar] [CrossRef]
- Houck, C.A.; Chandler, S.F.; Bogers, A.J.J.C.; Triedman, J.K.; Walsh, E.P.; de Groot, N.M.S.; Abrams, D.J. Arrhythmia Mechanisms and Outcomes of Ablation in Pediatric Patients With Congenital Heart Disease. Circ. Arrhythm. Electrophysiol. 2019, 12, e007663. [Google Scholar] [CrossRef]
- Hoffman, T.M.; Wernovsky, G.; Wieand, T.S.; Cohen, M.I.; Jennings, A.C.; Vetter, V.L.; Godinez, R.I.; Gaynor, J.W.; Spray, T.L.; Rhodes, L.A. The Incidence of Arrhythmias in a Pediatric Cardiac Intensive Care Unit. Pediatr. Cardiol. 2002, 23, 598–604. [Google Scholar] [CrossRef]
- Maisat, W.; Lapmahapaisan, S. Early Postoperative Tachyarrhythmias in Adult Congenital Heart Surgery: An Eight-Year Review at a Tertiary University Hospital in Thailand. J. Thorac. Dis. 2024, 16, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, J.-P.; Bachmann, D.C.G.; Wagner, B.P.; Pavlovic, M.; Berdat, P.; Carrel, T.; Pfenninger, J. Early Postoperative Arrhythmias after Open-Heart Procedures in Children with Congenital Heart Disease. Pediatr. Crit. Care Med. 2001, 2, 217. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Flack, E.C.; Borgman, K.Y.; Owen, J.P.; Fish, F.A.; Bichell, D.P.; Kannankeril, P.J. A Common Angiotensin-Converting Enzyme (ACE) Polymorphism and Preoperative ACE Inhibition Modify Risk of Tachyarrhythmias after Congenital Heart Surgery. Heart Rhythm Off. J. Heart Rhythm Soc. 2014, 11, 637–643. [Google Scholar] [CrossRef]
- Fuchs, S.R.; Smith, A.H.; Van Driest, S.L.; Crum, K.F.; Edwards, T.L.; Kannankeril, P.J. Incidence and Effect of Early Postoperative Ventricular Arrhythmias after Congenital Heart Surgery. Heart Rhythm 2019, 16, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Bossers, S.S.M.; Duppen, N.; Kapusta, L.; Maan, A.C.; Duim, A.R.; Bogers, A.J.J.C.; Hazekamp, M.G.; van Iperen, G.; Helbing, W.A.; Blom, N.A. Comprehensive Rhythm Evaluation in a Large Contemporary Fontan Population. Eur. J. Cardiothorac. Surg. 2015, 48, 833–841. [Google Scholar] [CrossRef]
- Manning, P.B.; Mayer, J.E.; Wernovsky, G.; Fishberger, S.B.; Walsh, E.P. Staged Operation to Fontan Increases the Incidence of Sinoatrial Node Dysfunction. J. Thorac. Cardiovasc. Surg. 1996, 111, 833–840. [Google Scholar] [CrossRef]
- Weindling, S.N.; Saul, J.P.; Gamble, W.J.; Mayer, J.E.; Wessel, D.; Walsh, E.P. Duration of Complete Atrioventricular Block after Congenital Heart Disease Surgery. Am. J. Cardiol. 1998, 82, 525–527. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A.M.; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. J. Am. Coll. Cardiol. 2008, 51, e1–e62. [Google Scholar] [CrossRef] [PubMed]
- Liberman, L.; Pass, R.H.; Hordof, A.J.; Spotnitz, H.M. Late Onset of Heart Block after Open Heart Surgery for Congenital Heart Disease. Pediatr. Cardiol. 2008, 29, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Butera, G.; Carminati, M.; Chessa, M.; Piazza, L.; Micheletti, A.; Negura, D.G.; Abella, R.; Giamberti, A.; Frigiola, A. Transcatheter Closure of Perimembranous Ventricular Septal Defects: Early and Long-Term Results. J. Am. Coll. Cardiol. 2007, 50, 1189–1195. [Google Scholar] [CrossRef]
- Predescu, D.; Chaturvedi, R.R.; Friedberg, M.K.; Benson, L.N.; Ozawa, A.; Lee, K.-J. Complete Heart Block Associated with Device Closure of Perimembranous Ventricular Septal Defects. J. Thorac. Cardiovasc. Surg. 2008, 136, 1223–1228. [Google Scholar] [CrossRef]
- O’Leary, E.T.; Feins, E.N.; Davee, J.; Baird, C.W.; Beroukhim, R.; del Nido, P.J.; Dionne, A.; Gauvreau, K.; Hoganson, D.M.; Triedman, J.K.; et al. Intraoperative Conduction Mapping to Reduce Postoperative Atrioventricular Block in Complex Congenital Heart Disease. JACC 2024, 84, 2102–2112. [Google Scholar] [CrossRef] [PubMed]
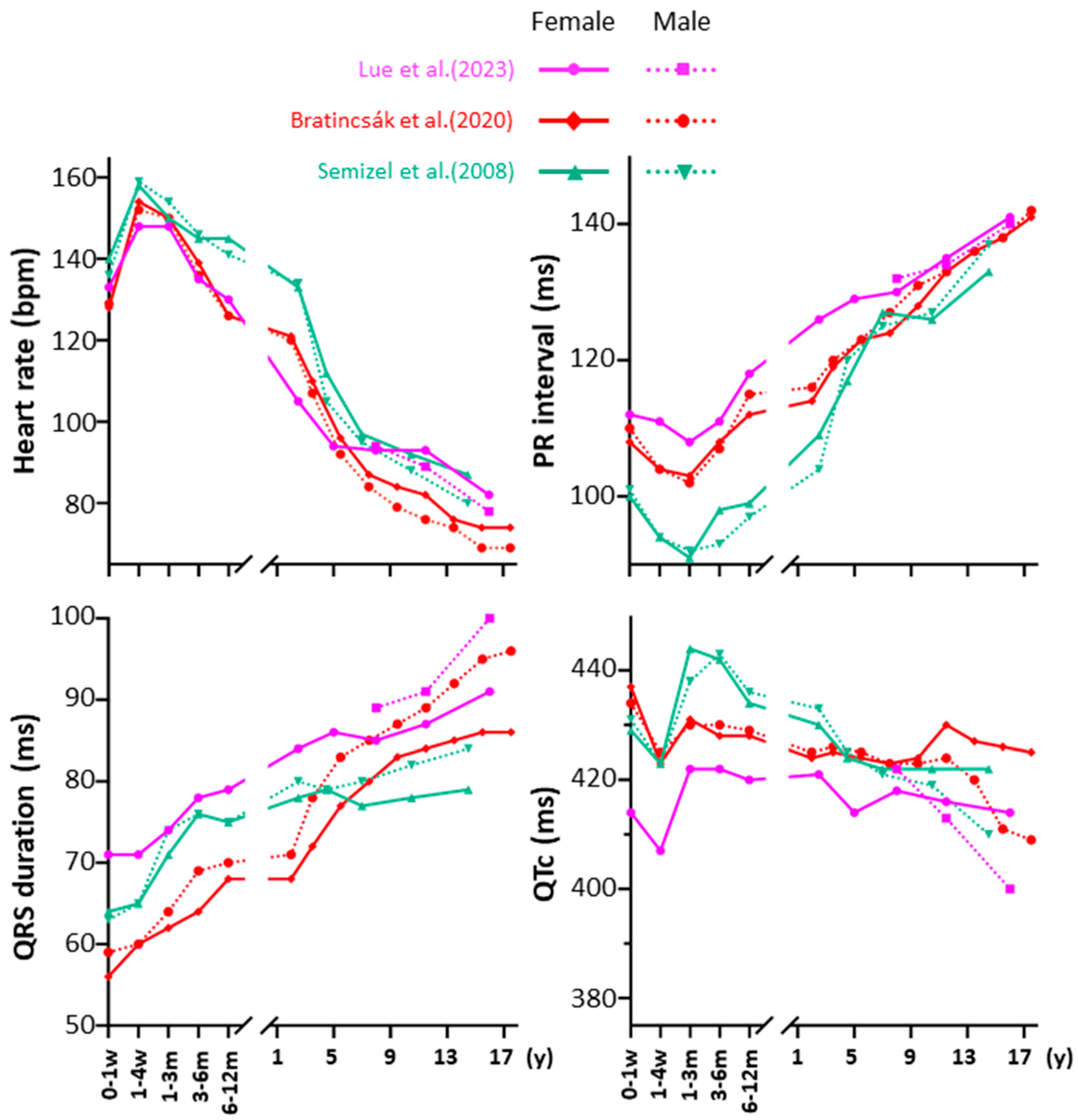

| CHD | Conduction System Abnormalities | Arrhythmias | References |
|---|---|---|---|
| ASD | Primum: posteroinferior displacement of the AVN and His bundle Secundum: AV nodal fast pathway Sinus venosus: close proximity to the SAN | AVB, SND AVNRT, SND SND | [7,51] |
| pmVSD | Posteroinferior displacement of the AVN and His bundle | AVB | [52,53] |
| AVSD | Posteroinferior displacement of the AVN and His bundle | AVB | [51,54,55] |
| ccTGA | Displacement of AVN, altered conduction axis | Complete AVB | [56,57] |
| Single ventricle | Displacement of AV conduction pathways | AVNRT | [55] |
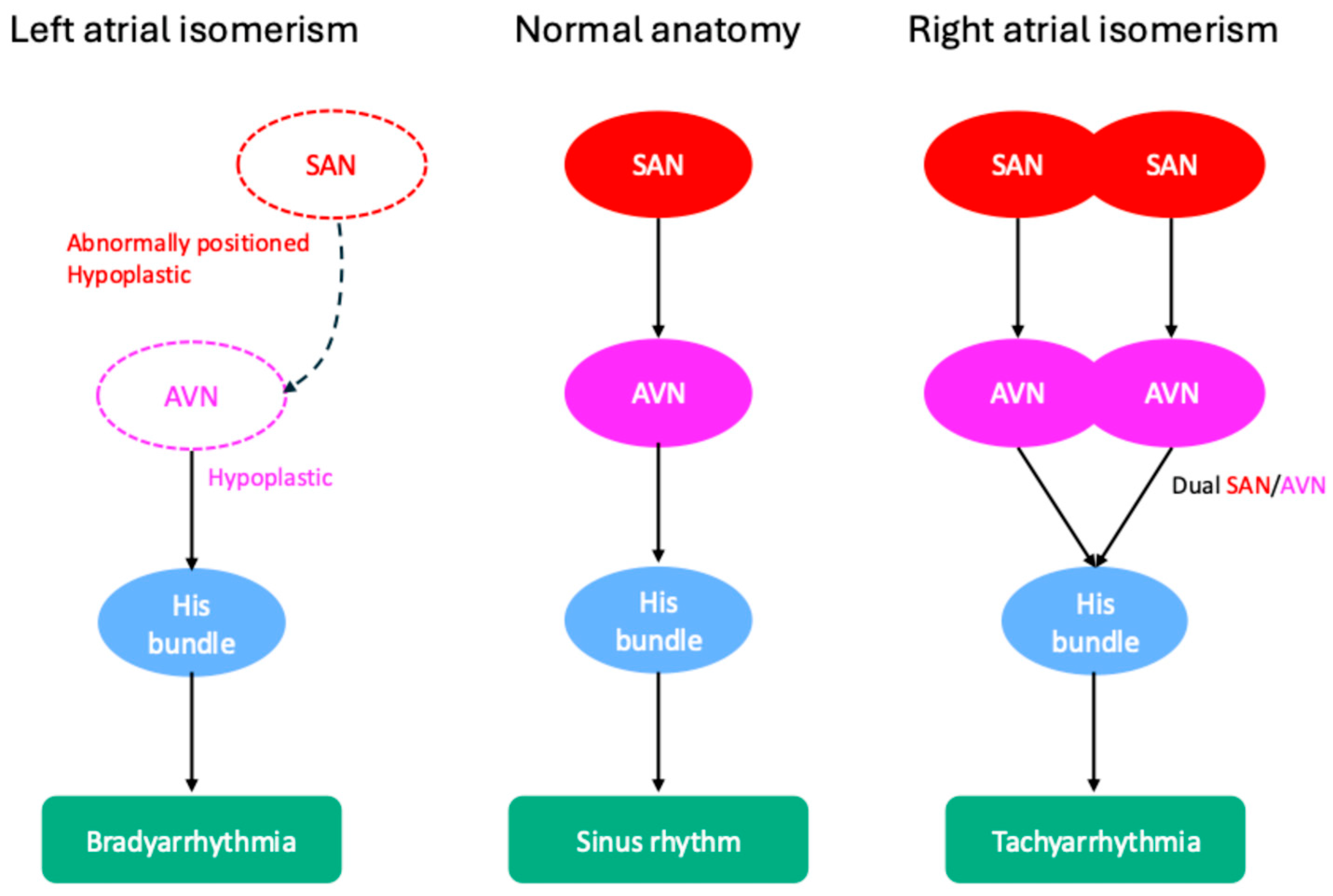
| Left atrial isomerism | Hypoplastic SAN and malformed AVN | AVB; SND | [58,59] |
| Right atrial somerism | Dual SAN and dual AVN, potential for twin AVN pathways | AFL, SVTs, and VT | [58,59] |
| Ebstein’s anomaly | Compressed AVN, malformed right bundle branch, accessory pathways | AVB, tachyarrhythmias | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Liu, W.; Yildirim, V.; van Schie, M.S.; Taverne, Y.J.H.J.; de Groot, N.M.S. Early Currents: Developmental Electrophysiology and Arrhythmia in Pediatric Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 386. https://doi.org/10.3390/jcdd12100386
Dai L, Liu W, Yildirim V, van Schie MS, Taverne YJHJ, de Groot NMS. Early Currents: Developmental Electrophysiology and Arrhythmia in Pediatric Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2025; 12(10):386. https://doi.org/10.3390/jcdd12100386
Chicago/Turabian StyleDai, Lixia, Weilin Liu, Vehpi Yildirim, Mathijs S. van Schie, Yannick J. H. J. Taverne, and Natasja M. S. de Groot. 2025. "Early Currents: Developmental Electrophysiology and Arrhythmia in Pediatric Congenital Heart Disease" Journal of Cardiovascular Development and Disease 12, no. 10: 386. https://doi.org/10.3390/jcdd12100386
APA StyleDai, L., Liu, W., Yildirim, V., van Schie, M. S., Taverne, Y. J. H. J., & de Groot, N. M. S. (2025). Early Currents: Developmental Electrophysiology and Arrhythmia in Pediatric Congenital Heart Disease. Journal of Cardiovascular Development and Disease, 12(10), 386. https://doi.org/10.3390/jcdd12100386









